Aortic Stenosis in End-Stage Renal Disease: Incidence, Prevalence, and Mortality in a National Korean Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Population
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
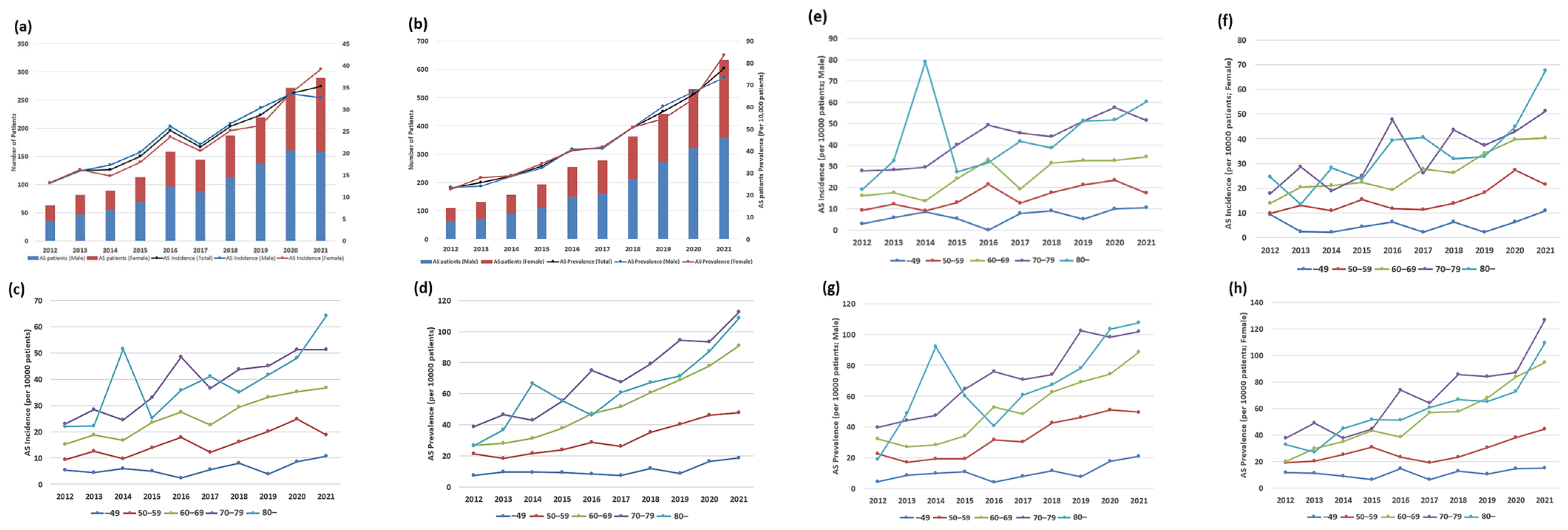
3.2. Temporal Trends in the Incidence and Prevalence of AS Among Patients with ESRD According to Sex and Age
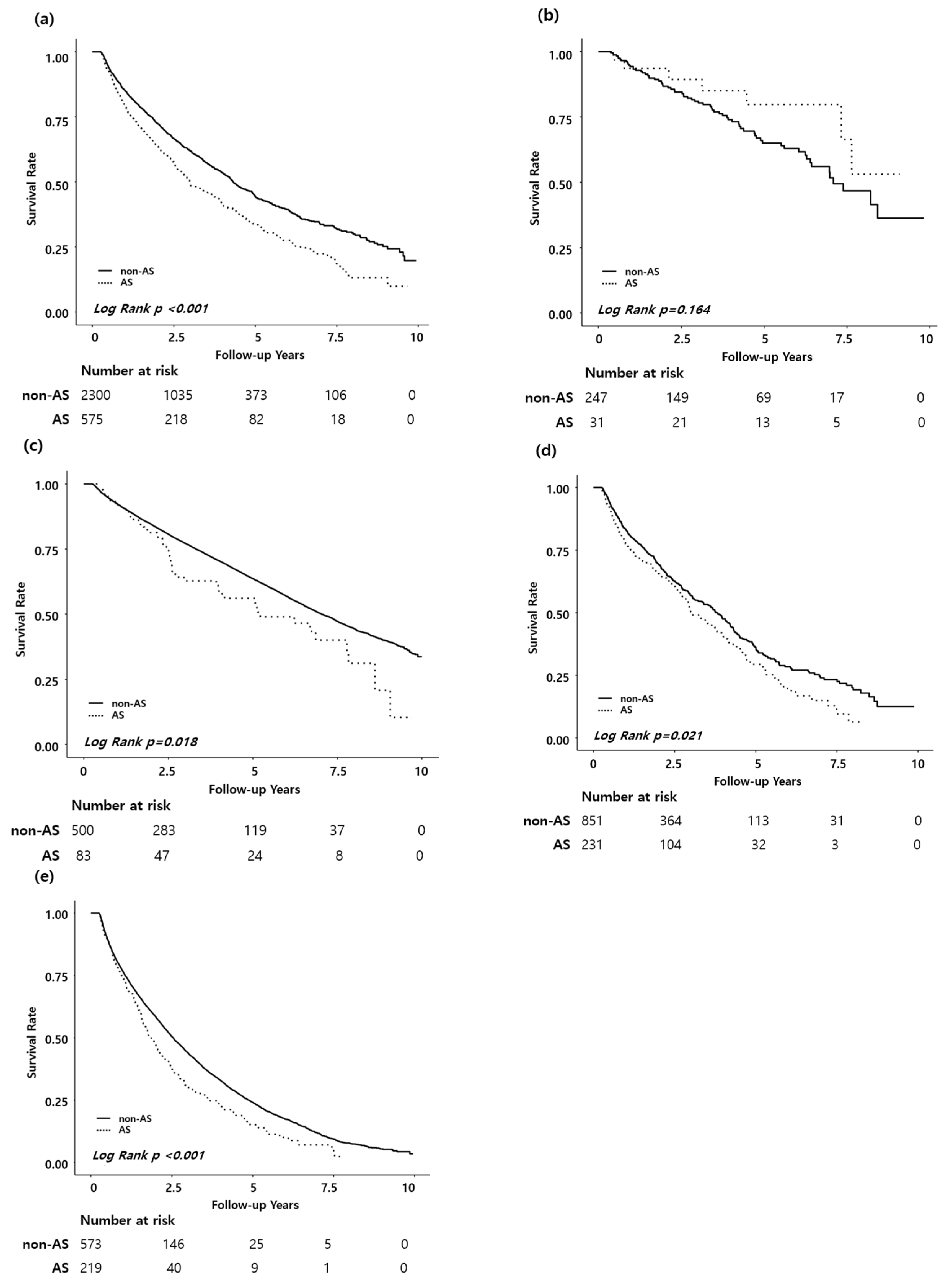
3.3. AS and Mortality Risk in ESRD
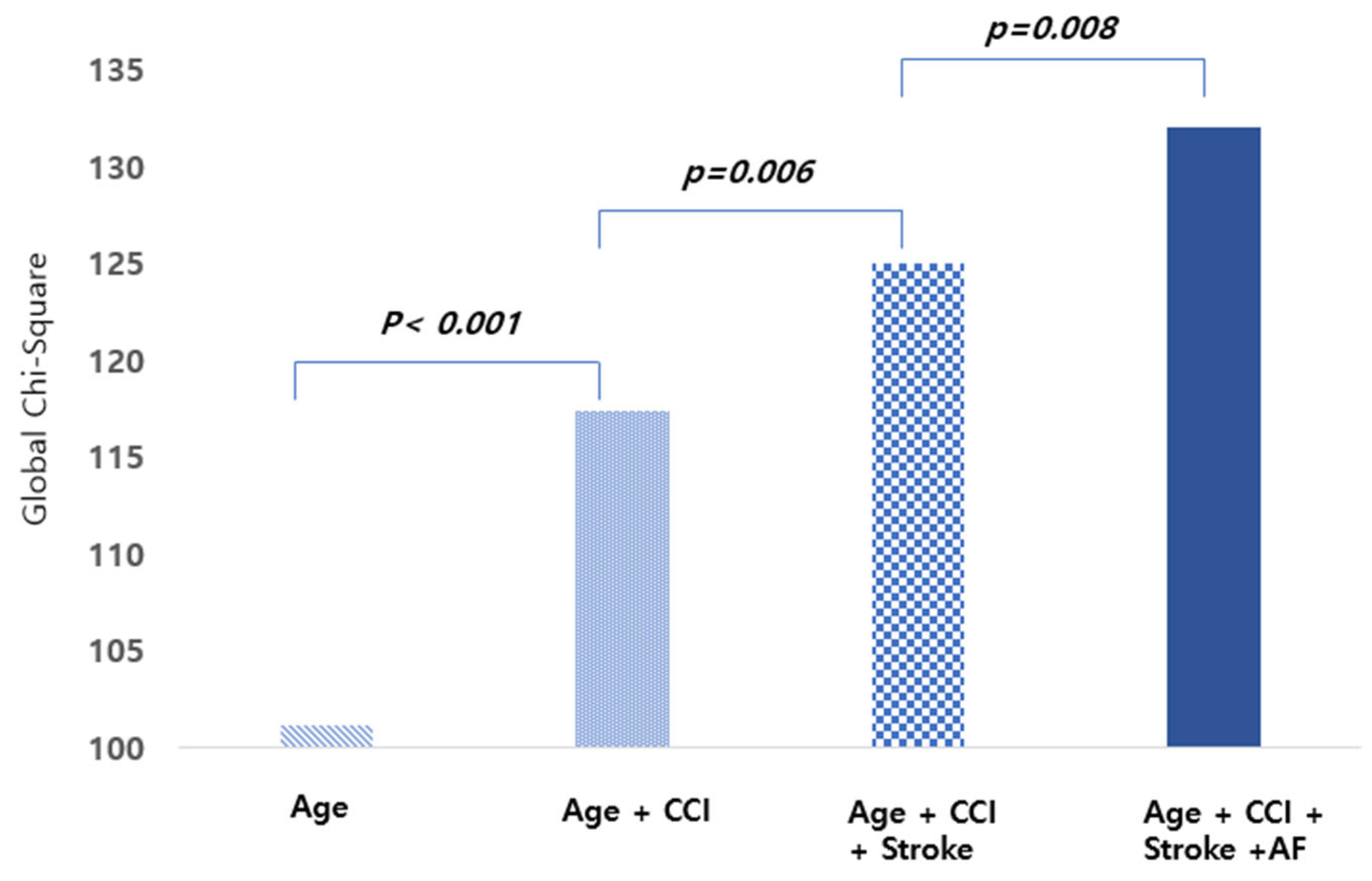
3.4. Predictors of Mortality Among Patients with ESRD and AS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| CVD | Cardiovascular disease |
| AS | Aortic stenosis |
| NHIS | Korean National Health Insurance Service |
| ICD-10 | International Classification of Diseases, 10th Revision |
| CCI | Charlson Comorbidity Index |
| HR | Hazard ratio |
| CI | Confidence interval |
| PD | Peritoneal dialysis |
| AF | Atrial fibrillation |
| HTN | Hypertension |
| DM | Diabetes mellitus |
| HLD | Hyperlipidemia |
| MI | Myocardial infarction |
| HF | Heart failure |
| PVD | Peripheral vascular disease |
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.; Muntner, P.; et al. Estimated GFR and albuminuria for prediction of cardiovascular outcomes. JAMA 2015, 313, 940–953. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F. 2020 ACC/AHA guideline for the management of valvular heart disease. Circulation 2020, 143, e35–e71. [Google Scholar] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. J. Am. Coll. Cardiol. 2016, 68, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; D O’Brien, K.; et al. Calcific aortic valve disease: Not simply a degenerative process. Nat. Rev. Cardiol. 2011, 8, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M. Calcification of bicuspid aortic valves. Heart 2002, 88, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Kawase, Y.; Taniguchi, T.; Morimoto, T.; Kadota, K.; Iwasaki, K.; Kuwayama, A.; Ohya, M.; Shimada, T.; Amano, H.; Maruo, T.; et al. Severe Aortic Stenosis in Dialysis Patients. J. Am. Heart. Assoc. 2017, 6, e004961. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shim, C.Y.; Hong, G.R.; Cho, I.J.; Chang, H.J.; Ha, J.W.; Chung, N. Effect of End-Stage Renal Disease on Rate of Progression of Aortic Stenosis. Am. J. Cardiol. 2016, 117, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.G.; Goldin, J.; Kuizon, B.D.; Yoon, C.; Gales, B.; Sider, D.; Wang, Y.; Chung, J.; Emerick, A.; Greaser, L.; et al. Coronary-artery calcification in young adults with ESKD. N. Engl. J. Med. 2000, 342, 1478–1483. [Google Scholar] [CrossRef]
- Hoshina, M.; Wada, H.; Sakakura, K.; Kubo, N.; Ikeda, N.; Sugawara, Y.; Yasu, T.; Ako, J.; Momomura, S. Determinants of progression of aortic valve stenosis and outcome of adverse events in hemodialysis patients. J. Cardiol. 2012, 59, 78–83. [Google Scholar] [CrossRef]
- Shroff, G.R.; Bangalore, S.; Bhave, N.M.; Chang, T.I.; Garcia, S.; Mathew, R.O.; Rangaswami, J.; Ternacle, J.; Thourani, V.H.; Pibarot, P.; et al. Evaluation and Management of Aortic Stenosis in Chronic Kidney Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e1088–e1114. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Bellamy, M.F.; Baker, C.S.R. Aortic Stenosis in Dialysis Patients. Semin. Dial. 2017, 30, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Park, S.J.; Kim, E.K.; Park, S.W. Temporal trends in incidence, prevalence, and death of aortic stenosis in Korea: A nationwide population-based study. ESC Heart Fail. 2022, 9, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, A.C.; AlBadri, A.; Wei, J.; Nelson, M.D.; Li, D.; Makkar, R.; Merz, C.N.B. Sex differences in aortic stenosis: Identification of knowledge gaps for sex-specific personalized medicine. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 21, 100197. [Google Scholar] [CrossRef] [PubMed]
- Bevan, G.H.; Zidar, D.A.; Josephson, R.A.; Al-Kindi, S.G. Mortality due to aortic stenosis in the United States. JAMA 2008, 321, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R. The mortality burden of untreated aortic stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Leow, A.; Ching, K.; Goh, F.Q.; Chew, N.W.S.; Kong, W.K.F.; Yeo, T.C.; Sia, C.H.; Poh, K.K. Long-term mortality out comes of southeast Asian patients with aortic stenosis. Eur. Heart. J. 2024, 45, ehae666.3136. [Google Scholar] [CrossRef]

| Variables | Total (%) | Non-AS (%) | AS (%) | p-Value |
|---|---|---|---|---|
| Patient number | 2875 | 2300 (80.0) | 575 (20.0) | |
| Male | 1572 (54.7) | 1274 (55.4) | 298 (51.8) | 0.072 |
| Age (years) | 71.90 ± 11.70 | 71.90 ± 11.79 | 75.61 ± 10.13 | <0.001 |
| <50 | 97 (3.4) | 83 (3.6) | 14 (2.4) | |
| 50–59 | 278 (9.7) | 247 (10.7) | 31 (5.4) | |
| 60–69 | 783 (27.2) | 637 (27.7) | 146 (25.4) | |
| 70–79 | 925 (32.2) | 760 (33.0) | 165 (28.7) | |
| ≥80 | 792 (27.5) | 573 (24.9) | 219 (38.1) | |
| Income | 0.004 | |||
| Low | 792 (27.5) | 658 (28.6) | 134 (23.3) | |
| Mid Low | 357 (12.4) | 278 (12.1) | 79 (13.7) | |
| Mid High | 505 (17.6) | 388 (16.9) | 117 (20.4) | |
| High | 1221 (42.5) | 976 (42.4) | 245 (42.6) | |
| Dialysis | 0.026 | |||
| Hemodialysis | 2806 (97.6) | 2243 (97.5) | 563 (97.9) | |
| Peritoneal dialysis | 69 (2.4) | 57 (2.5) | 12 (2.1) | |
| Atrial fibrillation | 671 (23.3) | 543 (23.6) | 132 (23.0) | 0.011 |
| Cancer | 720 (25.0) | 566 (24.6) | 154 (26.8) | 0.050 |
| Diabetes mellitus | 2521 (87.7) | 2029 (88.2) | 492 (85.6) | 0.159 |
| Heart failure | 1801 (62.6) | 1416 (61.6) | 385 (67.0) | 0.045 |
| Hyperlipidemia | 2725 (94.8) | 2179 (94.7) | 546 (95.0) | 0.010 |
| Hypertension | 2844 (98.9) | 2270 (98.7) | 574 (99.8) | 0.132 |
| Myocardial infarction | 398 (13.8) | 331 (14.4) | 67 (11.7) | 0.081 |
| Peripheral vascular disease | 1330 (46.3) | 1074 (46.7) | 256 (44.5) | 0.044 |
| Stroke | 941 (32.7) | 672 (29.2) | 172 (29.9) | 0.076 |
| Charlson Comorbidity Index | 7.77 ± 2.62 | 7.80 ± 2.72 | 7.63 ± 2.63 | 0.065 |
| Death | 1318 (45.8) | 1006 (43.7) | 312 (54.3) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Aortic stenosis | 1.41 | 1.24–1.60 | <0.001 | 1.23 | 1.08–1.40 | 0.002 |
| Male | 1.05 | 0.94–1.17 | 0.383 | |||
| Age (year) | 1.06 | 1.05–1.07 | <0.001 | 1.06 | 1.05–1.06 | <0.001 |
| <50 | ref | |||||
| 50–59 | 1.91 | 1.21–3.01 | 0.006 | |||
| 60–69 | 2.46 | 1.61–3.75 | <0.001 | |||
| 70–79 | 5.18 | 3.44–7.80 | <0.001 | |||
| ≥80 | 8.45 | 5.59–12.79 | <0.001 | |||
| Income | ||||||
| Low | ref | |||||
| Mid Low | 0.82 | 0.67–1.00 | 0.050 | |||
| Mid High | 0.92 | 0.78–1.09 | 0.344 | |||
| High | 1.09 | 0.95–1.24 | 0.209 | |||
| PD | 1.17 | 0.84–1.62 | 0.365 | |||
| Comorbidities | ||||||
| AF | 1.55 | 1.38–1.75 | <0.001 | 1.31 | 1.16–1.48 | <0.001 |
| HTN | 1.50 | 0.85–2.64 | 0.163 | |||
| DM | 1.45 | 1.22–1.71 | <0.001 | 1.08 | 0.89–1.31 | 0.436 |
| HLD | 1.27 | 1.01–1.61 | 0.044 | 0.78 | 0.61–0.99 | 0.045 |
| MI | 1.34 | 1.15–1.56 | <0.001 | 1.17 | 1.00–1.37 | 0.056 |
| HF | 1.39 | 1.25–1.56 | <0.001 | 1.02 | 0.90–1.16 | 0.724 |
| PVD | 1.14 | 1.02–1.27 | 0.023 | 0.87 | 0.77–0.98 | 0.023 |
| Stroke | 1.43 | 1.28–1.60 | <0.001 | 1.22 | 1.07–1.38 | 0.002 |
| Cancer | 1.35 | 1.19–1.53 | <0.001 | 1.02 | 0.87–1.20 | 0.786 |
| CCI | 1.11 | 1.09–1.13 | <0.001 | 1.06 | 1.03–1.10 | 0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Male | 1.23 | 1.01–1.49 | 0.038 | 1.01 | 0.83–1.23 | 0.932 |
| Age (year) | 1.06 | 1.05–1.08 | <0.001 | 1.06 | 1.05–1.07 | <0.001 |
| <50 | ref | |||||
| 50–59 | 1.43 | 0.47–4.40 | 0.530 | |||
| 60–69 | 2.69 | 0.97–7.44 | 0.057 | |||
| 70–79 | 5.48 | 2.02–14.87 | 0.001 | |||
| ≥80 | 8.69 | 3.20–23.63 | <0.001 | |||
| Income | ||||||
| Low | ref | |||||
| Mid Low | 1.00 | 0.71–1.41 | 1.000 | |||
| Mid High | 1.08 | 0.80–1.46 | 0.625 | |||
| High | 1.24 | 0.97–1.58 | 0.093 | |||
| PD | 1.43 | 0.80–2.53 | 0.227 | |||
| AF | 1.43 | 1.16–1.76 | 0.001 | 1.30 | 1.05–1.60 | 0.016 |
| HTN | 0.87 | 0.12–6.17 | 0.886 | |||
| DM | 1.28 | 0.99–1.67 | 0.064 | |||
| HLD | 1.19 | 0.77–1.82 | 0.436 | |||
| MI | 1.31 | 0.99–1.71 | 0.055 | |||
| HF | 1.63 | 1.32–2.01 | <0.001 | 1.14 | 0.91–1.43 | 0.266 |
| PVD | 1.28 | 1.05–1.56 | 0.014 | |||
| Stroke | 1.58 | 1.29–1.93 | <0.001 | 1.35 | 1.10–1.66 | 0.004 |
| Cancer | 1.18 | 0.94–1.47 | 0.155 | |||
| CCI | 1.13 | 1.09–1.17 | <0.001 | 1.07 | 1.03–1.12 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, M.-H.; Park, S.J.; Kim, H. Aortic Stenosis in End-Stage Renal Disease: Incidence, Prevalence, and Mortality in a National Korean Cohort. J. Clin. Med. 2025, 14, 6921. https://doi.org/10.3390/jcm14196921
Kim M, Kim M-H, Park SJ, Kim H. Aortic Stenosis in End-Stage Renal Disease: Incidence, Prevalence, and Mortality in a National Korean Cohort. Journal of Clinical Medicine. 2025; 14(19):6921. https://doi.org/10.3390/jcm14196921
Chicago/Turabian StyleKim, Minjeong, Min-Ho Kim, Sang Jun Park, and Hyangkyoung Kim. 2025. "Aortic Stenosis in End-Stage Renal Disease: Incidence, Prevalence, and Mortality in a National Korean Cohort" Journal of Clinical Medicine 14, no. 19: 6921. https://doi.org/10.3390/jcm14196921
APA StyleKim, M., Kim, M.-H., Park, S. J., & Kim, H. (2025). Aortic Stenosis in End-Stage Renal Disease: Incidence, Prevalence, and Mortality in a National Korean Cohort. Journal of Clinical Medicine, 14(19), 6921. https://doi.org/10.3390/jcm14196921






