Normative Optical Coherence Tomography Angiography Metrics of Macular Vessel Density and Foveal Avascular Zone in Healthy Children
Abstract
1. Introduction
2. Methods
2.1. Study Design & Ethics
2.2. Study Population
2.3. Optical Coherence Tomography (OCT)
2.4. Optical Coherence Tomography Angiography
2.5. Statistical Analysis
3. Results
3.1. Demographic, Clinical and Structural OCT Characteristics of the Study Cohort
3.2. OCT Angiography Findings
3.2.1. Macular VD and FAZ Area Metrics
3.2.2. Age-Stratified Vessel Density Percentiles
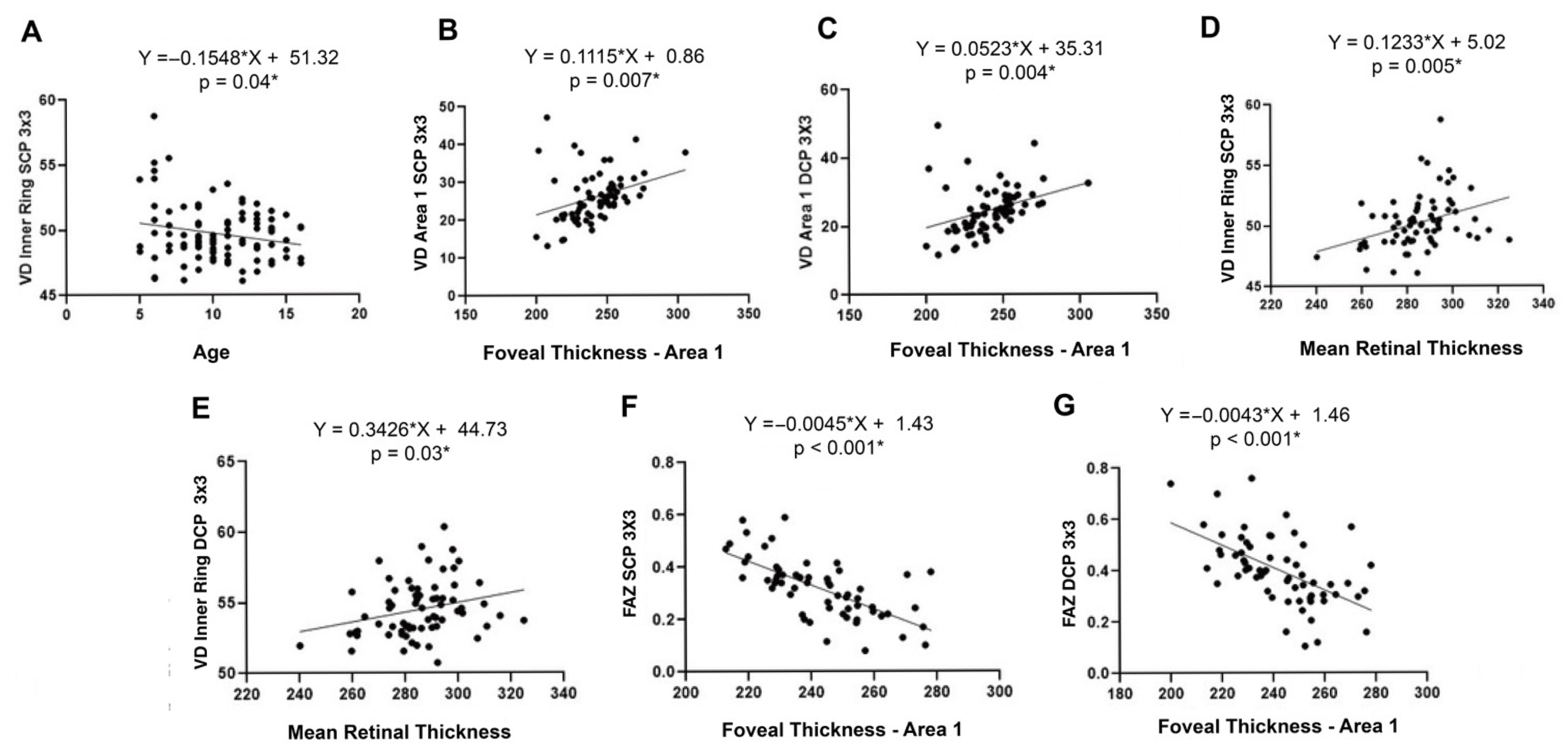
3.2.3. Correlation Studies and Multivariate Analyses
- a.
- Associations between age and vascular parameters
- b.
- Associations between axial length, spherical equivalent, and vascular metrics
- c.
- Associations between structural and vascular parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACD | Anterior chamber depth |
| AL | Axial length |
| CC | Choriocapillaris |
| DCP | Deep capillary plexus |
| ETDRS | Early treatment diabetic retinopathy study |
| FAZ | Foveal avascular zone |
| FEVR | Familial exudative vitreoretinopathy |
| GCC | Ganglion cell complex |
| IQR | Interquartile range |
| OCT | Optical Coherence Tomography |
| OCTA | Optical Coherence Tomography Angiography |
| RNFL | Retinal nerve fiber layer |
| ROP | Retinopathy of prematurity |
| SCP | Superficial capillary plexus |
| SD | Standard deviation |
| SE | Spherical equivalent |
| VD | Vessel density |
References
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G.; States, U.; Science, C.; States, U.; Angeles, L.; States, U.; et al. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A Review of Optical Coherence Tomography Angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R.; Rahimi, M.; Iafe, N.A.; Freund, K.B.; Sadda, S.R.; Sarraf, D. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Niestrata-Ortiz, M.; Fichna, P.; Stankiewicz, W.; Stopa, M. Enlargement of the Foveal Avascular Zone Detected by Optical Coherence Tomography Angiography in Diabetic Children without Diabetic Retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Hautz, W.; Gołȩbiewska, J.; Kocyła-Karczmarewicz, B. Optical Coherence Tomography and Optical Coherence Tomography Angiography in Monitoring Coats’ Disease. J. Ophthalmol. 2017, 2017, 7849243. [Google Scholar] [CrossRef]
- Rezar-Dreindl, S.; Eibenberger, K.; Told, R.; Neumayer, T.; Steiner, I.; Sacu, S.; Schmidt-Erfurth, U.; Stifter, E. Retinal Vessel Architecture in Retinopathy of Prematurity and Healthy Controls Using Swept-Source Optical Coherence Tomography Angiography. Acta Ophthalmol. 2021, 99, e232–e239. [Google Scholar] [CrossRef]
- Ong, S.S.; Hsu, S.T.; Grewal, D.; Arevalo, J.F.; El-Dairi, M.A.; Toth, C.A.; Vajzovic, L. Appearance of Pediatric Choroidal Neovascular Membranes on Optical Coherence Tomography Angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 89–98. [Google Scholar] [CrossRef]
- Ong, S.S.; Patel, T.P.; Singh, M.S. Optical Coherence Tomography Angiography Imaging in Inherited Retinal Diseases. J. Clin. Med. 2019, 8, 2078. [Google Scholar] [CrossRef]
- Pua, T.S.; Hairol, M.I. Evaluating Retinal Thickness Classification in Children: A Comparison between Pediatric and Adult Optical Coherence Tomography Databases. PLoS ONE 2024, 19, e0314395. [Google Scholar] [CrossRef]
- Kalra, G.; Zarranz-Ventura, J.; Chahal, R.; Bernal-Morales, C.; Lupidi, M.; Chhablani, J. Optical Coherence Tomography (OCT) Angiolytics: A Review of OCT Angiography Quantitative Biomarkers. Surv. Ophthalmol. 2022, 67, 1118–1134. [Google Scholar] [CrossRef]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. OCT-Angiography: A Qualitative and Quantitative Comparison of 4 OCT-A Devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef]
- Xu, S.; Gao, F.; Luan, R.; Liu, Y.; Li, X.; Liu, J. Normative Data and Correlation Parameters for Vessel Density Measured by 6 × 6-Mm Optical Coherence Tomography Angiography in a Large Chinese Urban Healthy Elderly Population: Date from the Beichen Eye Study. BMC Ophthalmol. 2024, 24, 298. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Odrobina, D.; Baszyńska-Wilk, M.; Groszek, A.; Szalecki, M.; Hautz, W. Optical Coherence Tomography Angiography Vessel Density in Children with Type 1 Diabetes. PLoS ONE 2017, 12, e0186479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, X.; Meng, X.; Chen, T.; Gu, Y.; Wu, Y.; Wu, Z. In Vivo Assessment of Macula in Eyes of Healthy Children 8 to 16 Years Old Using Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 8936. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewska, J.; Biała-Gosek, K.; Czeszyk, A.; Hautz, W. Optical Coherence Tomography Angiography of Superficial Retinal Vessel Density and Foveal Avascular Zone in Myopic Children. PLoS ONE 2019, 14, e0219785. [Google Scholar] [CrossRef]
- Wong, E.S.; Zhang, X.J.; Yuan, N.; Li, J.; Pang, C.P.; Chen, L.; Tham, C.C.; Cheung, C.Y.; Yam, J.C. Association of Optical Coherence Tomography Angiography Metrics with Detection of Impaired Macular Microvasculature and Decreased Vision in Amblyopic Eyes: The Hong Kong Children Eye Study. JAMA Ophthalmol. 2020, 138, 858–865. [Google Scholar] [CrossRef]
- Coscas, F.; Sellam, A.; Glacet-Bernard, A.; Jung, C.; Goudot, M.; Miere, A.; Souied, E.H. Normative Data for Vascular Density in Superficial and Deep Capillary Plexuses of Healthy Adults Assessed by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT211–OCT223. [Google Scholar] [CrossRef]
- Samara, W.A.; Say, E.A.T.; Khoo, C.T.L.; Higgins, T.P.; Magrath, G.; Ferenczy, S. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef]
- Borrelli, E.; Lonngi, M.; Balasubramanian, S.; Tepelus, T.C.; Baghdasaryan, E.; Iafe, N.A.; Pineles, S.L.; Velez, F.G.; Sarraf, D.; Sadda, S.R.; et al. Macular microvascular networks in healthy pediatric subjects. Retina 2019, 39, 1216–1224. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, C.; Wang, X.; Zhu, L.; Gu, R.; Xu, H.; Jia, Y.; Huang, D.; Sun, X. Macular Perfusion in Healthy Chinese: An Optical Coherence Tomography Angiogram Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3212–3217. [Google Scholar] [CrossRef]
- Shahlaee, A.; Pefkianaki, M.; Hsu, J.; Ho, A.C. Measurement of Foveal Avascular Zone Dimensions and Its Reliability in Healthy Eyes Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 161, 50–55.e1. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, M.; Gao, M.; Liu, H.; Sun, X. Factors Affecting the Foveal Avascular Zone Area in Healthy Eyes among Young Chinese Adults. BioMed Res. Int. 2020, 2020, 7361492. [Google Scholar] [CrossRef] [PubMed]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H.; Yoshida, M.; Ogura, Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.T.; Ngo, H.T.; Stinnett, S.S.; Cheung, N.L.; House, R.J.; Kelly, M.P.; Chen, X.; Enyedi, L.B.; Prakalapakorn, S.G.; Materin, M.A.; et al. Assessment of Macular Microvasculature in Healthy Eyes of Infants and Children Using OCT Angiography. Ophthalmology 2019, 126, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.; Fan, M.; Gao, X.; Wen, X.; Li, Z.; Zeng, P.; Tan, W.; Lan, Y. The Vascular Densities of the Macula and Optic Disc in Normal Eyes from Children by Optical Coherence Tomography Angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 437–444. [Google Scholar] [CrossRef]
- İçel, E.; Yılmaz, H.; Uçak, T.; Taşlı, N.G.; Uğurlu, A.; Karakurt, Y. Evaluation of the Optic Disc and Macula in Healthy Children Using Optical Coherence Tomography Angiography. Turk. J. Ophthalmol. 2020, 50, 228–233. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Li, M.; Sun, L.; Zhao, X.; Wang, Q.; Huang, S.; Chen, C.; Wang, Z.; Luo, X.; et al. Developmental Changes in Retinal Microvasculature in Children: A Quantitative Analysis Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2020, 219, 231–239. [Google Scholar] [CrossRef]
- Ghassemi, F.; Hatami, V.; Salari, F.; Bazvand, F.; Shamouli, H.; Mohebbi, M.; Sabour, S. Quantification of Macular Perfusion in Healthy Children Using Optical Coherence Tomography Angiography. Int. J. Retin. Vitr. 2021, 7, 56. [Google Scholar] [CrossRef]
- Xiang, L.; Zhou, Y.; Chen, Y.; Jiang, S.; Fei, C.; Wang, Y.; Bai, Y.; Zhang, X.; Li, K.; Shen, X. Assessment of the Retinal Vasculature in Healthy Chinese Preschool Children Aged 4–6 Years Old Using Optical Coherence Tomography Angiography. BMC Ophthalmol. 2021, 21, 415. [Google Scholar] [CrossRef]
- Kurumoğlu İncekalan, T.; Naz Şimdivar, G.H.; Çelik, Ü.; Alyamaç Sukgen, E.; Özdemir, U. Optical Coherence Tomography Angiography in Healthy Children: Normative Data and Age–Related Changes in Microvascular Structure of the Optic Disk and Macula. Int. Ophthalmol. 2022, 42, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Bajtl, D.; Bjeloš, M.; Bušić, M.; Križanović, A.; Marković, L.; Kuzmanović Elabjer, B. Macular Perfusion Normative Data Acquired with Optical Coherence Tomography Angiography in Healthy Four-Year-Old Caucasian Children. BMC Ophthalmol. 2021, 21, 354. [Google Scholar] [CrossRef]
- Chen, R.; Lian, H.; McAlinden, C.; Skiadaresi, E.; Liu, S.; Wan, T.; Diao, K.; Pan, H.; Qu, J.; Huang, J.; et al. Normative Data and Determinants of Macular, Disc, and Peripapillary Vascular Density in Healthy Myopic Children Using Optical Coherence Tomography Angiography. Front. Med. 2022, 9, 890294. [Google Scholar] [CrossRef]
- Plaitano, C.; Periti, F.; Guagliano, R.; Bertone, C.; Barillà, D.; Arpa, C.; Tinelli, C.; Gallo, F.G.; Bianchi, A.; Magli, A. Optical Coherence Tomography Angiography in Healthy Children: A Comparison of Macular Structure. Eur. J. Ophthalmol. 2022, 32, 2005–2010. [Google Scholar] [CrossRef]
- Diao, K.; Huang, X.; Yao, M.; Li, J.; Fan, F.; Pan, H.; Yu, J.; Yang, Y.; Lu, W.; Lian, H.; et al. Inter-Examiner and Intra-Examiner Reliability of Optical Coherence Tomography Angiography in Vascular Density Measurement of Retinal and Choriocapillaris Plexuses in Healthy Children Aged 6–15 Years. Front. Med. 2023, 10, 1161942. [Google Scholar] [CrossRef]
- Yilmaz, H.; Karakurt, Y.; Icel, E.; Ugurlu, A.; Ucak, T.; Tasli, N.G.; Elpeze, S.B. Normative Data Assessment of Vessel Density and Foveal Avascular Zone Metrics Using AngioScan Software. Curr. Eye Res. 2019, 44, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Kuehlewein, L.; Tepelus, T.C.; An, L.; Durbin, M.K.; Srinivas, S.; Sadda, S.R. Noninvasive Visualization and Analysis of the Human Parafoveal Capillary Network Using Swept Source OCT Optical Microangiography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3984–3988. [Google Scholar] [CrossRef] [PubMed]
- Niestrata-Ortiz, M.; Fichna, P.; Stankiewicz, W.; Stopa, M. Sex-Related Variations of Retinal and Choroidal Thickness and Foveal Avascular Zone in Healthy and Diabetic Children Assessed by Optical Coherence Tomography Imaging. Ophthalmologica 2019, 241, 173–177. [Google Scholar] [CrossRef]
- Wysocka-Mincewicz, M.; Gołębiewska, J.; Baszyńska-Wilk, M.; Olechowski, A.; Byczyńska, A.; Szalecki, M. Influence of Puberty on Retinal Microcirculation in Children with Type 1 Diabetes without Retinopathy Using Optical Coherence Tomography Angiography. Diabetes Vasc. Dis. Res. 2021, 18, 14791641211004427. [Google Scholar] [CrossRef]
- Iafe, N.A.; Phasukkijwatana, N.; Chen, X.; Sarraf, D. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5780–5787. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, L.; Chen, Q.; Hu, G.; Yin, Y.; Fan, Y.; Zhu, J.; He, J.; Zheng, Z.; Zou, H.; et al. Macular Vessel Density Changes in Young Adults With High Myopia: A Longitudinal Study. Front. Med. 2021, 8, 648644. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Chua, J.; Ke, M.; Tan, B.; Yu, M.; Hu, Q.; Cheung, C.M.G.; Ang, M.; Lee, S.Y.; Wong, T.Y.; et al. Quantitative OCT Angiography of the Retinal Microvasculature and Choriocapillaris in Highly Myopic Eyes with Myopic Macular Degeneration. Br. J. Ophthalmol. 2022, 106, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Verticchio Vercellin, A.; Harris, A.; Oddone, F.; Carnevale, C.; Siesky, B.A.; Arciero, J.; Fry, B.; Eckert, G.; Sidoti, P.A.; Antman, G.; et al. Diagnostic Capability of OCTA-Derived Macular Biomarkers for Early to Moderate Primary Open Angle Glaucoma. J. Clin. Med. 2024, 13, 4190. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, M.H.; Cho, H.Y.; Lim, H.W.; Seong, M. Comparative Study of Macular Ganglion Cell Complex Thickness Measured by Spectral-Domain Optical Coherence Tomography in Healthy Eyes, Eyes with Preperimetric Glaucoma, and Eyes with Early Glaucoma. Jpn. J. Ophthalmol. 2014, 58, 244–251. [Google Scholar] [CrossRef]
- Morales, D.; Wu, A.; Wu, L. The Foveal Avascular Zone Area in Healthy Eyes Measured by Ocular Coherence Tomography Angiography Using a Full Spectrum Probabilistic Algorithm. Int. Ophthalmol. 2021, 41, 2187–2196. [Google Scholar] [CrossRef]
- Fu, D.; Li, M.; Zeng, L.; Shang, J.; Yu, Z.; Zhou, X. The Role of Magnification Correction in Macular Vessel Density Assessment: A Contralateral Eye Study in Anisometropia Patients. Ann. Transl. Med. 2021, 9, 380. [Google Scholar] [CrossRef]
- Viehland, C.; Chen, X.; Tran-Viet, D.; Jackson-Atogi, M.; Ortiz, P.; Waterman, G.; Vajzovic, L.; Toth, C.A.; Izatt, J.A. Ergonomic Handheld OCT Angiography Probe Optimized for Pediatric and Supine Imaging. Biomed. Opt. Express 2019, 10, 2623–2638. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Visual Acuity (Decimal VA)
|
|
Spherical equivalent (SE)
| |
Astigmatism
|
| Study Sample (n = 118) | Boys (n = 56) | Girls (n = 62) | p | |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 10 ± 3 | 10 ± 2.7 | 10 ± 3.3 | p = 0.72 |
| Median (IQR) | 10 (5 to 17) | |||
| Sex, n (%) | ||||
| Girls | 62 (52.5%) | 56 (47.5%) | 62 (52.5%) | p = 0.7 |
| Boys | 56 (47.5%) | |||
| BCVA, logMAR | ||||
| Mean ± SD | 1 ± 0.057 | 1.0 ± 0.06 | 1.0 ± 0.056 | p = 0.52 |
| Median (IQR) | 1.00 (0.70 to 1.00) | |||
| AL, mm | ||||
| Mean ± SD | 23 ± 1.3 | 24 ± 1.1 | 23.19 ± 1.33 | p = 0.03 * |
| Median (IQR) | 23 (20–26 mm) | |||
| K1, D | ||||
| Mean ± SD | 43 ± 1.6 | 42.50 ± 1.62 | 43.25 ± 1.44 | p = 0.01 * |
| Median (IQR) | 43 (38 to 48) | |||
| K2, D | ||||
| Mean ± SD | 44 ± 1.7 | 43.63 ± 1.64 | 44.39 ± 1.59 | p = 0.01 * |
| Median (IQR) | 44 (40 to 48) | |||
| ACD, mm | ||||
| Mean ± SD | 3.5 ± 0.31 | 3.56 ± 0.28 | 3.53 ± 0.32 | p = 0.57 |
| Median (IQR) | 3.5 (2.8 to 4.3) | |||
| Spherical Equivalent, D | ||||
| Mean ± SD | +0.036 ± 2.3 | 0.01 ± 2.4 | 0.06 ± 2.2 | p = 0.89 |
| Median (IQR) | +0.50 (−6.00 to +4.80) | |||
| Average macular thickness | ||||
| Mean ± SD | 259 ± 64 | 238 ± 57 | 275 ± 64 | |
| Median (IQR) | 263 (134 to 402) | 255 (134 to 336) | 263 (170 to 402) | p = 0.02 * |
| Central macular thickness | ||||
| Mean ± SD | 279 ± 73 | 251 ± 63 | 300 ± 74 | p = 0.001 * |
| Median (IQR) | 280 (141 to 469) | 264 (141 to 355) | 301 (149 to 469) | |
| GCL average thickness | ||||
| Mean ± SD | 74 ± 5.2 | 74 ± 4.9 | 74 ± 5.5 | p = 0.77 |
| Median (IQR) | 74(58 to 85) | 75 (63 to 84) | 77 (58 to 85) | |
| GCL central thickness | ||||
| Mean ± SD | 13 ± 7.4 | 14 ± 7.3 | 12 ± 7.4 | p = 0.33 |
| Median (IQR) | 12(7.3 to 35) | 15 (8 to 28) | 12 (6 to 35) | |
| RNFL macula layer | ||||
| Mean ± SD | 110 ± 9.9 | 111 ± 9.9 | 110 ± 10 | p = 0.68 |
| Median (IQR) | 111(88 to 139) | 111(88 to 139) | 110 (88 to 132) |
| 3 × 3 mm | 6 × 6 mm | |||||||||
| SCP | DCP | SCP | DCP | |||||||
| Fovea | Inner Ring | FAZ | Fovea | Inner Ring | FAZ | Fovea | Inner Ring | Fovea | Inner Ring | |
| Overall | 25.93 ± 5.9 | 49.71 ± 2.1 | 0.31 ± 0.12 | 24.78 ± 6.2 | 53.91 ± 2 | 0.4 ± 0.13 | 25.04 ± 5.8 | 49.09 ± 2.7 | 23.91 ± 6 | 48.01 ± 4 |
| Boys | 26.40 ± 5.1 | 49.77 ± 1.9 | 0.31 ± 0.1 | 24.99 ± 5.5 | 53.68 ± 2 | 0.4 ± 0.1 | 25.71 ± 5.6 | 49.30 ± 3.3 | 24.49 ± 5.9 | 47.91 ± 4.9 |
| Girls | 25.48 ± 6.5 | 49.65 ± 2.4 | 0.29 ± 0.11 | 24.58 ± 7 | 54.13 ± 2 | 0.39 ± 0.1 | 24.42 ± 5.94 | 48.9 ± 2.1 | 23.38 ± 6.1 | 48.10 ± 3 |
| p (Boys vs. Girls) | p = 0.45 | p = 0.79 | p = 0.43 | p = 0.75 | p = 0.28 | p = 0.69 | p = 0.21 | p = 0.56 | p = 0.22 | p = 0.96 |
| SCP 3 × 3 | DCP 3 × 3 | ||||||||||||
| Age | Area | x ± SD | P5 | P25 | P50 | P75 | P95 | x ± SD | P5 | P25 | P50 | P75 | P95 |
| <7 (n = 25) | Fovea | 29 ± 9 | 14.66 | 21.46 | 28.07 | 37.75 | 47.13 | 28 ± 9.8 | 13.12 | 18.78 | 25.51 | 34.83 | 49.54 |
| Inner Ring | 51 ± 3.4 | 46.28 | 48.40 | 50.37 | 53.96 | 58.75 | 55 ± 3 | 50.41 | 53.15 | 54.48 | 57.92 | 60.36 | |
| 8–9 (n= 25) | Fovea | 24 ± 5.9 | 13.31 | 20.03 | 23.73 | 28.23 | 37.44 | 21.4 ± 5.4 | 11.83 | 17.69 | 21.04 | 24.84 | 33.11 |
| Inner Ring | 49.3 ± 1.5 | 46.20 | 48.67 | 49.10 | 50.49 | 51.95 | 53.7 ± 1.5 | 50.20 | 52.81 | 53.59 | 54.82 | 56.66 | |
| 10–11 (n = 27) | Fovea | 25.3 ± 4.5 | 17.53 | 20.97 | 25.80 | 28.50 | 35.18 | 24.2 ± 4.5 | 16.09 | 20 | 24.70 | 28.44 | 32.21 |
| Inner Ring | 49.3 ± 1.6 | 47.46 | 48.02 | 49.06 | 50.11 | 53.48 | 53.5 ± 1.8 | 50.54 | 52.65 | 53.08 | 53.88 | 58.40 | |
| 12–13 (n = 22) | Fovea | 25.5 ± 3.1 | 18.92 | 24.09 | 25.44 | 29.18 | 29.55 | 24.8 ± 3.4 | 17.24 | 22.80 | 25.43 | 27.90 | 29.12 |
| Inner Ring | 49.7 ± 1.9 | 46.09 | 48.57 | 50.09 | 51.20 | 52.38 | 54 ± 1.6 | 51.36 | 52.89 | 54.00 | 55.28 | 56.22 | |
| >14 (n = 19) | Fovea | 26.1 ± 4.4 | 14.84 | 23.62 | 26.07 | 29.43 | 32.36 | 26.1 ± 5 | 13.68 | 22.65 | 27.10 | 29.92 | 33.85 |
| Inner Ring | 49.3 ± 1.3 | 47.35 | 47.82 | 49.52 | 50.21 | 51.45 | 43.4 ± 1.6 | 50.45 | 51.88 | 53.62 | 54.61 | 55.85 | |
| SCP 6 × 6 | DCP 6 × 6 | ||||||||||||
| Age | Area | x ± SD | P5 | P25 | P50 | P75 | P95 | x ± SD | P5 | P25 | P50 | P75 | P95 |
| <7 (n = 25) | Fovea | 29 ± 9.1 | 17.45 | 22.29 | 26.31 | 33.30 | 49.73 | 28.03 ± 9.9 | 16.21 | 20.59 | 24.48 | 33.77 | 50.59 |
| Inner Ring | 50 ± 4.7 | 45.35 | 47.55 | 49.90 | 52.49 | 65.19 | 48 ± 6.5 | 27.18 | 46.91 | 48.13 | 49.95 | 57.47 | |
| 8–9 (n = 25) | Fovea | 22.5 ± 4.8 | 12.99 | 19.29 | 22.74 | 27.03 | 28.83 | 21.3 ± 4.6 | 12.47 | 17.89 | 21.31 | 24.79 | 29.19 |
| Inner Ring | 48.9 ± 1.6 | 45.79 | 47.99 | 48.50 | 50.08 | 52.75 | 48.2 ± 3.47 | 44.64 | 46.02 | 46.57 | 48.98 | 57.25 | |
| 10–11 (n = 27) | Fovea | 24.7 ± 4.41 | 18.44 | 20.33 | 24.47 | 27.76 | 34.55 | 23.2 ± 4 | 16.61 | 16.93 | 23.20 | 26.21 | 29.78 |
| Inner Ring | 48.6 ± 2.2 | 44.88 | 47.28 | 48.50 | 50.12 | 53 | 47.7 ± 3.18 | 43.59 | 46.02 | 46.70 | 48.66 | 56.18 | |
| 12–13 (n = 22) | Fovea | 24.2 ± 2.92 | 18.60 | 22.19 | 23.97 | 25.22 | 29.92 | 23.1 ± 3.54 | 16.52 | 20.50 | 23.17 | 25.51 | 29.90 |
| Inner Ring | 48.5 ± 1.40 | 46.53 | 47.25 | 48.20 | 49.49 | 51.27 | 48.1 ± 3.07 | 44.96 | 45.83 | 46.95 | 48.77 | 54.74 | |
| >14 (n = 19) | Fovea | 25 ± 3.5 | 16.58 | 23.09 | 24.81 | 27.13 | 31.29 | 24.3 ± 3.7 | 15.45 | 21.93 | 23.79 | 27.33 | 30.72 |
| Inner Ring | 49 ± 2.2 | 43.40 | 47.44 | 49.31 | 50.22 | 53.44 | 48.2 ± 2.8 | 43.51 | 46.82 | 47.69 | 49.44 | 57.17 | |
| Author | Year | Device | Software (Version) | Scan (mm) | Sample Size (n) | Age (years) | Ethnicity | SCP (mean ± SD) | DCP (mean ± SD) | FAZ mm2 (SCP or DCP) |
| Zhang et al. [16] | 2017 | Optove RTVue Avanti | AngioVue (v2016.1.0.26) | 3 × 3 | 75 | 8–16 | Asian (Chinese) | 54.29 ± 2.25 VD% | 60.19 ± 1.76 VD% | SCP: 0.29 ± 0.109 |
| Hsu et al. [26] | 2019 | Spectralis | MATLAB * | 10º × 10º | 89 | 9 weeks–17 | Caucasian, African, Hispanic, Asian, other | 35 ± 2.5 VAD% 28.2 ± 3.3 VLD% | 39.4 ± 1.5 VAD% 33.1 ± 2.1 VLD% | DCP: 0.35 ± 0.17 |
| Borrelli et al. [21] | 2019 | Optove RTVue Avanti | AngioVue (v2016.1.0.26) | 3 × 3 | 77 | 5–17 | Caucasian, African, Hispanic, Asian |
0.33 ± 0.24 PD% 0.12 ± 0.01 VD% |
0.35 ± 0.13 PD% 0.13 ± 0.01 VD% | SCP: 0.261 ± 0.149 |
| Zhang et al. [27] | 2020 | Optove RTVue Avanti | AngioVue (v2018.0.0.18) | 6 × 6 | 71 | 4.5–20.5 | Asian (Chinese) | 20.10 ± 7.13 VD% | 36.19 ± 7.68 VD% | SCP: 0.28 ± 0.10 |
| Içel E et al. [28] | 2020 | Nidek RS-3000 | Angioscan * | 3 × 3 | 146 | 6–16 | Not specified | 43.88 ± 3.4 VD% | 39.6 ± 3.55 VD% | SCP: 0.3 ± 0.09 |
| Li S et al. [29] | 2020 | Zeiss Cirrus 5000 | AngioPlex * | 3 × 3 | 333 | 4–16 | Asian (Chinese) | 4–6.9 years: 20.81 ± 1.56 7–9.9 years: 21.57 ± 1.05 10–12.9 years: 21.61 ± 1.36 13–15.9 years: 22.01 ± 0.89 | SCP 4–6.9 years: 0.22 ± 0.084 7–9.9 years: 0.24 ± 0.085 10–12.9 years: 0.24 ± 0.093 13–15.9 years: 0.25 ± 0.089 | |
| Ghassemi et al. [30] | 2021 | Optovue RTVue XR Avanti | AngioVue (v2016.1.0.23) | 6 × 6 | 54 patients (108 eyes) | 3–18 | Not specified | Total: 50.66 (21.04–56.74) VD% <7 years: 51.83 (21.04–56.74) VD% 7–10 years: 51.76 (44.09–55.29) VD% 11–14 years:51.17 (46.58–55.68) VD% >14 years: 49.53 (35.81–55.01) VD% | Total: 51.15 (16.36–63.32) VD% <7 years: 50.23 (16.36–63.32) VD% 7–10 years: 51.41 (43.53–61.12) VD% 11–14 years: 52.93 (42.62–61.51) VD% >14 years: 49.14 (28.51–58.65) VD% | SCP Total:0.28 (0.04–4.20) <7 years: 0.27 (0.05–1.10) 7–10 years: 0.28 (0.10–5.05) 11–14 years: 0.29 (0.09–0.40) >14 years: 0.28 (0.04–4.20) |
| Xiang et al. [31] | 2021 | Optove RTVue Avanti | AngioVue (v2017.1.0.155) | 6 × 6 | 242 | 4–6 | Asian (Chinese) | 48.10 ± 2.92 | 48.74 ± 6.51 | SCP 0.3 ± 0.13 |
| Kurumoğlu Incekalan et al. [32] | 2021 | Optove RTVue Avanti | AngioVue (v2017.1.0.151) | 3 × 3 | 185 | 7–18 | Not Specified | 7–9 years: 18.22 ± 6.1 VD% 10–12 years: 18.71 ± 4.77 VD% 13–15 years: 18.69 ± 5.77 VD% 16–18 years: 18.34 ± 5.27 VD% | 7–9 years: 35.16 ± 7.58 VD% 10–12 years: 35.48 ± 6.88 VD% 13–15 years: 35.43 ± 7.14 VD% 16–18 years: 35.54 ± 5.96 VD% | SCP 7–9 years: 0.28 ± 0.09 10–12 years: 0.27 ± 0.11 13–15 years: 0.27 ± 0.11 16–18 years: 0.26 ± 0.08 |
| Bajtl et al. [33] | 2021 | Spectralis OCTA | AngioTool (v0.6a,02.18.14) | 10º × 10º | 62 | 4–5 | Caucasian | Boys: 65.12 Girls: 64.84 | Boys: 68.02 Girls: 66.81 | SCP Boys: 0.55/Girls: 0.52 DCP Boys: 0.52/Girls: 0.53 |
| Chen R et al. [34] | 2022 | Optove RTVue Avanti | AngioVue (v2018.0.0.14) | 6 × 6 | 370 | 7–18 | Not Specified | 7–9 years: 18.22 ± 6.1 VD% 10–12 years: 18.71 ± 4.77 VD% 13–15 years: 18.69 ± 5.77 VD% 16–18 years: 18.34 ± 5.27 VD% | 7–9 years: 35.16 ± 7.58 VD% 10–12 years: 35.48 ± 6.88 VD% 13–15 years: 35.43 ± 7.14 VD% 16–18 years: 35.54 ± 5.96 VD% | SCP 7–9 years: 0.28 ± 0.09 10–12 years: 0.27 ± 0.11 13–15 years: 0.27 ± 0.11 16–18 years: 0.26 ± 0.08 |
| Plaitano et al. [35] | 2022 | DRI SS-OCT-A Triton plus | IMAGENET 6 | 4.5 × 4.5 | 206 | 4–16 | Caucasian | Fovea 17.1 ± 4.26 PD% | Fovea 13.55 ± 5.23 PD% | SCP:234 ± 106.39 DCP:298.32 ± 112.37 |
| Diao et al. [36] | 2023 | Optove RTVue XR-Avanti | AngioVue * | 6 × 6 | 90 | 6–15 | Asian (Chinese) | Whole retina: 49.934 ± 2.362 VD% Fovea: 22.668 ± 6.728 VD% Parafovea: 52.536 ± 3.083 VD% Perifovea: 50.570 ± 2.473 VD% | Whole retina 55.015 ± 4.764VD% Fovea: 35.155 ± 7.636 VD% Parafovea: 54.676 ± 5.676 VD% Perifovea: 56.373 ± 5.353 VD% |
SCP 0.261 ± 0.104 |
| THIS STUDY Guirao-Navarro et al. | 2025 | DRI OCT Triton | IMAGENET 6 | 3 × 3 6 × 6 | 118 | 4–17 | Caucasian | 3 × 3 A1: 25.93 ± 5.9 AI: 39.71 ± 2.1 6 × 6 A1:25.04 ± 5.8 AI: 49.09 ± 2.7 | 3 × 3 A1: 24.78 ± 6.2 AI: 53.91 ± 2 6 × 6 A1:23.91 ± 6 AI: 48.01 ± 4 | 3 × 3 SCP: 0.31 ± 0.12 DCP: 0.40 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guirao-Navarro, M.C.; Viñeta-Garcia, P.; Zarranz-Ventura, J.; Barrio-Barrio, J. Normative Optical Coherence Tomography Angiography Metrics of Macular Vessel Density and Foveal Avascular Zone in Healthy Children. J. Clin. Med. 2025, 14, 6911. https://doi.org/10.3390/jcm14196911
Guirao-Navarro MC, Viñeta-Garcia P, Zarranz-Ventura J, Barrio-Barrio J. Normative Optical Coherence Tomography Angiography Metrics of Macular Vessel Density and Foveal Avascular Zone in Healthy Children. Journal of Clinical Medicine. 2025; 14(19):6911. https://doi.org/10.3390/jcm14196911
Chicago/Turabian StyleGuirao-Navarro, María Concepción, Pablo Viñeta-Garcia, Javier Zarranz-Ventura, and Jesús Barrio-Barrio. 2025. "Normative Optical Coherence Tomography Angiography Metrics of Macular Vessel Density and Foveal Avascular Zone in Healthy Children" Journal of Clinical Medicine 14, no. 19: 6911. https://doi.org/10.3390/jcm14196911
APA StyleGuirao-Navarro, M. C., Viñeta-Garcia, P., Zarranz-Ventura, J., & Barrio-Barrio, J. (2025). Normative Optical Coherence Tomography Angiography Metrics of Macular Vessel Density and Foveal Avascular Zone in Healthy Children. Journal of Clinical Medicine, 14(19), 6911. https://doi.org/10.3390/jcm14196911







