From Emergency Department to Operating Room: The Role of Early Prehabilitation and Perioperative Care in Emergency Laparotomy: A Scoping Review and Practical Proposal
Abstract
1. Introduction
2. Materials and Methods
2.1. Rationale and Design
2.2. Research Questions
- What ERAS standards and guidelines currently exist for emergency laparotomy?
- What evidence supports the feasibility and effectiveness of ERAS-aligned interventions in emergency surgery?
- Which components of pre-optimization are feasible to initiate in the emergency department (ED) without delaying surgery?
- Which tools (e.g., ESS, sarcopenia, frailty indices) are available for rapid risk stratification in ED settings?
- What evidence exists regarding the contextual application in oncological emergencies, particularly in obstructive or complicated colorectal cancer?
2.3. Eligibility Criteria (PCC Framework)
- Population: Adults (≥18 years) undergoing emergency gastrointestinal or hepatopancreatobiliary surgery, with an emphasis on EL.
- Concept: Interventions or care bundles aligned with ERAS and perioperative optimization, including nutrition, respiratory training, mobilization, delirium prevention, anemia/micronutrient correction, fluid and analgesia strategies, and implementation science. Also included were risk stratification tools (ESS, frailty, sarcopenia).
- Context: EDs, acute surgical admission units, and perioperative emergency pathways (including oncological emergencies).
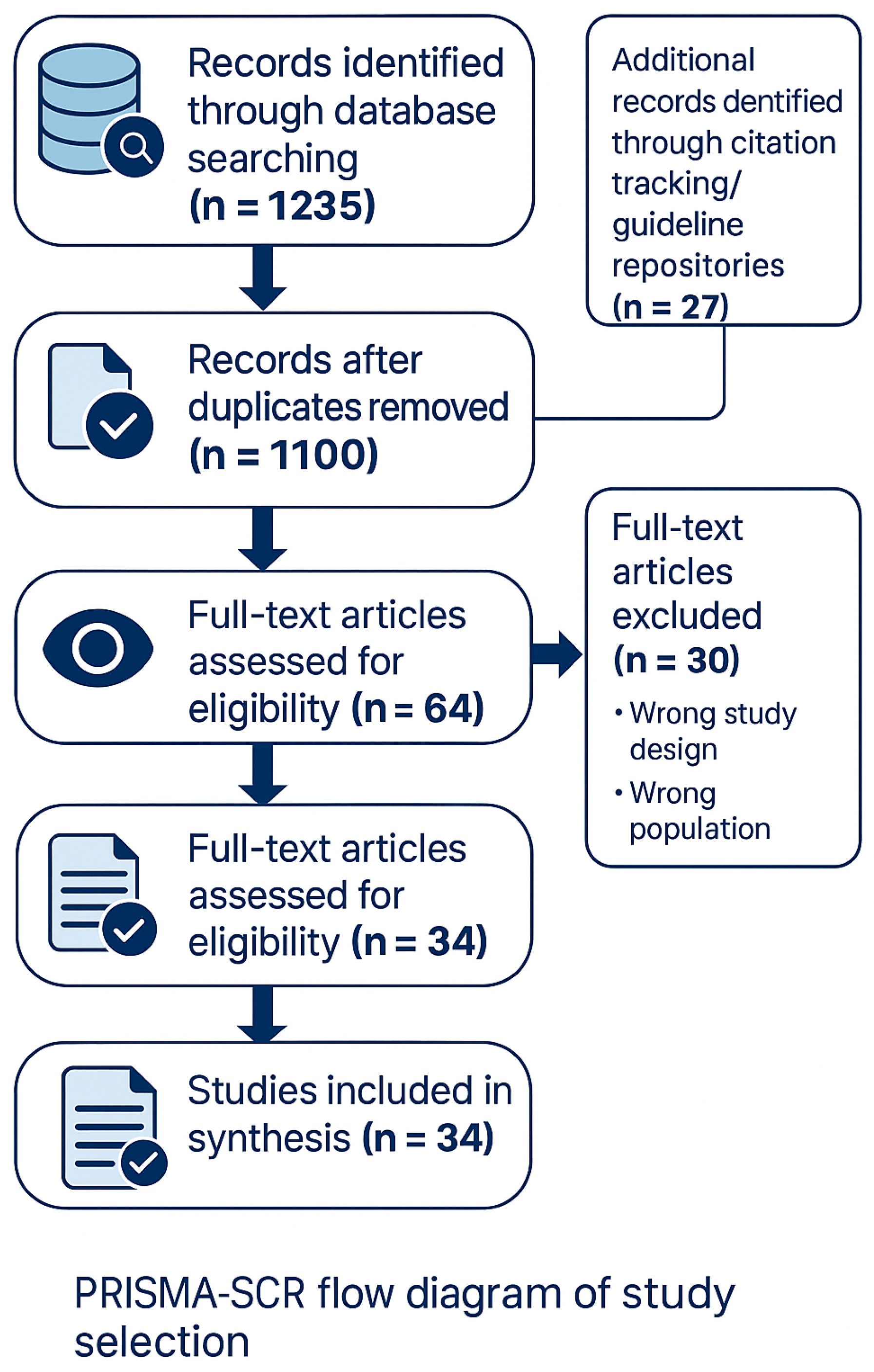
2.4. Search Details and Reviewer Agreement
2.5. Study Selection
2.6. Data Extraction and Charting
2.7. Synthesis of Results
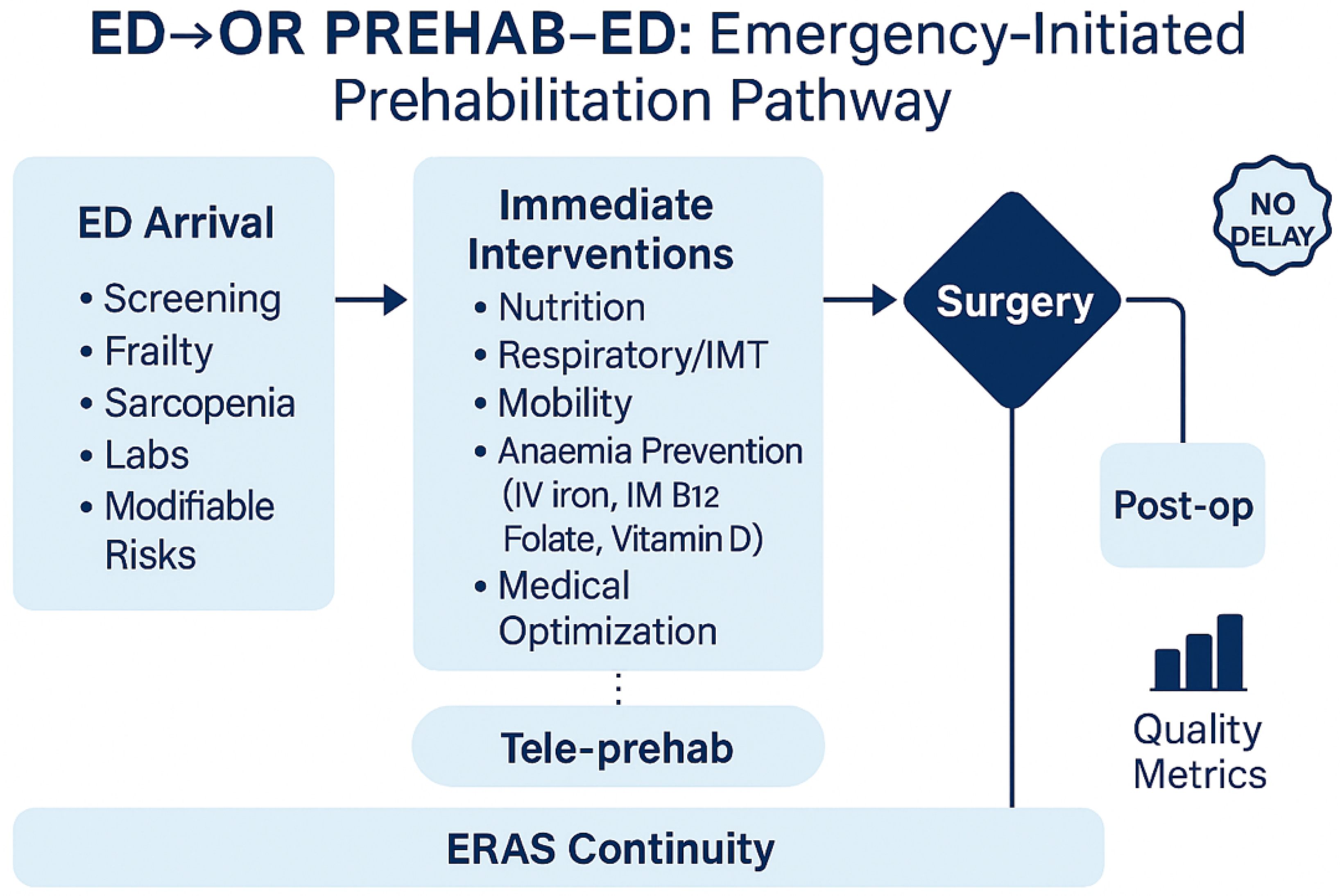
2.8. Patient Selection and Decision Rules for ED Activation
- Full bundle if ESS ≥ 7 or Clinical Frailty Scale (CFS) ≥ 5 or documented sarcopenia (rectus femoris ultrasound below age/sex thresholds), and expected time-to-OR ≥ 3 h.
- Partial bundle (analgesia/fluids ± respiratory coaching) if ESS 4–6 or CFS 4; defer nutrition if aspiration risk or bowel obstruction is suspected.
- Contraindications: imminent transfer to OR (<2 h); sepsis-induced hemodynamic instability (defer IV iron); high aspiration risk/bowel obstruction (no ONS); severe hypoxemia or agitation precluding coaching.
- Concurrency rules: when ≥2 high-risk arrivals compete, prioritize by (a) time-to-OR and (b) respiratory risk (e.g., SpO…OR and (b) respiratory risk (e.g., SpO2 < 92%, COPD) for early physiotherapy; nutrition tasks can shift to ward handover.
3. Results
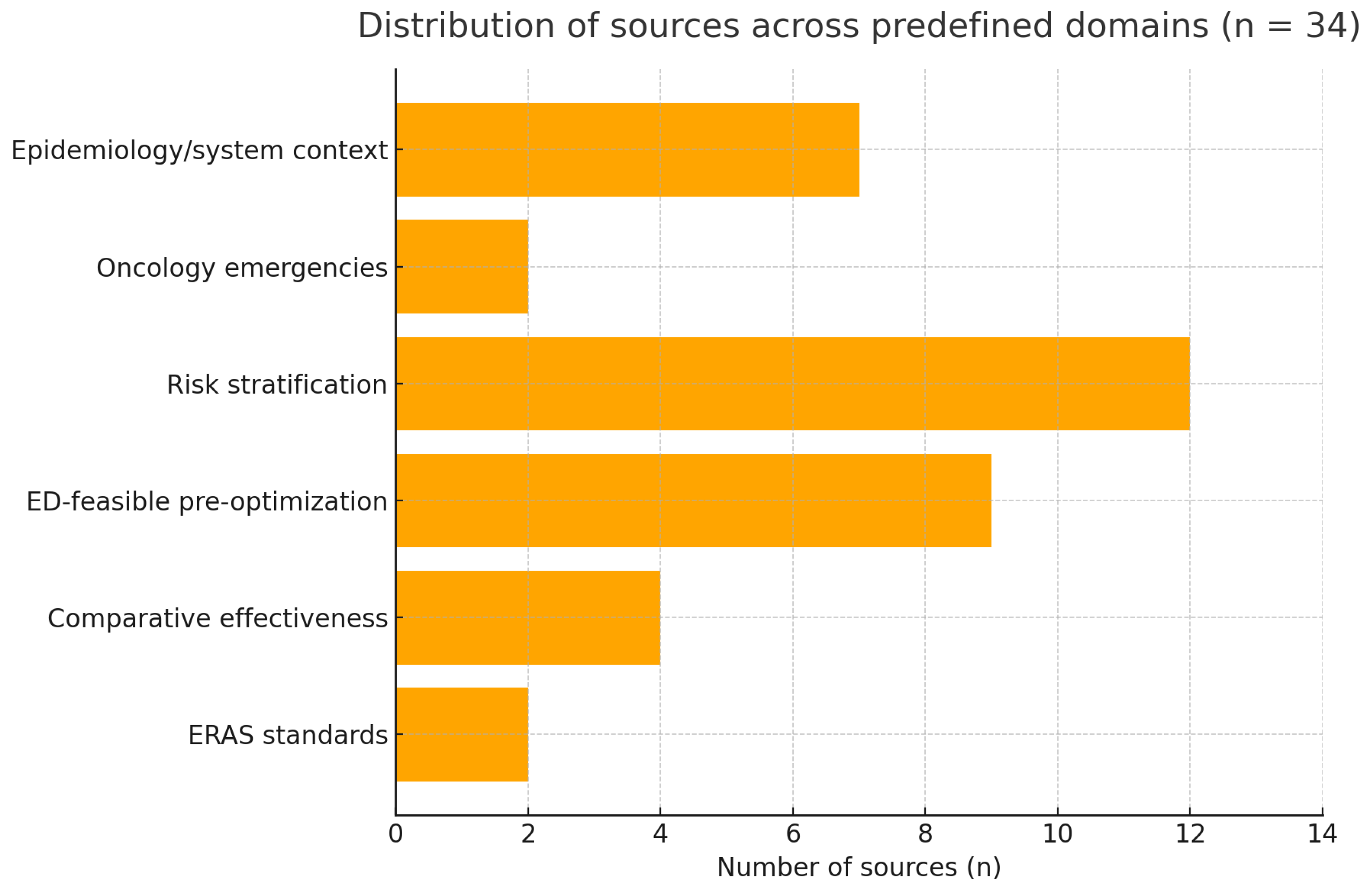
3.1. Evidence Base
3.2. ERAS Standards for EL
3.3. Evidence of Effect
- Shorter length of stay (LOS) (typically 1–3 days)
3.4. Pre-Optimization Is Feasible in ED
3.5. Targeting High-Risk Patients
3.6. Oncology Emergencies and Local Programs
3.7. Advanced Risk Stratification and Emerging Predictors
4. Discussion
4.1. Principal Findings and Interpretation
4.2. Why an ED-Initiated Bundle Matters
4.3. Mechanistic Rationale for the Bundle
- Analgesia and fluids. Goal-directed fluid therapy and multimodal, opioid-sparing analgesia are core ERAS elements that attenuate physiological stress and reduce pulmonary and ileus-related complications [5,6,7,8]. In emergencies, the aim is not elaborate prehabilitation but fast, protocolized resuscitation and pain control that stabilize physiology without delaying source control [5,9].
- Nutrition. Early, safe nutrition is linked to preserved lean mass and immune function in elective ERAS. In emergencies, oral nutritional supplements (ONS) can be initiated when the aspiration risk is acceptable and the obstruction physiology allows, with continuation postoperatively [5,9]. Recent program experience supports the feasibility of combined nutrition-exercise interventions and their potential to improve perioperative readiness [31] and generate cost savings [32].
- Respiratory preparation. Incentive spirometer and brief inspiratory muscle training (IMT), when feasible, are low-risk and may reduce atelectasis-related complications, particularly in high-risk or painful upper-abdominal presentations [9]. Given the compressed timelines, even short exposure alongside coaching can be justified if it does not interfere with OR timing.
- Pragmatic comorbidity optimization (patient blood management, PBM). Anemia and iron deficiency are common and are associated with transfusions and adverse outcomes. The international consensus advocates early screening and intravenous iron (IVI) when timelines and logistics permit [29]. In emergency pathways, a single-dose IVI strategy during ED/ward wait can be considered for iron-deficiency anemia when it will not delay OR. Where feasible and indicated, targeted correction of vitamin B12, folate, and vitamin D can be incorporated, recognizing that the evidence is extrapolated and the timing is short [29]. The unifying principle is opportunism without delay.
4.4. Targeting: Who Stands to Benefit Most
4.5. Integrating Morphometric and Biomarker Data into ED Triage
4.6. Frailty, Discharge Planning and End-of-Life Implications
4.7. Context: Oncological Emergencies
4.8. Operative Conduct and Team Configuration
4.9. Implementation: From Concept to Routine Practice
- Ready-to-use kits and roles. Respiratory devices at triage bays, ONS stock, patient leaflets, and a one-page protocol reduce friction. Role clarity (ED nursing, physiotherapy, dietetics, anesthesia, surgery) mitigates “everyone/no-one” ownership problems and aligns with ERAS Part 2 accountability [6,9].
4.10. Implementation Roadmap (Tiered)
- Bronze (Minimal): one-page protocol; ESS/CFS triage prompt; stock of ONS and incentive spirometers; analgesia/fluids order set with no-delay hard stop; basic CBC/ferritin/TSAT; named owner for ED → OR handover.
- Silver (Standard): on-call grid for physio/dietitian (response ≥ 2 h); micro-learning modules (20 min); shared ultrasound access; weekly dashboard of process metrics.
- Gold (Enhanced): automated CT-morphometrics; bedside rectus femoris ultrasound with competency log; single-dose IV iron pathway; embedded delirium-risk tool (PIPRA); monthly review of morbidity, costs, and equity.
4.11. Operational Risks and Safeguards
4.12. Contextual Adaptation
- Rural/remote: Use the Bronze bundle; paper checklist; shared ultrasound if available or no-tech proxies (hand-grip, calf circumference); tele-physio and tele-dietitian; ONS trolley in ED.
- Resource-constrained/LMIC: Prioritize time-neutral actions (analgesia/fluids, early mobilization intent, delirium prevention); substitute IV iron with early post-op oral iron + folate when IV supply/logistics limit delivery; use CFS when ESS/EHR tools are unavailable; monitor two core metrics (time-to-OR, LOS).
- Different organizational structures: If anesthesia leads pre-operative assessment, ED nursing initiates and transfers the bundle via a preformatted handover; if pharmacy controls stock, create ED kits (ONS, spirometers).
4.13. Measurement and Learning
4.14. Evaluation Framework (For Research and QI)
- Process: completion rates of ESS/CFS; ONS started when eligible; respiratory coaching delivered; ED → OR handover completed; no-delay breaches.
- Clinical (30-day): pulmonary complications, postoperative ileus, delirium proxy, transfusion exposure, length of stay, discharge destination, readmissions, mortality.
- Implementation: fidelity (bundle adherence %), acceptability (staff survey), cost (pharmacy/physio time, ONS/IV iron), and equity (age/frailty strata).
4.15. Equity and Context Sensitivity
4.16. Safety Considerations and “No-Delay” Governance
4.17. How This Review Advances the Field
4.18. Limitations of the Evidence Base and of This Review
4.19. Practice Recommendations
- Activate a minimal, ED-initiated, ERAS-aligned bundle comprising multi-modal analgesia, goal-directed fluids, early safe nutrition, respiratory preparation, mobilization intent, and pragmatic PBM (CBC, ferritin/TSAT; consider IV iron when indicated/logistically neutral; correct B12/folate/vitamin D as appropriate) under an explicit no-delay rule [5,6,9,16].
4.20. Research Agenda
4.21. Ethical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFS | Clinical Frailty Scale |
| EL | Emergency Laparotomy |
| ERAS | Enhanced Recovery After Surgery |
| ESS | Emergency Surgery Score |
| IMT | Inspiratory muscle training |
| ONS | Oral Nutritional Supplements |
| PBM | Patient Blood Management |
| PIPRA | Pre-Interventional Preventive Risk Assessment |
| RF-US | Rectus Femoris ultrasound |
| suPAR | Soluble Urokinase Plasminogen Activator Receptor |
References
- Lee, K.C.; Sturgeon, D.; Lipsitz, S.; Weissman, J.S.; Mitchell, S.; Cooper, Z. Mortality and health care utilization among Medicare patients undergoing emergency general surgery vs those with acute medical conditions. JAMA Surg. 2020, 155, 216–223. [Google Scholar] [CrossRef]
- Ingraham, A.M.; Cohen, M.E.; Raval, M.V.; Ko, C.Y.; Nathens, A.B. Variation in quality of care after emergency general surgery procedures in the elderly. J. Am. Coll. Surg. 2011, 212, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Haider, A.H.; Riviello, R.; Zogg, C.K.; Zafar, S.N.; Latif, A.; Rios Diaz, A.J.; Rehman, Z.; Zafar, H. Geriatric emergency general surgery: Survival and outcomes in a low-middle income country. Surgery 2015, 158, 562–569. [Google Scholar] [CrossRef]
- Harji, D.P.; Griffiths, B.; Stocken, D.; Pearse, R.; Blazeby, J.; Brown, J.M. Key interventions and outcomes in perioperative care pathways in emergency laparotomy: A systematic review. World J. Emerg. Surg. 2025, 20, 20. [Google Scholar] [CrossRef]
- Peden, C.J.; Aggarwal, G.; Aitken, R.J.; Anderson, I.D.; Foss, N.B.; Cooper, Z.; Dhesi, J.K.; French, W.B.; Grant, M.C.; Hammarqvist, F.; et al. Guidelines for Perioperative Care for Emergency Laparotomy Enhanced Recovery After Surgery (ERAS) Society Recommendations: Part 1—Preoperative. World J. Surg. 2021, 45, 1272–1290. [Google Scholar] [CrossRef]
- Scott, M.J.; Aggarwal, G.; Aitken, R.J.; Anderson, I.D.; Balfour, A.; Foss, N.B.; Cooper, Z.; Dhesi, J.K.; French, W.B.; Grant, M.C.; et al. Consensus Guidelines for Perioperative Care for Emergency Laparotomy Enhanced Recovery After Surgery (ERAS) Society Recommendations Part 2—Intra- and Postoperative Care. World J. Surg. 2023, 47, 1850–1880. [Google Scholar] [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Bill, V.; Satyadas, T. Meta-analysis of Enhanced Recovery After Surgery (ERAS) Protocols in Emergency Abdominal Surgery. World J. Surg. 2020, 44, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Wisely, J.C.; Barclay, K.L. Effects of an Enhanced Recovery After Surgery programme on emergency surgical patients. ANZ J. Surg. 2016, 86, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Poulton, T.; Murray, D. Pre-optimisation of patients undergoing emergency laparotomy: A review of best practice. Anaesthesia 2019, 74, 100–107. [Google Scholar] [CrossRef]
- Humphry, N.; Jones, M.; Goodison, S.; Carter, B.; Hewitt, J. The Effect of Sarcopenia on Postoperative Outcomes Following Emergency Laparotomy: A Systematic Review and Meta-Analysis. J. Frailty Aging 2023, 12, 305–310. [Google Scholar] [CrossRef]
- Shrestha, A.; Dani, M.; Kemp, P.; Fertleman, M. Acute Sarcopenia after Elective and Emergency Surgery. Aging Dis. 2022, 13, 1759–1769. [Google Scholar] [CrossRef]
- Leiner, T.; Nemeth, D.; Hegyi, P.; Ocskay, K.; Virag, M.; Kiss, S.; Rottler, M.; Vajda, M.; Varadi, A.; Molnar, Z. Frailty and emergency surgery: Results of a systematic review and meta-analysis. Front. Med. 2022, 9, 811524. [Google Scholar] [CrossRef]
- Saxena, P.; Nair, A. Emergency Surgery Score as an Effective Risk Stratification Tool for Patients Undergoing Emergency Surgeries: A Narrative Review. Cureus 2022, 14, e26226. [Google Scholar] [CrossRef]
- Mihailescu, A.A.; Gradinaru, S.; Kraft, A.; Blendea, C.D.; Capitanu, B.S.; Neagu, S.I. Enhanced rehabilitation after surgery: Principles in the treatment of emergency complicated colorectal cancers—A narrative review. J. Med. Life 2025, 18, 179–186. [Google Scholar] [CrossRef]
- Shah, P.M.; Johnston, L.; Sarosiek, B.; Harrigan, A.; Friel, C.M.; Thiele, R.H.; Hedrick, T.L. Reducing Readmissions While Shortening Length of Stay: The Positive Impact of an Enhanced Recovery Protocol in Colorectal Surgery. Dis. Colon Rectum 2017, 60, 219–227. [Google Scholar] [CrossRef]
- Munoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Baikady, R.R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Carson, H.; Gana, T.; Lockwood, S.; Murray, D.; Tierney, G.M. A review of surgical and peri-operative factors to consider in emergency laparotomy care. Anaesthesia 2020, 75, e75–e82. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; De Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Ramírez-Reyes, L.G.; Carrillo-Torres, O.; Brito-Ramírez, F. Complicaciones postoperatorias por descontrol lipídico perioperatorio. Revisión sistemática. Rev. Mex. Anestesiol. 2021, 44, 34–42. [Google Scholar] [CrossRef]
- Halle-Smith, J.M.; Naumann, D.N.; Powell, S.L.; Naumann, L.K.; Griffiths, E.A. Improving outcomes for elderly patients following emergency surgery: A cutting-edge review. Curr. Anesthesiol. Rep. 2021, 11, 396–404. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.J.; Souviron-Dixon, V.E.; Roque-Rojas, F.; Mudarra-Garcia, N. Assessment of Sarcopenia Using Rectus Femoris Ultrasound in Emergency Patients—A Cross-Sectional Study. J. Clin. Med. 2025, 14, 3932. [Google Scholar] [CrossRef]
- Body, S.; Ligthart, M.A.P.; Rahman, S.; Ward, J.; May-Miller, P.; Pucher, P.H.; Curtis, N.J.; West, M.A. Sarcopenia and Myosteatosis Predict Adverse Outcomes After Emergency Laparotomy: A Multi-Center Observational Cohort Study. Ann. Surg. 2022, 275, 1103–1111. [Google Scholar] [CrossRef]
- Carter, B.; Law, J.; Hewitt, J.; Parmar, K.L.; Boyle, J.M.; Casey, P.; Maitra, I.; Pearce, L.; Moug, S.J.; Ross, B.; et al. Association between preadmission frailty and care level at discharge in older adults undergoing emergency laparotomy. Br. J. Surg. 2020, 107, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Petring Hasselager, R.; Foss, N.B.; Andersen, O.; Cihoric, M.; Bay-Nielsen, M.; Nielsen, H.J.; Camilla Andresen, L.; Toft Tengberg, L. Mortality and major complications after emergency laparotomy: A pilot study of risk prediction model development by preoperative blood-based immune parameters. Acta Anaesthesiol. Scand. 2021, 65, 151–161. [Google Scholar] [CrossRef]
- Hewitt, J.N.; Milton, T.J.; Jeanes, J.; Murshed, I.; Nann, S.; Wells, S.; Gupta, A.K.; Ovenden, C.D.; Kovoor, J.G.; Bacchi, S.; et al. Emergency laparotomy preoperative risk assessment tool performance: A systematic review. Surg. Pract. Sci. 2024, 19, 100264. [Google Scholar] [CrossRef]
- Dodsworth, B.T.; Reeve, K.; Falco, L.; Hueting, T.; Sadeghirad, B.; Mbuagbaw, L.; Goettel, N.; Schmutz Gelsomino, N. Development and validation of an international preoperative risk assessment model for postoperative delirium. Age Ageing 2023, 52, afad086. [Google Scholar] [CrossRef]
- Fehlmann, C.A.; Taljaard, M.; McIsaac, D.I.; Suppan, L.; Anderegg, E.; Dupuis, A.; Rouyer, F.; Eagles, D.; Perry, J.J. Incidence and outcomes of emergency department patients requiring emergency general surgery: A 5-year retrospective cohort study. Swiss Med. Wkly. 2024, 154, 3729. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, K.G.; Kim, M.K. Trends and outcomes of emergency general surgery in elderly and highly elderly population in a single regional emergency center. Ann. Surg. Treat. Res. 2023, 104, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Yamanaka, K.; Kurimoto, M.; Aoki, H.; Shinkura, A.; Hanabata, Y.; Kayano, M.; Tashima, M.; Tamura, J. Effect of emergency general surgery on postoperative performance status in patients aged over 90 years. Surg. Open Sci. 2024, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sokas, C.; Lee, K.C.; Sturgeon, D.; Streid, J.; Lipsitz, S.R.; Weissman, J.S.; Kim, D.H.; Cooper, Z. Preoperative Frailty Status and Intensity of End-of-Life Care Among Older Adults After Emergency Surgery. J. Pain Symptom Manag. 2021, 62, 66–74.e3. [Google Scholar] [CrossRef]
- Mudarra-Garcia, N.; Roque-Rojas, F.; Nieto-Ramos, A.; Izquierdo-Izquierdo, V.; Garcia-Sanchez, F.J. Feasibility of a Pre-Operative Morphofunctional Assessment and the Effect of an Intervention Program with Oral Nutritional Supplements and Physical Exercise. Nutrients 2025, 17, 1509. [Google Scholar] [CrossRef] [PubMed]
- Mudarra-Garcia, N.; Roque-Rojas, F.; Izquierdo-Izquierdo, V.; Garcia-Sanchez, F.J. Prehabilitation in Major Surgery: An Evaluation of Cost Savings in a Tertiary Hospital. J. Clin. Med. 2025, 14, 2460. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, F.J.; Mudarra-Garcia, N. Evaluation of Postoperative Results after a Presurgical Optimisation Programme. Perioper. Med. 2024, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Reeve, K.A.; Schmutz Gelsomino, N.; Venturini, M.; Buddeberg, F.; Zozman, M.; Stocker, R.; Kedda, M.A.; Meier, P.; Möller, M.; Wildhaber, S.P.; et al. Prospective external validation of the automated PIPRA multivariable prediction model for postoperative delirium on real-world data from a consecutive cohort of non-cardiac surgery inpatients. BMJ Health Care Inform. 2025, 32, e101291. [Google Scholar] [CrossRef]
| Domain | Bronze (Minimal) | Silver (Standard) | Gold (Enhanced) |
|---|---|---|---|
| Staffing & roles | ED nurse triggers checklist; surgeon/ED physician co-own; basic physio/dietitian on-call | Named “bundle owner” per shift; physio/dietitian response ≤2 h | Perioperative team huddle; dual consultant availability for high-risk |
| Training | 20 min micro-learning; one-page protocol | Annual refresh; IMT coaching script | RF ultrasound competency log; CT-morphometrics auto-feed |
| Equipment/supplies | ONS stock; incentive spirometers; printed checklists | Shared ultrasound; single-dose IV iron kit; stoma nurse pager | Point-of-care RF-US; spirometry counters; digital leaflets |
| IT/EHR | ESS/CFS prompt; order set with no-delay hard stop | Auto handover ED → OR; weekly process dashboard | CT auto-read (L3 area/attenuation); PIPRA integration |
| Quality assurance | Track time-to-OR, screening rates | Add LOS, pulmonary events, ileus, readmission | Add fidelity %, cost, staff acceptability, equity |
| Cost levers | Minimal (stock + training) | Pharmacy kits + part-time physio/dietitian | Ultrasound devices; analytics support |
| Change management | Local champion; brief huddles | Audit–feedback monthly | Formal QI cycles; cross-site learning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sánchez, F.J.; Roque-Rojas, F.; Mudarra-García, N. From Emergency Department to Operating Room: The Role of Early Prehabilitation and Perioperative Care in Emergency Laparotomy: A Scoping Review and Practical Proposal. J. Clin. Med. 2025, 14, 6922. https://doi.org/10.3390/jcm14196922
García-Sánchez FJ, Roque-Rojas F, Mudarra-García N. From Emergency Department to Operating Room: The Role of Early Prehabilitation and Perioperative Care in Emergency Laparotomy: A Scoping Review and Practical Proposal. Journal of Clinical Medicine. 2025; 14(19):6922. https://doi.org/10.3390/jcm14196922
Chicago/Turabian StyleGarcía-Sánchez, Francisco Javier, Fernando Roque-Rojas, and Natalia Mudarra-García. 2025. "From Emergency Department to Operating Room: The Role of Early Prehabilitation and Perioperative Care in Emergency Laparotomy: A Scoping Review and Practical Proposal" Journal of Clinical Medicine 14, no. 19: 6922. https://doi.org/10.3390/jcm14196922
APA StyleGarcía-Sánchez, F. J., Roque-Rojas, F., & Mudarra-García, N. (2025). From Emergency Department to Operating Room: The Role of Early Prehabilitation and Perioperative Care in Emergency Laparotomy: A Scoping Review and Practical Proposal. Journal of Clinical Medicine, 14(19), 6922. https://doi.org/10.3390/jcm14196922






