Respiratory System Compliance Predicts Outcome After Lung Transplantation—A Retrospective Single Center Study
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Patients
2.3. Data Collection
2.4. Perioperative Management
2.5. Definitions and Calculations
2.6. Study Endpoints
2.7. Statistical Analysis
3. Results
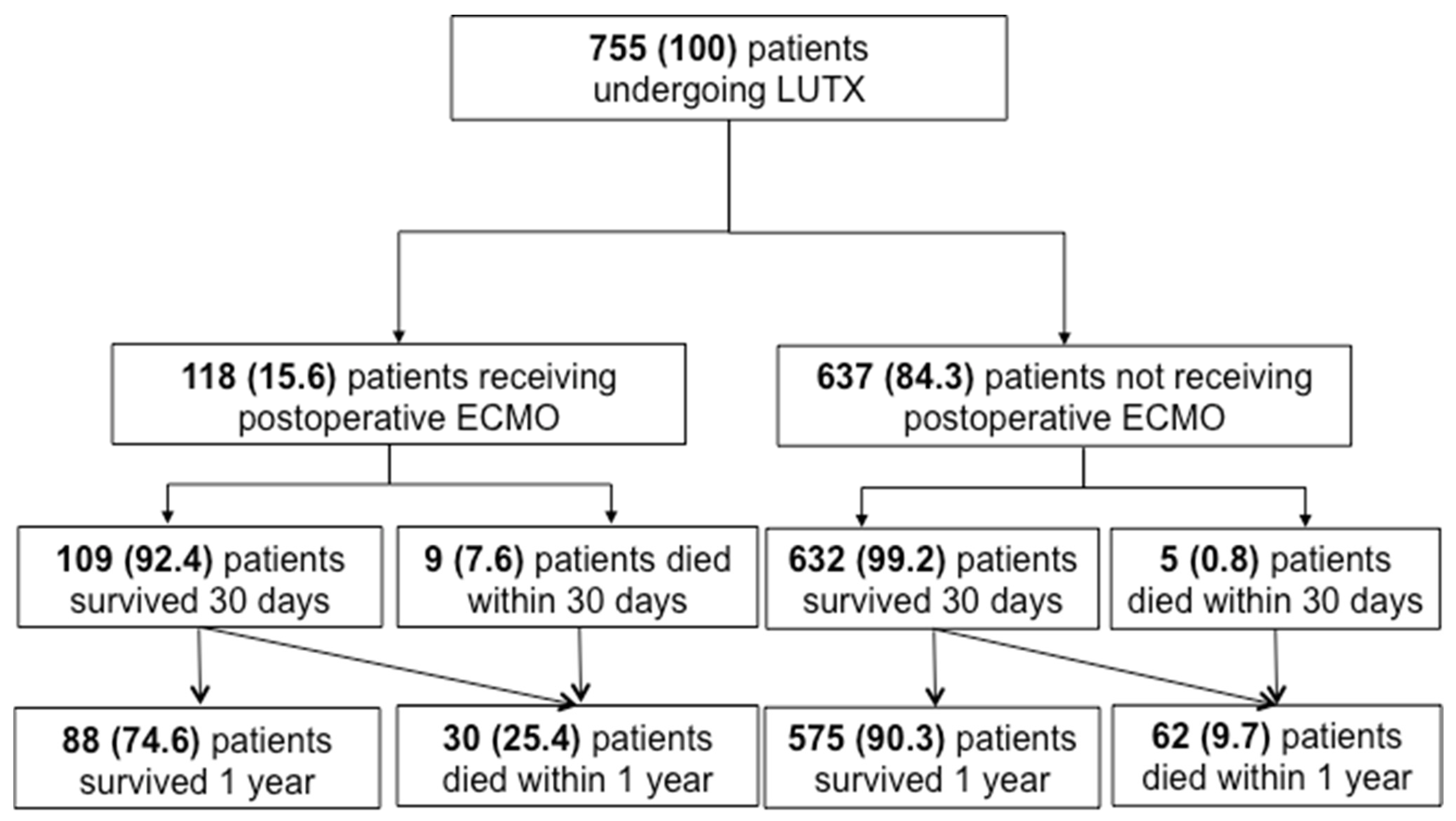
3.1. Clinical and Demographic Data
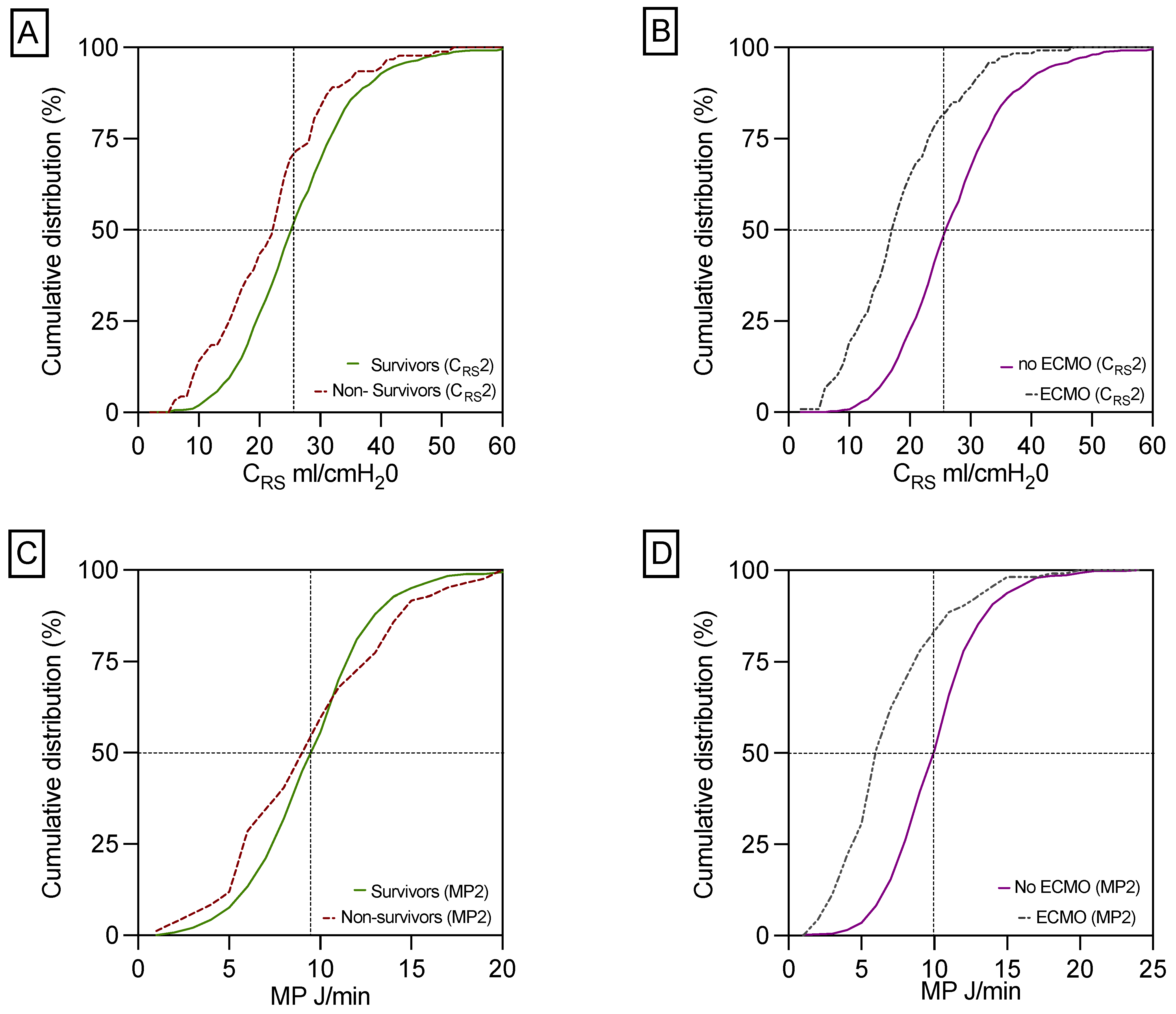
3.2. CRS and MP in Survivors and Non-Survivors
3.3. Independent Predictors for Mortality in the Multivariate Analysis
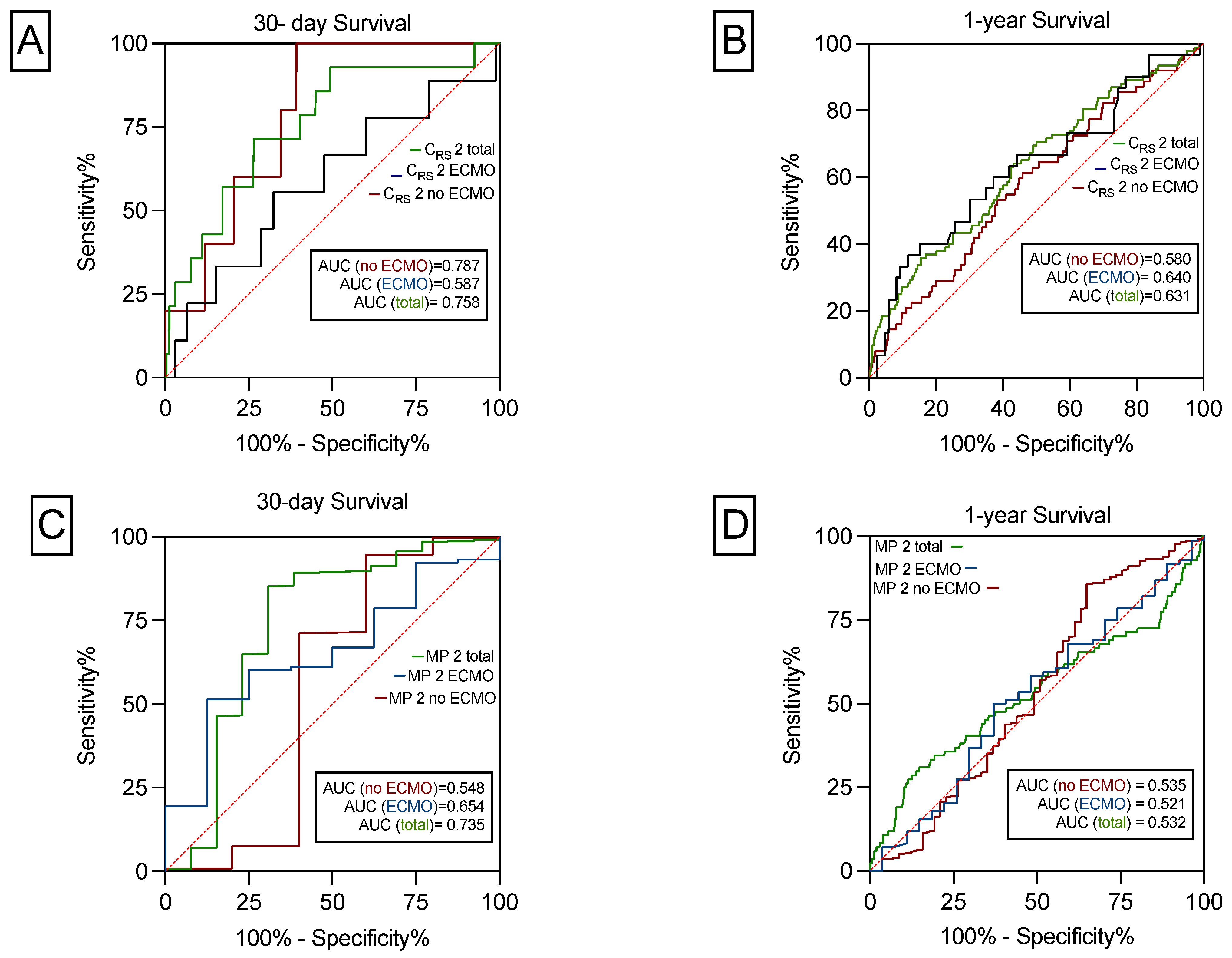
3.4. ROC Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARDS | Acute Respiratory Distress Syndrome |
| CRS | Respiratory System Compliance |
| CLAD | Chronic Lung Allograft Dysfunction |
| ECMO | Extracorporeal Membrane Oxygenation |
| FFP | Fresh Frozen Plasma |
| FN | False negative |
| HB | Hemoglobin values |
| ISHLT | International Society for Heart and Lung Transplantation |
| IQR | Interquartile Range |
| LUTX | Lung Transplantation |
| MP | Mechanical Power of Ventilation |
| OR | Operating Room |
| PBW | Predicted Body Weight |
| PGD | Primary Graft Dysfunction |
| ΔP | Driving Pressure |
| PRBC | Packed Red Blood Cells |
| TN | True Negative |
| TP | True Positive |
| VT | Tidal Volume |
| RR | Respiratory Rate |
| ROC | Receiver Operating Characteristic |
References
- Raskin, J.; Vanstapel, A.; Verbeken, E.K.; Beeckmans, H.; Vanaudenaerde, B.M.; Verleden, S.E.; Neyrinck, A.P.; Ceulemans, L.J.; Van Raemdonck, D.E.; Verleden, G.M.; et al. Mortality after lung transplantation: A single-centre cohort analysis. Transpl. Int. 2020, 33, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Kirschner, E.; Schwarz, S.; Jaksch, P.; Hoetzenecker, K.; Tschernko, E.; Dworschak, M.; Ankersmit, H.J.; Moser, B. Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO. Biology 2022, 11, 1475. [Google Scholar] [CrossRef]
- Veraar, C.; Schwarz, S.; Thanner, J.; Direder, M.; Boehm, P.M.; Harnoncourt, L.; Ortmayr, J.; Veraar, C.; Mascherbauer, J.; Klepetko, W.; et al. Transient perioperative inflammation following lung transplantation and major thoracic surgery with elective extracorporeal support: A prospective observational study. Ann. Transl. Med. 2021, 9, 385. [Google Scholar] [CrossRef]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef]
- Schwarz, S.; Benazzo, A.; Dunkler, D.; Muckenhuber, M.; Del Sorbo, L.; Di Nardo, M.; Sinn, K.; Moser, B.; Matilla, J.R.; Lang, G.; et al. Ventilation parameters and early graft function in double lung transplantation. J. Heart Lung Transplant. 2021, 40, 4–11. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Taylor Thompson, B.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Sjoding, M.W.; Hofer, T.P.; Co, I.; Courey, A.; Cooke, C.R.; Iwashyna, T.J. Interobserver Reliability of the Berlin ARDS Definition and Strategies to Improve the Reliability of ARDS Diagnosis. Chest 2018, 153, 361–367. [Google Scholar] [CrossRef]
- Benazzo, A.; Schwarz, S.; Frommlet, F.; Schweiger, T.; Jaksch, P.; Schellongowski, P.; Staudinger, T.; Klepetko, W.; Lang, G.; Hoetzenecker, K.; et al. Twenty-year experience with extracorporeal life support as bridge to lung transplantation. J. Thorac. Cardiovasc. Surg. 2019, 157, 2515–2525.e10. [Google Scholar] [CrossRef]
- Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Beer, L.; Gittler, F.; Prosch, H.; Röhrich, S.; Milos, R.; Schweiger, T.; Jaksch, P.; et al. Interobserver variability impairs radiologic grading of primary graft dysfunction after lung transplantation. J. Thorac. Cardiovasc. Surg. 2019, 158, 955–962.e1. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A.; Avalli, L.; Rossi, F.; Bombino, M. Pressure-volume curve of total respiratory system in acute respiratory failure: Computed tomographic scan study. Am. Rev. Respir. Dis. 1987, 136, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A. The concept of “baby lung”. Intensive Care Med. 2005, 31, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.E.; Schiefer, J.; Maleczek, M.; Schwarz, S.; Benazzo, A.; Rath, A.; Kulu, A.; Hritcu, R.; Faybik, P.; Schaden, E.; et al. The Effect of Prone Positioning After Lung Transplantation. Ann. Thorac. Surg. 2023, 117, 1045–1051. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Nikolla, K.; et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Belliato, M.; Epis, F.; Cremascoli, L.; Ferrari, F.; Quattrone, M.G.; Fisser, C.; Malfertheiner, M.V.; Taccone, F.S.; Di Nardo, M.; Broman, L.M.; et al. Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study. Membranes 2021, 11, 30. [Google Scholar] [CrossRef]
- Grieco, D.L.; Maggiore, S.M.; Bellani, G.; Spadaro, S.; Spinelli, E.; Tonetti, T.; Menga, L.S.; Pozzi, M.; Battaglini, D.; Di Mussi, R.; et al. Individualized positive end-expiratory pressure guided by end-expiratory lung volume in early acute respiratory distress syndrome: Study protocol for the multicenter, randomized IPERPEEP trial. Trials 2022, 23, 63. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W.; Kifjak, D.; Baron, D.; Hager, H.; et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e1. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving Pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Paudel, R.; Trinkle, C.A.; Waters, C.M.; Robinson, L.E.; Cassity, E.; Sturgill, J.L.; Broaddus, R.; Morris, P.E. Mechanical Power: A New Concept in Mechanical Ventilation. Am. J. Med. Sci. 2021, 362, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.-C.; Lin, S.-W.; Chuang, L.-P.; Li, H.-H.; Liu, P.-H.; Tsai, F.-C.; Chang, C.-H.; Hung, C.-Y.; Lee, C.-S.; Leu, S.-W.; et al. Mechanical power during extracorporeal membrane oxygenation and hospital mortality in patients with acute respiratory distress syndrome. Crit. Care 2021, 25, 13. [Google Scholar] [CrossRef]
| Total n = 755 | Survivors n = 663 | Non-Survivors n = 92 | p-Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Female # | 346 (45.8) | 308 (46.5) | 38 (41.3) | 0.382 |
| Age (years) ## | 50 ± 15.4 | 49.9 ± 15.4 | 50.9 ± 15.9 | 0.271 |
| BMI (kg/m2) ## | 22.4 ± 4.2 | 22.2 ± 4.2 | 23.0 ± 4.4 | 0.051 |
| Diagnosis | ||||
| COPD # | 282 (37.4) | 258 (38.9) | 24 (26.1) | |
| ILD # | 185 (24.5) | 153 (23.1) | 32 (34.8) | |
| CF # | 125 (16.6) | 112 (16.9) | 13 (14.1) | |
| IPAH # | 52 (6.9) | 45 (6.8) | 7 (7.6) | |
| Alpha-1 antitrypsin deficiency # | 30 (4.0) | 26 (3.9) | 4 (4.3) | |
| COVID ARDS # | 27 (3.6) | 18 (2.7) | 9 (9.8) | |
| Miscellaneous # | 21 (2.8) | 21 (3.2) | 0 (0.0) | |
| Sarcoidosis # | 9 (1.2) | 9 (1.4) | 0 (0.0) | |
| LAM # | 7 (0.9) | 6 (0.9) | 1 (1.1) | |
| Histiozytosis # | 4 (0.5) | 4 (0.6) | 0 (0.0) | |
| Bronchiectasis # | 4 (0.5) | 3 (0.5) | 1 (1.1) | |
| CTEPH # | 3 (0.4) | 2 (0.3) | 1 (1.1) | |
| Alveolarproteinosis # | 3 (0.4) | 3 (0.5) | 0 (0.0) | |
| Post-COVID fibrosis # | 3 (0.4) | 3 (0.5) | 0 (0.0) | |
| Perioperative data | ||||
| Lactate max (mmol/L) ## | 3.3 ± 1.5 | 3.2 ± 1.5 | 3.6 ± 1.9 | 0.035 |
| HB min (g/dL) ## | 9.2 ±1.3 | 9.2 ± 1.3 | 8.9 ± 1.5 | 0.091 |
| PRBC * | 8 (4.6, 13.3) | 7.8 (4.0, 12.6) | 9.0 (6.0, 13.0) | <0.001 |
| FFP * | 10 (6.0, 14.0) | 9.0 (6.0, 13.0) | 10.0 (6.9, 20,0) | 0.037 |
| Crystalloids (mL) * | 1500 (1000, 2500) | 1500 (1000, 2500) | 1500 (1000, 2200) | 0.199 |
| Extracorporeal circulation | ||||
| CPB # | 10 (1.3) | 9 (1.4) | 1 (1.1) | 0.306 |
| preoperative ECMO # | 85 (11.3) | 62 (9.4) | 23 (25.0) | <0.001 |
| intraoperative ECMO # | 738 (97.7) | 647 (97.3) | 91 (8.9) | 0.631 |
| postoperative ECMO # | 118 (15.6) | 88 (13.3) | 30 (32.6) | <0.001 |
| no ECMO # | 7 (0.9) | 7 (0.9) | - | 0.116 |
| Respiratory parameters | ||||
| Before LuTX (native lung) | ||||
| Pmax (cmH2O) * | 24.1 (19.3, 30.5) | 23.8 (19.0, 30.1) | 27.1 (21.9, 35.0) | <0.001 |
| PEEP (cmH2O) * | 4.6 (3.3, 5.2) | 4.6 (3.4, 5.1) | 4.7 (3.2, 6.0) | 0.328 |
| ΔP (cmH2O) * | 19.6 (15.0, (25.7) | 19.2 (14.7, 25.5) | 22.3 (16.5, 29.7) | <0.001 |
| VT (mL/kg) * | 6.8 (5.4, 8.3) | 6.9 (5.5, 8.4) | 6.4 (4.7,7.9) | 0.375 |
| RR (per min) * | 14 (12, 16) | 14 (12, 16) | 15 (12, 17) | 0.074 |
| CRS1 (mL/cmH2O) * | 21.9 (14.2, 32.6) | 22.5 (14.9, 33.1) | 17.7 (10.4, 24.8) | <0.001 |
| MP1 (J/min) * | 8.2 (5.9, 11.4) | 8.2 (5.8, 11.1) | 8.8 (6.4, 12.3) | 0.158 |
| At the end of LuTX (transplanted lung) | ||||
| Pmax (cmH2O) * | 24.5 (22.1, 26.8) | 24.2 (22.0, 26.5) | 25.8 (23.1, 28.0) | <0.001 |
| PEEP (cmH2O) * | 7.0 (6.0, 8.0) | 7.0 (5.8, 8.0) | 7.0 (6.1, 8.0) | 0.028 |
| ΔP (cmH2O) * | 17.6 (15.0, 20.3) | 17.3 (14.8, 20.2) | 18.1 (15.7, 21.2) | 0.034 |
| VT (mL/kg) * | 6.9 (5.7, 8.2) | 7.0 (5.8, 8.3) | 6.0 (4.6, 7.4) | <0.001 |
| RR (per min) * | 15 (13, 16) | 15 (14, 16) | 15 (13, 18) | 0.516 |
| CRS2 (mL/cmH2O) * | 25.1 (19.4, 31.5) | 25.8 (20.1, 32.1) | 22.5 (15.2, 28.4) | <0.001 |
| MP2 (J/min) * | 9.9 (7.7, 12.0) | 10.0 (7.8, 12.0) | 9.3 (6.2, 13.1) | 0.329 |
| Outcome | ||||
| PGD time point 0 h | ||||
| PGD 0 | 494 (65.4) | 441 (66.5) | 53 (57.6) | |
| PGD 1 | 30 (4.0) | 27 (4.1) | 3 (3.3) | |
| PGD 2 | 40 (5.3) | 39 (5.9) | 1 (1.1) | |
| PGD 3 | 91 (12.1) | 75 (11.3) | 15 (17.4) | |
| PGD ungradable | 84 (11.1) | 67 (10.1) | 17 (18.5) | |
| PGD missing | 16 (2.1) | 14 (2.1) | 2 (2.2) | 0.016 |
| PGD time point 24 h | ||||
| PGD 0 | 594 (72.7) | 493 (74.4) | 56 (60.9) | |
| PGD 1 | 53 (7.0) | 50 (7.5) | 3 (3.3) | |
| PGD 2 | 30 (4.0) | 27 (4.1) | 3 (3.3) | |
| PGD 3 | 33 (4.4) | 22 (3.3) | 11 (12.0) | |
| PGD ungradable | 73 (9.7) | 58 (8.7) | 15 (16.3) | |
| PGD missing | 17 (2.3) | 13 (2.0) | 4 (4.3) | <0.001 |
| Ventilation support and length of stay | ||||
| Duration of MV (days) | 7 (4, 16) | 6 (4, 12) | 9 (6, 18) | <0.001 |
| ICU length of stay (days) | 9 (6, 21) | 9 (18, 18) | 22 (9, 49) | <0.001 |
| Hospital length of stay (days) | 27 (20, 42) | 27 (20, 40) | 36 (23, 71) | <0.001 |
| At End of TX | No Postoperative ECMO | ECMO Prolongation | ||||
|---|---|---|---|---|---|---|
| Survivors n = 592 | Non-Survivors n = 64 | p-Value | Survivor n = 89 | Non-Survivor n = 33 | p-Value | |
| Pmax (cmH2O) * | 24.1 (22.0, 26.2) | 25.1 (22.2, 27.6) | 0.056 | 25.0 (22.6, 28.0) | 26.9 (24.8, 30.0) | 0.047 |
| PEEP (cmH2O) * | 6.8 (5.6, 7.8) | 6.9 (5.9, 7.7) | 0.767 | 7.5 (6.1, 8.1) | 8.0 (7.0, 9.9) | 0.040 |
| ΔP (cmH2O) * | 17.3 (15.0, 20.1) | 17.8 (15.2, 20.9) | 0.130 | 17.5 (14.9, 20.9) | 18.6 (16.0, 21.5) | 0.459 |
| VT (mL/kg) * | 7.2 (6.1, 8.4) | 6.9 (5.9, 7.7) | 0.011 | 5.1 (4.0, 6.7) | 4.6 (2.8, 6.5) | 0.167 |
| RR (per min) * | 15 (14, 16) | 15 (14, 18) | 0.261 | 14 (11, 16) | 14 (12, 18) | 0.450 |
| CRS (mL/cmH2O) * | 26.5 (21.2, 32.7) | 24.1 (19.2, 30.3) | 0.037 | 18.6 (13.6, 24.4) | 15.8 (9.6, 23.0) | 0.034 |
| MP (J/min) * | 10.4 (8.4, 12.1) | 10.6 (8.2, 13.8) | 0.382 | 6.8 (4.9, 9.1) | 6.2 (4.5, 9.8) | 0.741 |
| A | Univariate Model | Multivariate Model | |||||
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | ||
| 30-day all-cause mortality | |||||||
| CRS | ≥25.1 mL/mbar | 1.0 | |||||
| <25.1 mL/mbar | 6.0 | 1.3–27.0 | 0.005 | 5.2 | 0.7–38.2 | 0.099 | |
| MP | ≥9.9 J/min | 1.0 | |||||
| <9.9 J/min | 3.4 | 0.9–12.6 | 0.058 | ||||
| Gender | male | 1.0 | |||||
| female | 1.8 | 0.6–5.1 | 0.257 | ||||
| Age | ≤20 years | 1.0 | 1.0 | ||||
| 21–40 years | 0.6 | 0.1–2.5 | 0.489 | 0.1 | 0.0–0.7 | 0.024 | |
| 41–60 years | 0.1 | 0.0–0.7 | 0.018 | 0.0 | 0.0–0.6 | 0.022 | |
| 61–75 years | 0.2 | 0.0–1.1 | 0.068 | 0.1 | 0.0–2.1 | 0.168 | |
| sCR | ≤1 mg/dL | 1.0 | |||||
| 1–2 mg/dL | 6.4 | 2.1–19.1 | <0.001 | 4.1 | 1.1–14.6 | 0.027 | |
| Diagnosis | COPD | 1.0 | 1.0 | ||||
| ILD | 9.2 | 1.1–76.5 | 0.040 | 2.8 | 0.2–2.0 | 0.586 | |
| CF | 6.7 | 0.7–65.8 | 0.093 | 0.5 | 0.0–13.6 | 0.686 | |
| IPAH | 16.6 | 1.7–160 | 0.015 | 0.8 | 0.0–18.0 | 0.918 | |
| Alpha 1 | 0.0 | 0.0– | 0.996 | 0.0 | 0.0– | 0.993 | |
| COVID ARDS | 0.0 | 0.0 | <0.001 | 0.0 | 0.0 | 0.998 | |
| Miscellaneous | 0.0 | 0.0– | 0.992 | 0.0 | 0.0– | 0.991 | |
| Sarcoidosis | 0.0 | 0.0– | 0.989 | 0.0 | 0.0– | 0.993 | |
| LAM | 0.0 | 0.0– | 0.995 | 0.0 | 0.0– | 0.997 | |
| Histiozytosis | 0.0 | 0.0– | 0.996 | 0.0 | 0.0– | 0.992 | |
| Bronchiectasis | 0.0 | 0.0– | 0.994 | 0.0 | 0.0– | 0.996 | |
| CTEPH | 112 | 7.0–1803 | <0.001 | 85.5 | 2.6–319.0 | 0.006 | |
| Alveolarproteinosis | 0.0 | 0.0– | 0.997 | 0.0 | 0.0– | 0.997 | |
| Post-COVID fibrosis | 0.0 | 0.0– | 0.990 | 0.0 | 0.0– | 0.993 | |
| ECMO | postop ECMO (yes) | 9.9 | 3.3–29.7 | <0.001 | 1.8 | 0.4–8.1 | 0.168 |
| preop ECMO (yes) | 2.1 | 0.6–7.2 | 0.239 | ||||
| HB min | >9.2 g/dL | 1.0 | |||||
| ≤9.2 g/dL | 1.8 | 0.7–6.2 | 0.253 | ||||
| BL max | <3.0 mmol/L | 1.0 | |||||
| 3.0–6.0 mmol/L | 2.1 | 0.6–7.0 | 0.219 | ||||
| >6.0 mmol/L | 4.3 | 0.8–23.9 | 0.088 | ||||
| PRBCs | <8 units | 1.0 | 1.0 | ||||
| >8–16 units | 6.2 | 0.7–56.0 | 0.097 | 3.8 | 0.4–37.4 | 0.241 | |
| >16 units | 23.0 | 2.8–184 | <0.001 | 9.7 | 1.0–94.4 | 0.050 | |
| FFPs | <5 units | 1.0 | |||||
| >5–10 units | 0.4 | 0.0–6.4 | 0.522 | ||||
| >10–15 units | 0.8 | 0.0–12.8 | 0.875 | ||||
| >15–20 units | 0.0 | 0.0– | 0.989 | ||||
| >20 units | 5.9 | 0.6–56.8 | 0.124 | ||||
| B | Univariate Model | Multivariate Model | |||||
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | ||
| 1-year all-cause mortality | |||||||
| CRS | ≥25.1 mL/mbar | 1.0 | |||||
| <25.1 mL/mbar | 1.9 | 1.2–3.0 | 0.002 | 2.1 | 1.1–4.8 | 0.048 | |
| MP | ≥9.9 J/min | 1.0 | |||||
| <9.9 J/min | 1.1 | 0.7–1.8 | 0.437 | ||||
| Gender | male | 1.0 | |||||
| female | 1.1 | 0.7–1.7 | 0.417 | ||||
| Age | ≤20 years | 1.0 | |||||
| 21 < 40 years | 0.6 | 0.2–1.5 | 0.367 | ||||
| 41–60 years | 0.6 | 0.3–1.4 | 0.332 | ||||
| 61–75 years | 1.0 | 0.4–2.2 | 0.887 | ||||
| sCR | ≤1 mg/dL | 1.0 | |||||
| 1–2 mg/dL | 2.4 | 1.5–3.9 | <0.001 | 2.9 | 1.3–6.5 | 0.008 | |
| Diagnosis | COPD | 1.0 | 1.0 | ||||
| ILD | 2.0 | 1.2–3.5 | 0.006 | 0.7 | 0.2–2.0 | 0.586 | |
| CF | 1.1 | 0.6–2.3 | 0.522 | 0.7 | 0.2–2.1 | 0.547 | |
| IPAH | 1.6 | 0.6–3.6 | 0.273 | 0.3 | 0.0–1.6 | 0.197 | |
| Alpha 1 | 1.3 | 0.4–3.9 | 0.565 | 1.1 | 0.1–9.1 | 0.891 | |
| COVID ARDS | 4.4 | 2.0–9.4 | <0.001 | 1.5 | 0.9–14.9 | 0.412 | |
| Miscellaneous | 0.0 | 0.0–2.4e279 | 0.971 | 0.0 | 0.0– | 0.981 | |
| Sarcoidosis | 0.0 | 0.0– | 0.981 | 0.0 | 0.0– | 0.989 | |
| LAM | 1.7 | 0.2–12.8 | 0.588 | 2.1 | 0.2–18.0 | 0.490 | |
| Histiozytosis | 0.0 | 0.0– | 0.986 | 0.0 | 0.0– | 0.992 | |
| Bronchiectasis | 4.0 | 0.5–29.8 | 0.171 | 4.3 | 0.7–23.7 | 0.091 | |
| CTEPH | 5.0 | 0.6–37.6 | 0.111 | 3.1 | 0.3–24.9 | 0.279 | |
| Alveolarproteinosis | 0.0 | 0.0– | 0.989 | 0.0 | 0.0– | 0.981 | |
| Post-COVID fibrosis | 0.0 | 0.0– | 0.991 | 0.0 | 0.0– | 0.993 | |
| ECMO | postop ECMO (yes) | 3.1 | 2.0–4.7 | <0.001 | 1.2 | 0.5–2.9 | 0.592 |
| preop ECMO (yes) | 2.8 | 1.7–4.5 | <0.001 | 0.6 | 0.2–2.2 | 0.547 | |
| HB min | >9.2 g/dL | 1.0 | |||||
| ≤9.2 g/dL | 1.6 | 1.1–2.5 | 0.015 | ||||
| BL max | <3.0 mmol/L | 1.0 | 1.0 | ||||
| 3.0–6.0 mmol/L | 1.2 | 0.8–1.8 | 0.316 | 2.1 | 1.0–4.6 | 0.043 | |
| >6.0 mmol/L | 2.1 | 1.0–4.3 | 0.044 | 1.1 | 0.3–4.2 | 0.786 | |
| PRBCs | <4 units | 1.0 | 1.0 | ||||
| >4–8 units | 2.2 | 0.7–6.5 | 0.135 | 2.2 | 0.4–10.2 | 0.296 | |
| >8–16 units | 4.2 | 1.4–11.8 | 0.006 | 3.9 | 0.7–20.2 | 0.107 | |
| >16 units | 7.3 | 2.6–20.9 | <0.001 | 7.1 | 11–4.5 | 0.027 | |
| FFPs | <5 units | 1.0 | 1.0 | ||||
| >5–10 units | 0.7 | 0.2–1.7 | 0.446 | 1.0 | 0.3–3.0 | 0.867 | |
| >10–15 units | 0.5 | 0.2–1.6 | 0.327 | 0.5 | 0.1–1.6 | 0.270 | |
| >15–20 units | 1.2 | 0.4–3.8 | 0.693 | 0.7 | 0.2–2.7 | 0.692 | |
| >20 units | 2.6 | 1.0–6.7 | 0.040 | 0.7 | 0.2–2.6 | 0.668 | |
| CRS 30-Day Survival | CRS 1-Year Survival | MP 30-Day Survival | MP 1-Year Survival | PGD (No) 30-Day Survival | PGD (No) 1-Year Survival | |
|---|---|---|---|---|---|---|
| PPV | 99% | 91% | 99% | 88% | 98% | 89% |
| NPV | 3% | 15% | 2% | 10% | 3% | 15% |
| Sensitivity | 50% | 52% | 51% | 51% | 67% | 67% |
| Specificity | 85% | 65% | 75% | 52% | 64% | 41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veraar, C.; Schwarz, S.; Wohlrab, P.; Geilen, J.; Fischer, A.; Neugebauer, T.; Hillebrand, C.; Moser, B.; Hoetzenecker, K.; Dworschak, M.; et al. Respiratory System Compliance Predicts Outcome After Lung Transplantation—A Retrospective Single Center Study. J. Clin. Med. 2025, 14, 6941. https://doi.org/10.3390/jcm14196941
Veraar C, Schwarz S, Wohlrab P, Geilen J, Fischer A, Neugebauer T, Hillebrand C, Moser B, Hoetzenecker K, Dworschak M, et al. Respiratory System Compliance Predicts Outcome After Lung Transplantation—A Retrospective Single Center Study. Journal of Clinical Medicine. 2025; 14(19):6941. https://doi.org/10.3390/jcm14196941
Chicago/Turabian StyleVeraar, Cecilia, Stefan Schwarz, Peter Wohlrab, Johannes Geilen, Arabella Fischer, Thomas Neugebauer, Caroline Hillebrand, Bernhard Moser, Konrad Hoetzenecker, Martin Dworschak, and et al. 2025. "Respiratory System Compliance Predicts Outcome After Lung Transplantation—A Retrospective Single Center Study" Journal of Clinical Medicine 14, no. 19: 6941. https://doi.org/10.3390/jcm14196941
APA StyleVeraar, C., Schwarz, S., Wohlrab, P., Geilen, J., Fischer, A., Neugebauer, T., Hillebrand, C., Moser, B., Hoetzenecker, K., Dworschak, M., Schultz, M. J., & Tschernko, E. M. (2025). Respiratory System Compliance Predicts Outcome After Lung Transplantation—A Retrospective Single Center Study. Journal of Clinical Medicine, 14(19), 6941. https://doi.org/10.3390/jcm14196941








