POTEC (Platelet Count, Oxygen Saturation, Time of CPR, Elective Surgery, and Initial ETCO2) Score for Predicting 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Development and Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
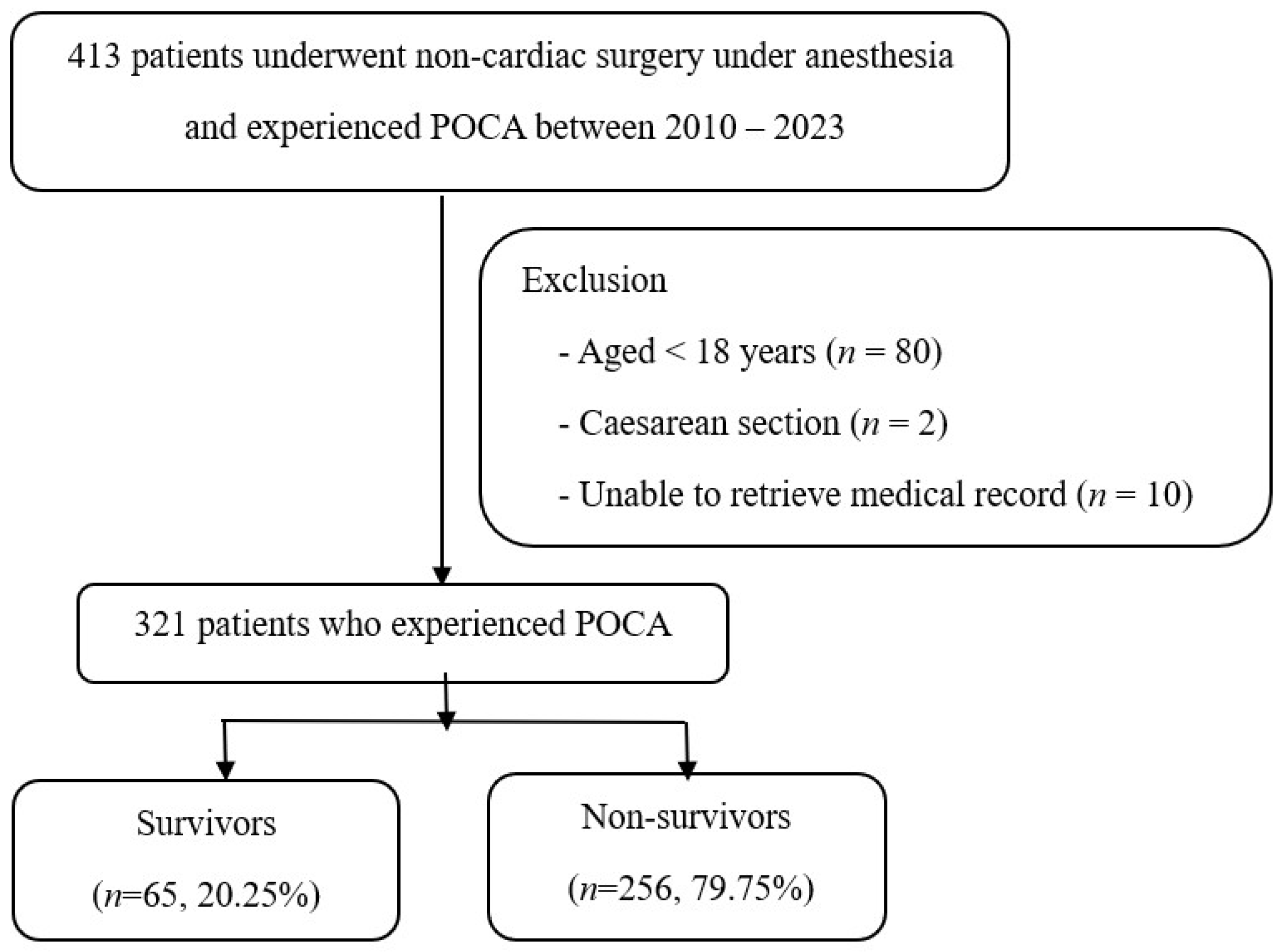
2.2. Participants
2.3. Outcome
2.4. Predictors
2.5. Sample Size
2.6. Missing Data
2.7. Statistical Analysis
2.8. Model Development
2.9. Model Performance and Internal Validation
2.10. Model Presentation
3. Results
4. Discussion
4.1. Limitations
4.2. Implications of All Available Evidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| POCA | Perioperative Cardiac Arrest |
| CPR | Perioperative cardiopulmonary resuscitation |
| ROSC | The return of spontaneous circulation |
| TRIPOD | The Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines |
| GA | General anesthesia |
| RA | Regional anesthesia |
| MAC | Monitored anesthesia care |
| ASA | American Society of Anesthesiologists physical status |
| MAP | Mean arterial pressure |
| SpO2 | Peripheral oxygen saturation |
| ETCO2 | End-tidal carbon dioxide |
| AuROC | The receiver operating characteristic curve |
| SD | Standard deviation |
| IQR | Interquartile range |
| ORs | Adjusted odds ratios |
| CIs | Confidence intervals |
| CASPRI | The cardiac arrest survival post-resuscitation in-hospital |
| GO-FAR | The good outcome following attempted resuscitation |
| CART | The cardiac arrest risk triage score |
| MEWS | The modified early warning score |
References
- Hinkelbein, J.; Andres, J.; Böttiger, B.W.; Brazzi, L.; De Robertis, E.; Einav, S.; Gwinnutt, C.; Kuvaki, B.; Krawczyk, P.; McEvoy, M.D.; et al. Cardiac arrest in the perioperative period: A consensus guideline for identification, treatment, and prevention from the European Society of Anaesthesiology and Intensive Care and the European Society for Trauma and Emergency Surgery. Eur. J. Trauma. Emerg. Surg. 2023, 49, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.A.; Bobrow, B.J.; Mancini, M.E.; Christenson, J.; de Caen, A.R.; Bhanji, F.; Abella, B.S.; Kleinman, M.E.; Edelson, D.P.; Berg, R.A.; et al. Cardiopulmonary Resuscitation Quality: Improving Cardiac Resuscitation Outcomes Both Inside and Outside the Hospital. Circulation 2013, 128, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Jindawatthana, I.; Pathomkajonkul, N.; Wongsripuemtet, P. Factors associated with 30-day mortality after perioperative cardiac arrest in adults undergoing non-cardiac surgery: A seven-year observational study from Siriraj Hospital. Ann. Transl. Med. 2023, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Franzmeier, L.; Cheseaux-Carrupt, M.; Kaempfer, M.; Disma, N.; Pietsch, U.; Huber, M.; Riva, T.; Greif, R. Characteristics and neurological survival following intraoperative cardiac arrest in a Swiss University Hospital: A 7-year retrospective observational cohort study. Front. Med. 2023, 10, 1198078. [Google Scholar] [CrossRef]
- Nunnally, M.E.; O’Connor, M.F.; Kordylewski, H.; Westlake, B.; Dutton, R.P. The incidence and risk factors for perioperative cardiac arrest observed in the national anesthesia clinical outcomes registry. Anesth. Analg. 2015, 120, 364–370. [Google Scholar] [CrossRef]
- Goswami, S.; Brady, J.E.; Jordan, D.A.; Li, G. Intraoperative Cardiac Arrests in Adults Undergoing Noncardiac Surgery: Incidence, Risk Factors, and Survival Outcome. Anesthesiology 2012, 117, 1018–1026. [Google Scholar] [CrossRef]
- Braz, L.G.; Carlucci, M.T.O.; Braz, J.R.C.; Módolo, N.S.P.; do Nascimento, P., Jr.; Braz, M.G. Perioperative cardiac arrest and mortality in trauma patients: A systematic review of observational studies. J. Clin. Anesth. 2020, 64, 109813. [Google Scholar] [CrossRef]
- Charuluxananan, S.; Sriraj, W.; Punjasawadwong, Y.; Pitimana-aree, S.; Lekprasert, V.; Werawatganon, T.; Wasinwong, W.; Ratanachai, P.; Sriramatr, D.; Atichat, S.; et al. Perioperative and Anesthetic Adverse events in Thailand (PAAd Thai) incident reporting study: Anesthetic profiles and outcomes. Asian Biomed. 2017, 11, 21–32. [Google Scholar] [CrossRef]
- Ofoma, U.R.; Basnet, S.; Berger, A.; Kirchner, H.L.; Girotra, S. Trends in Survival After In-Hospital Cardiac Arrest During Nights and Weekends. J. Am. Coll. Cardiol. 2018, 71, 402–411. [Google Scholar] [CrossRef]
- Zhao, B.-C.; Liu, W.-F.; Deng, Q.-W.; Zhuang, P.-P.; Liu, J.; Li, C.; Liu, K.-X.; on behalf of the PREvention of Vascular Events after Non-cardiac surGEry (PREVENGE) investigators. Meta-analysis of preoperative high-sensitivity cardiac troponin measurement in non-cardiac surgical patients at risk of cardiovascular complications. Br. J. Surg. 2020, 107, e81–e90. [Google Scholar] [CrossRef]
- Rodseth, R.N.; Biccard, B.M.; Le Manach, Y.; Sessler, D.I.; Lurati Buse, G.A.; Thabane, L.; Schutt, R.C.; Bolliger, D.; Cagini, L.; Cardinale, D.; et al. The Prognostic Value of Pre-Operative and Post-Operative B-Type Natriuretic Peptides in Patients Undergoing Noncardiac Surgery: B-Type Natriuretic Peptide and N-Terminal Fragment of Pro-B-Type Natriuretic Peptide: A Systematic Review and Individual Patient Data Meta-Analysis. J. Am. Coll. Cardiol. 2014, 63, 170–180. [Google Scholar] [CrossRef]
- Havmöller, R.; Chugh, S.S. Plasma biomarkers for prediction of sudden cardiac death: Another piece of the risk stratification puzzle? Circ. Arrhythm. Electrophysiol. 2012, 5, 237–243. [Google Scholar] [CrossRef]
- Humaloja, J.; Ashton, N.J.; Skrifvars, M.B. Brain Injury Biomarkers for Predicting Outcome After Cardiac Arrest. Crit. Care 2022, 26, 81. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Park, S.Y.; Meltzer, D.O.; Hall, J.B.; Edelson, D.P. Derivation of a cardiac arrest prediction model using ward vital signs. Crit. Care Med. 2012, 40, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Huber, M.T.; Park, S.Y.; Hall, J.B.; Edelson, D.P. Predicting cardiac arrest on the wards: A nested case-control study. Chest 2012, 141, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, I.; Price, C.; Sitch, A.; Banda, P.; Kellett, J.; Nyirenda, M.; Rylance, J. Early warning scores generated in developed healthcare settings are not sufficient at predicting early mortality in Blantyre, Malawi: A prospective cohort study. PLoS ONE 2013, 8, e59830. [Google Scholar] [CrossRef]
- Guo, K.; Xu, F.; Li, Y.; Ma, M.; Li, J.; Wang, L. Mortality and cardiac arrest rates of emergency surgery in developed and developing countries: A systematic review and meta-analysis. BMC Anesthesiol. 2024, 24, 178. [Google Scholar] [CrossRef]
- Shang, H.; Chu, Q.; Ji, M.; Guo, J.; Ye, H.; Zheng, S.; Yang, J. A retrospective study of mortality for perioperative cardiac arrests toward a personalized treatment. Sci. Rep. 2022, 12, 13709. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ (Clin. Res. Ed.) 2024, 385, e078378. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujino, Y.; Amato, M.B.; Kavanagh, B.P. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am. J. Respir. Crit. Care Med. 2017, 195, 985–992. [Google Scholar] [CrossRef]
- Okubo, M.; Komukai, S.; Andersen, L.W.; Berg, R.A.; Kurz, M.C.; Morrison, L.J.; Callaway, C.W. Duration of cardiopulmonary resuscitation and outcomes for adults with in-hospital cardiac arrest: Retrospective cohort study. BMJ (Clin. Res. Ed.) 2024, 384, e076019. [Google Scholar] [CrossRef]
- Matsuyama, T.; Ohta, B.; Kiyohara, K.; Kitamura, T. Cardiopulmonary resuscitation duration and favorable neurological outcome after out-of-hospital cardiac arrest: A nationwide multicenter observational study in Japan (the JAAM-OHCA registry). Crit. Care 2022, 26, 120. [Google Scholar] [CrossRef]
- Chungsaengsatitayaporn, S.; Pipanmekaporn, T.; Khorana, J.; Leurcharusmee, P.; Boonsri, S.; Siriphuwanun, V. Predictive Factors for 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Single-Center Retrospective Cohort Study. J. Clin. Med. 2025, 14, 599. [Google Scholar] [CrossRef] [PubMed]
- Kilgannon, J.H.; Jones, A.E.; Shapiro, N.I.; Angelos, M.G.; Milcarek, B.; Hunter, K.; Parrillo, J.E.; Trzeciak, S.; Emergency Medicine Shock Research Network Investigators. Association Between Arterial Hyperoxia Following Resuscitation From Cardiac Arrest and In-Hospital Mortality. JAMA 2010, 303, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Funada, A.; Goto, Y. Relationship Between the Duration of Cardiopulmonary Resuscitation and Favorable Neurological Outcomes After Out-of-Hospital Cardiac Arrest: A Prospective, Nationwide, Population-Based Cohort Study. J. Am. Heart Assoc. 2016, 5, e002819. [Google Scholar] [CrossRef]
- Andersen, L.W.; Grossestreuer, A.V.; Donnino, M.W. “Resuscitation time bias”-A unique challenge for observational cardiac arrest research. Resuscitation 2018, 125, 79–82. [Google Scholar] [CrossRef]
- Weil, I.A.; Kumar, P.; Seicean, S.; Neuhauser, D.; Seicean, A. Platelet count abnormalities and peri-operative outcomes in adults undergoing elective, non-cardiac surgery. PLoS ONE 2019, 14, e0212191. [Google Scholar] [CrossRef]
- De Sario Velasquez, G.D.; Forte, A.J.; McLeod, C.J.; Bruce, C.J.; Pacheco-Spann, L.M.; Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Borna, S.; et al. Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review. J. Clin. Med. 2023, 12, 7430. [Google Scholar] [CrossRef]
- Jung, J.; Ryu, J.H.; Shon, S.; Min, M.; Hyun, T.G.; Chun, M.; Lee, D.; Lee, M. Predicting in-hospital cardiac arrest outcomes: CASPRI and GO-FAR scores. Sci. Rep. 2023, 13, 18087. [Google Scholar] [CrossRef]

| Parameters | n Missing | Survivor N (%) | Non-Survivor N (%) | OR (95% CI) | p-Value | AuROC (95% CI) |
|---|---|---|---|---|---|---|
| Age, mean ± SD (years) | 0 | 60.05 ± 19.57 | 53.41 ± 20.25 | |||
| Age ≥ 65 years | 32 (49.23) | 74 (28.91) | 2.58 (1.39–4.79) | 0.003 | 0.602 (0.53–0.67) | |
| Female | 0 | 33 (50.77) | 81 (31.64) | 2.23 (1.28–3.87) | 0.005 | 0.596 (0.53–0.66) |
| ASA 1–2 | 0 | 19 (29.23) | 7 (2.73) | 14.69 (5.84–36.92) | <0.001 | 0.633 (0.58–0.69) |
| Elective surgery | 0 | 16 (24.62) | 17 (6.64) | 4.59 (2.17–9.71) | <0.001 | 0.589 (0.53–0.64) |
| Preoperative Spontaneous breathing | 0 | 23 (35.38) | 53 (21.09) | 2.05 (1.14–3.69) | 0.017 | 0.572 (0.51–0.64) |
| Preoperative no sign of shock | 0 | 53 (81.54) | 146 (57.03) | 3.33 (1.69–6.52) | <0.001 | 0.623 (0.57–0.68) |
| Hemoglobin (g/dL), mean ± SD | 3 | 10.57 ± 2.70 | 8.87 ± 3.43 | |||
| ≥8 | 57 (87.69) | 152 (60.08) | 4.73 (2.16–10.34) | <0.001 | 0.638 (0.59–0.69) | |
| Platelet count (109/L), median [Q1, Q3] | 3 | 202 [162, 291] | 138 [87, 217] | |||
| ≥ 100 × 109/L | 58 (89.23) | 171 (67.59) | 3.97 (1.74–9.08) | 0.001 | 0.608 (0.56–0.65) | |
| Non-trauma surgery | 0 | 43 (66.15) | 113 (44.14) | 2.47 (1.39–4.37) | 0.02 | 0.610 (0.54–0.67) |
| Site of operation 0 | ||||||
| Upper intra—abdominal | 22 (33.85) | 100 (39.06) | 1.25 (0.71–2.21) | 0.440 | 0.526 (0.46–0.59) | |
| Major vascular | 9 (13.85) | 43 (16.80) | 1.26 (0.58–2.73) | 0.565 | 0.515 (0.47–0.56) | |
| Intracranial | 10 (15.38) | 56 (21.88) | 1.54 (0.74–3.22) | 0.250 | 0.533 (0.48–0.58) | |
| Intrathoracic | 12 (18.46) | 44 (17.19) | 0.92 (0.45–1.85) | 0.809 | 0.506 (0.46–0.52) | |
| Orthopedic | 2 (3.08) | 9 (3.52) | 1.15 (0.24–5.45) | 0.862 | 0.502 (0.48–0.53) | |
| Others | 13 (20.00) | 18 (7.03) | 3.31 (1.52–7.17) | 0.002 | 0.565 (0.54–0.63) | |
| Supine position | 0 | 54 (84.38) | 236 (92.16) | 0.46 (0.20–1.03) | 0.060 | 0.453 (0.40–0.50) |
| Preoperative SpO2, median, [Q1, Q3] | 14 | 98 [95, 100] | 95.5 [82, 100] | |||
| ≥90% | 55 (84.62) | 145 (59.92) | 3.68 (1.79–7.57) | <0.001 | 0.624 (0.57–0.68) | |
| The initial ETCO2 (mmHg), | 14 | |||||
| mean ± SD | 28.09 ± 8.62 | 23.17 ± 10.53 | ||||
| <35 | 49 (75.38) | 214 (88.43) | ||||
| 35–45 | 16 (24.62) | 25 (10.33) | ref | |||
| >45 | 0 | 3 (1.24) | 2.79 (1.38–5.63) | 0.004 | 0.571 (0.52–0.63) | |
| Time of POCA: Working hours | 0 | 31 (47.69) | 153 (59.77) | 1.63 (0.94–2.82) | 0.08 | 0.560 (0.49–0.63) |
| Initial rhythm before POCA | 0 | |||||
| Shockable | 14 (21.54) | 35 (13.67) | 1.73 (0.87–3.46) | 0.118 | 0.539 (0.48–0.59) | |
| Non shockable | 51 (78.46) | 221 (86.33) | ||||
| Duration of CPR (min), Median [Q1, Q3] | 0 | 10 [5, 20] | 20 [10, 35] | |||
| ≤30 | 61 (93.85) | 180 (70.31) | 6.44 (2.26–18.34) | <0.001 | 0.618 (0.58–0.66) | |
| Potential Predictors | ORs | 95% CI | p-Value | Coefficients | Score |
|---|---|---|---|---|---|
| P = Platelet count 100 × 109/L | 4.24 | 1.72–10.41 | 0.002 | 1.44 | 1 |
| O = Oxygen saturation ≥ 90% upon OR arrival (SpO2) | 2.83 | 1.31–6.14 | 0.008 | 1.04 | 1 |
| T = Duration of CPR ≤ 30 min | 11.26 | 3.57–35.47 | <0.001 | 2.42 | 2 |
| E = Elective surgery | 4.92 | 1.97–12.32 | 0.001 | 1.59 | 2 |
| C = ETCO2 level 35–45 mmHg after intubation | 2.99 | 1.33–6.70 | 0.008 | 1.09 | 1 |
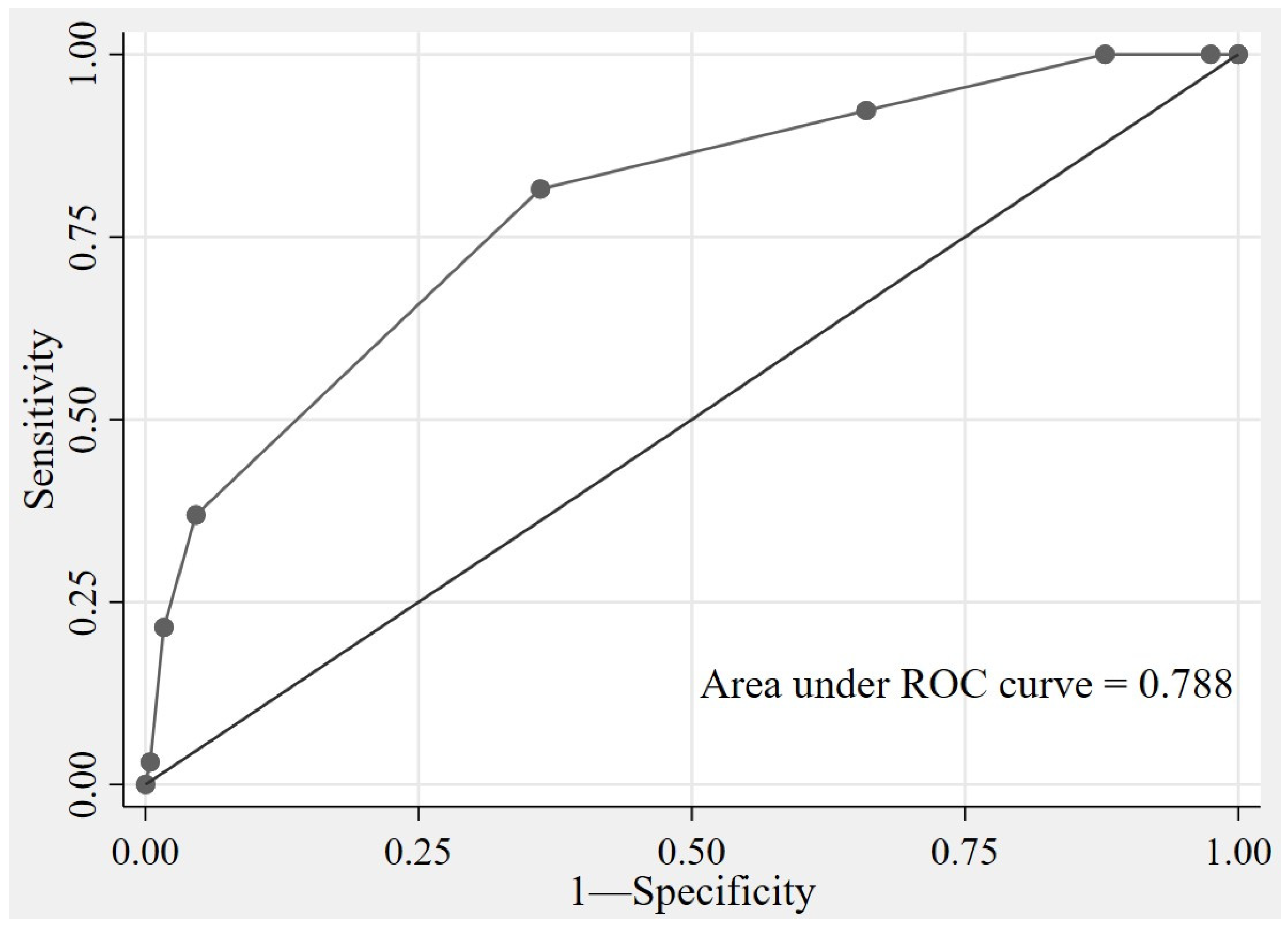
| Probability Categories | Total | Low Probability N (%) (score 0–4) | High Probability N (%) (score 5–7) |
|---|---|---|---|
| Total | 303 | ||
| Survived | 65 | 41 (15.30) | 24 (68.57) |
| Died | 238 | 227 (84.70) | 11 (31.43) |
| Prognosis performance | |||
| Sensitivity | 81.54 | 36.92 | |
| Specificity | 63.87 | 95.38 | |
| PPV | 15.30 | 68.57 | |
| LR+ | 0.66 | 7.99 | |
| LR− | 0.103 | 0.741 | |
| 95% CI | 0.23–0.40 | 1.94–3.53 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chungsaengsatitayaporn, S.; Pipanmekaporn, T.; Khorana, J.; Leurcharusmee, P.; Siriphuwanun, V.; Boonsri, S. POTEC (Platelet Count, Oxygen Saturation, Time of CPR, Elective Surgery, and Initial ETCO2) Score for Predicting 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Development and Validation. J. Clin. Med. 2025, 14, 6915. https://doi.org/10.3390/jcm14196915
Chungsaengsatitayaporn S, Pipanmekaporn T, Khorana J, Leurcharusmee P, Siriphuwanun V, Boonsri S. POTEC (Platelet Count, Oxygen Saturation, Time of CPR, Elective Surgery, and Initial ETCO2) Score for Predicting 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Development and Validation. Journal of Clinical Medicine. 2025; 14(19):6915. https://doi.org/10.3390/jcm14196915
Chicago/Turabian StyleChungsaengsatitayaporn, Soontarin, Tanyong Pipanmekaporn, Jiraporn Khorana, Prangmalee Leurcharusmee, Visith Siriphuwanun, and Settapong Boonsri. 2025. "POTEC (Platelet Count, Oxygen Saturation, Time of CPR, Elective Surgery, and Initial ETCO2) Score for Predicting 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Development and Validation" Journal of Clinical Medicine 14, no. 19: 6915. https://doi.org/10.3390/jcm14196915
APA StyleChungsaengsatitayaporn, S., Pipanmekaporn, T., Khorana, J., Leurcharusmee, P., Siriphuwanun, V., & Boonsri, S. (2025). POTEC (Platelet Count, Oxygen Saturation, Time of CPR, Elective Surgery, and Initial ETCO2) Score for Predicting 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Development and Validation. Journal of Clinical Medicine, 14(19), 6915. https://doi.org/10.3390/jcm14196915





