Sarcopenia in Hemodialysis Patients: Prevalence, Independent Risk Factors, and Functional Implications—A Multicenter Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Sample Size Calculation
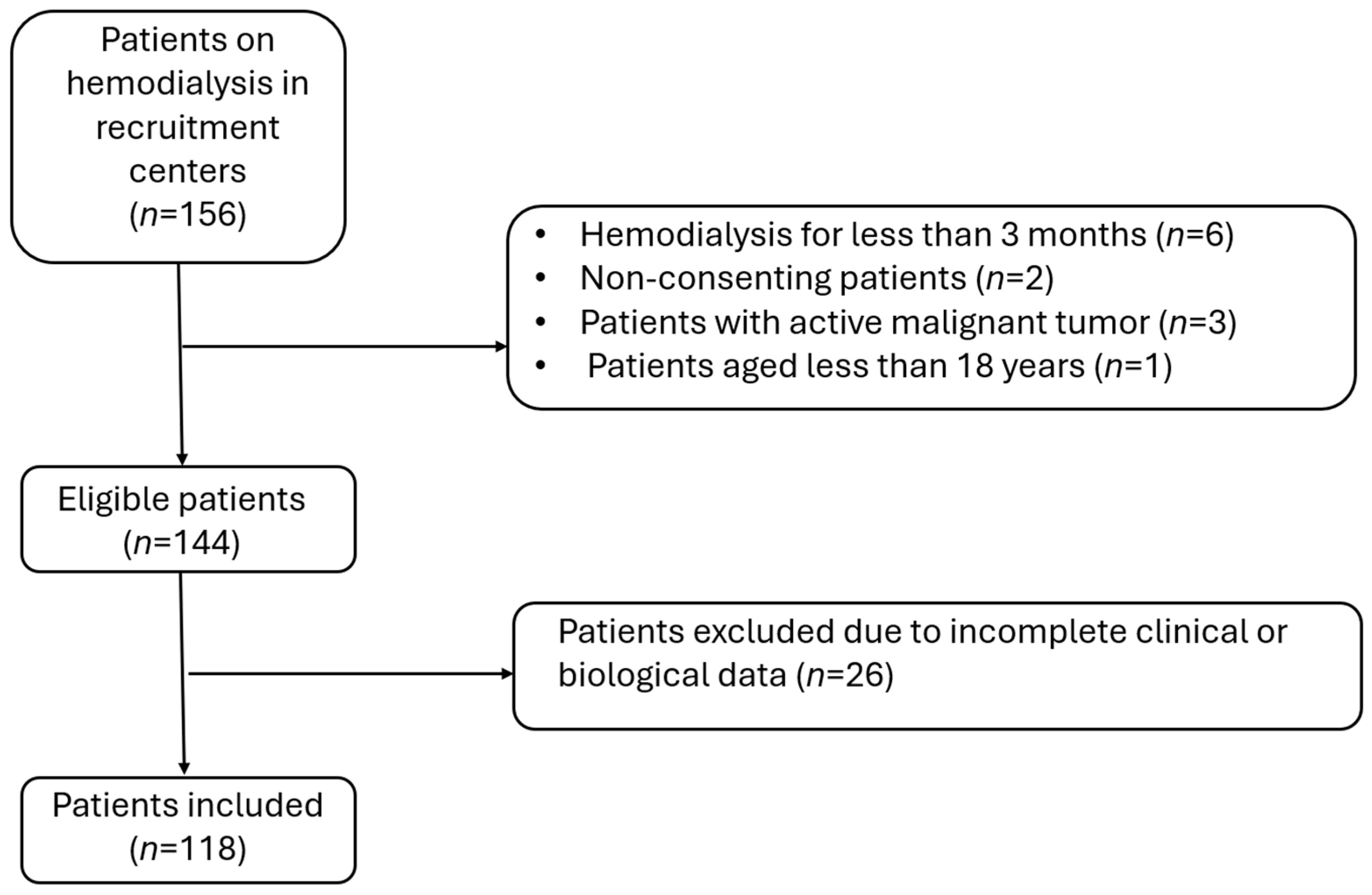
2.4. Participants
2.5. Experimental Procedures
2.5.1. Anthropometric Measurements
2.5.2. Muscle Strength Assessment
2.5.3. Physical Performance Testing
2.5.4. Sarcopenia Screening
2.5.5. Quality of Life Assessment
2.5.6. Laboratory Analyses
2.5.7. Nutritional Assessment
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Sarcopenia Screening and Diagnosis
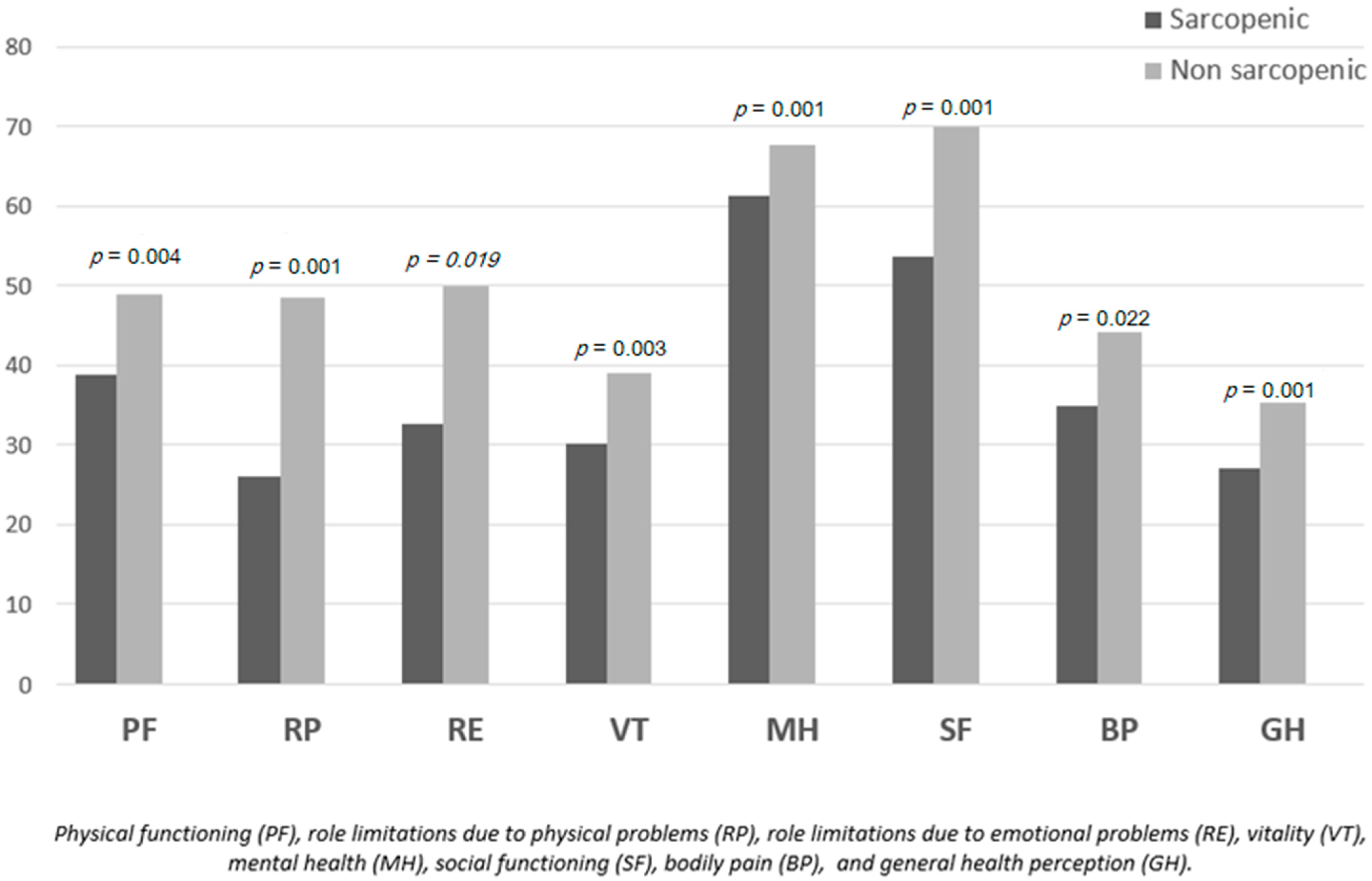
3.3. Quality of Life Subscale Scores
3.4. Nutritional Analysis
3.5. Multivariate Analysis
4. Discussion
4.1. Principal Findings
4.2. Sarcopenia Prevalence in Hemodialysis Populations
4.3. Determining Muscle Mass in the Absence of BIA
4.4. Diabetes Mellitus as a Risk Factor
4.5. Hemodialysis Duration and Sarcopenia Development
4.6. Physical Performance and Sarcopenia
4.7. Quality of Life Implications
4.8. Nutritional Considerations
4.9. Clinical Implications and Screening Recommendations
4.10. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Epidemiologic and methodologic problems in determining nutritional status of older persons (Summary comments). Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Zicarelli, M.; Duni, A.; Leivaditis, K.; Lin, Y.-L.; Baciga, F.; Pugliese, S.; Fiorentino, M.; Hsu, B.-G.; Roumeliotis, S.; Battaglia, Y.; et al. Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches. Medicina 2025, 61, 449. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef]
- Souweine, J.-S.; Kuster, N.; Chenine, L.; Rodriguez, A.; Patrier, L.; Morena, M.; Badia, E.; Chalabi, L.; Raynal, N.; Ohresser, I.; et al. Physical inactivity and protein energy wasting play independent roles in muscle weakness in maintenance haemodialysis patients. PLoS ONE 2018, 13, e0200061. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Mitch, W.E. Review of muscle wasting associated with chronic kidney disease. Am. J. Clin. Nutr. 2010, 91, 1128S–1132S. [Google Scholar] [CrossRef]
- Slee, A.; McKeaveney, C.; Adamson, G.; Davenport, A.; Farrington, K.; Fouque, D.; Kalantar-Zadeh, K.; Mallett, J.; Maxwell, A.P.; Mullan, R.; et al. Estimating the Prevalence of Muscle Wasting, Weakness, and Sarcopenia in Hemodialysis Patients. J. Ren. Nutr. 2020, 30, 313–321. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Li, J.-Y.; Xia, Q.-J.; Gao, Y.-Y.; Zhang, C.; Xu, P.-J.; Liu, J.; Zhang, H.-J.; Yu, R.-Z. Analysis of Risk Factors of Sarcopenia in Patients with Maintenance Hemodialysis and Its Correlation with Emotional Status and Quality of Life. J. Multidiscip. Healthc. 2024, 17, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P. Reconnaître et traiter la dénutrition en ambulatoire. In Post’U FMC-HE; Lévy, P., Ed.; Springer: Paris, France, 2011; pp. 1–8. [Google Scholar]
- Abro, A.; Delicata, L.-A.; Vongsanim, S.; Davenport, A. Differences in the prevalence of sarcopenia in peritoneal dialysis patients using hand grip strength and appendicular lean mass: Depends upon guideline definitions. Eur. J. Clin. Nutr. 2018, 72, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Woessner, M.N.; Sim, M.; Levinger, I. Sarcopenia definition: Does it really matter? Implications for resistance training. Ageing Res. Rev. 2022, 78, 101617. [Google Scholar] [CrossRef] [PubMed]
- Guelmami, N.; Aissa, M.B.; Ammar, A.; Dergaa, I.; Trabelsi, K.; Jahrami, H. Guidelines for applying psychometrics in sports science: Transitioning from traditional methods to the AI Era. Tunis. J. Sports Sci. Med. 2023, 1, 32–47. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef]
- Kiss, C.M.; Bertschi, D.; Beerli, N.; Berres, M.; Kressig, R.W.; Fischer, A.M. Calf circumference as a surrogate indicator for detecting low muscle mass in hospitalized geriatric patients. Aging Clin. Exp. Res. 2024, 36, 25. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Validating the SARC-F: A suitable community screening tool for sarcopenia? J. Am. Med. Dir. Assoc. 2014, 15, 630–634. [Google Scholar] [CrossRef]
- Guermazi, M.; Allouch, C.; Yahia, M.; Huissa, T.; Ghorbel, S.; Damak, J.; Mrad, M.; Elleuch, M. Translation in Arabic, adaptation and validation of the SF-36 Health Survey for use in Tunisia. Ann. Phys. Rehabil. Med. 2012, 55, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Guidarelli, G.; Giampieri, E.; Lanzarini, C.; Berendsen, A.A.M.; Januszko, O.; Jennings, A.; Lyon, N.; Caumon, E.; Gillings, R.; et al. Cross-sectional analysis of the correlation between daily nutrient intake assessed by 7-day food records and biomarkers of dietary intake among participants of the NU-AGE study. Front. Physiol. 2018, 9, 1359. [Google Scholar] [CrossRef] [PubMed]
- Therrien, M.; Byham-Gray, L.; Beto, J. A Review of Dietary Intake Studies in Maintenance Dialysis Patients. J. Ren. Nutr. 2015, 25, 329–338. [Google Scholar] [CrossRef]
- Les Références Nutritionnelles en Vitamines et Minéraux. Anses—Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail 2025. Available online: https://www.anses.fr/fr/content/les-references-nutritionnelles-en-vitamines-et-mineraux (accessed on 24 April 2025).
- Alimentation Saine n.d. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/healthy-diet (accessed on 28 April 2025).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Stubbs, B.; Veronese, N.; Manzato, E. Measurement of lean body mass using bioelectrical impedance analysis: A consideration of the pros and cons. Aging Clin. Exp. Res. 2017, 29, 591–597. [Google Scholar] [CrossRef]
- Duarte, C.K.; De Abreu Silva, L. What is the value of anthropometry for estimating muscle mass? Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 403–407. [Google Scholar] [CrossRef]
- Souweine, J.-S.; Pasquier, G.; Morena, M.; Patrier, L.; Rodriguez, A.; Raynal, N.; Ohresser, I.; Benomar, R.; Hayot, M.; Mercier, J.; et al. Beyond sarcopenia: Frailty in chronic haemodialysis patients. Clin. Kidney J. 2024, 17, sfae069. [Google Scholar] [CrossRef]
- Souza, A.B.F.; Nascimento, D.A.C.; Rodrigues, I.J.M.; Charone, C.C.O.; Lopes, G.L.; Lima, R.S.; Sá, A.A.; Carneiro, T.X.; Moraes, N.S. Association between sarcopenia and diabetes in community dwelling elderly in the Amazon region—Viver Mais Project. Arch. Gerontol. Geriatr. 2019, 83, 121–125. [Google Scholar] [CrossRef]
- Sugimoto, K.; Wang, C.-C.; Rakugi, H. Sarcopenia in diabetes mellitus. In Musculoskeletal Disease Associated with Diabetes Mellitus; Springer: Tokyo, Japan, 2016; pp. 237–252. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obes. 2023, 16, 1541–1554. [Google Scholar] [CrossRef]
- Purnamasari, D.; Tetrasiwi, E.N.; Kartiko, G.J.; Astrella, C.; Husam, K.; Laksmi, P.W. Sarcopenia and Chronic Complications of Type 2 Diabetes Mellitus. Rev. Diabet. Stud. 2022, 18, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Y.; Zhao, X.; Shi, R.; Lu, T.; Yu, R.; Wang, D. Prevalence and risk factors of sarcopenia in patients on maintenance hemodialysis: A retrospective cohort study. BMC Musculoskelet. Disord. 2024, 25, 424. [Google Scholar] [CrossRef] [PubMed]
- Ozen, A.; Cakmak, E.; Bilen, N.; Akel, M.; Gurel, A.; Selcuk, M. Investigation of sarcopenia in hemodialysis patients in Adiyaman province. Med.-Sci. 2021, 10, 895. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef]
- Kim, J.C.; Kalantar-Zadeh, K.; Kopple, J.D. Frailty and Protein-Energy Wasting in Elderly Patients with End Stage Kidney Disease. J. Am. Soc. Nephrol. 2013, 24, 337. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Flakoll, P.J.; Parker, R.A.; Hakim, R.M. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994, 46, 830–837. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Hu, H.; Chau, P.H.; Choi, E.P.H. Physical activity, exercise habits and health-related quality of life in maintenance hemodialysis patients: A multicenter cross-sectional study. J. Nephrol. 2024, 37, 1881–1891. [Google Scholar] [CrossRef]
- Olvera-Soto, M.G.; Valdez-Ortiz, R.; Alvarenga, J.C.L.; Espinosa-Cuevas, M.Á. Effect of resistance exercises on the indicators of muscle reserves and handgrip strength in adult patients on hemodialysis. J. Ren. Nutr. 2016, 26, 53–60. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Yeh, M.-L.; Liu, Y.-M. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: A systematic review and meta-analysis of randomised controlled trials. J. Clin. Nurs. 2017, 26, 1801–1813. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Wells, J.C.K.; Smith, S.R.; Stephan, B.C.M.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Mori, K. Maintenance of Skeletal Muscle to Counteract Sarcopenia in Patients with Advanced Chronic Kidney Disease and Especially Those Undergoing Hemodialysis. Nutrients 2021, 13, 1538. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Kamimura, M.A.; Lamarca, F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Association of Sarcopenia With Nutritional Parameters, Quality of Life, Hospitalization, and Mortality Rates of Elderly Patients on Hemodialysis. J. Ren. Nutr. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.W.; Watson, E.L.; Wilkinson, T.J.; Wormleighton, J.; Xenophontos, S.; Viana, J.L.; Smith, A.C. Ultrasound assessment of muscle mass in response to exercise training in chronic kidney disease: A comparison with MRI. J. Cachexia Sarcopenia Muscle 2019, 10, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Teo, S.S.L.; Che Din, N.; Abdul Gafor, A.H.; Ismail, R. The Role of Personality and Social Support in Health-Related Quality of Life in Chronic Kidney Disease Patients. PLoS ONE 2015, 10, e0129015. [Google Scholar] [CrossRef]
- Spiegel, B.M.R.; Melmed, G.; Robbins, S.; Esrailian, E. Biomarkers and health-related quality of life in end-stage renal disease: A systematic review. Clin. J. Am. Soc. Nephrol. 2008, 3, 1759–1768. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia—What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-W.; Chen, Y.-Y.; Chen, W.-L. Association between oral intake magnesium and sarcopenia: A cross-sectional study. BMC Geriatr. 2022, 22, 816. [Google Scholar] [CrossRef] [PubMed]
- Suranto, A.; Hermina, S.; Dwi, N.; Lusiana, B. Correlation between serum magnesium level and sarcopenia occurence in the elderly women: Study with dual-energy x-ray absorptiometry (DXA). Malays. J. Med. Health Sci. 2020, 16, 61–65. [Google Scholar]
- Cui, C.; Bao, Z.; Chow, S.K.-H.; Wong, R.M.Y.; Welch, A.; Qin, L.; Cheung, W.H. Coapplication of Magnesium Supplementation and Vibration Modulate Macrophage Polarization to Attenuate Sarcopenic Muscle Atrophy through PI3K/Akt/mTOR Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12944. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Yin, L.; Li, G.; Liang, W.; Chen, G. Characterization of the gut microbiota in hemodialysis patients with sarcopenia. Int. Urol. Nephrol. 2022, 54, 1899–1906. [Google Scholar] [CrossRef]
- Cai, G.; Ying, J.; Pan, M.; Lang, X.; Yu, W.; Zhang, Q. Development of a risk prediction nomogram for sarcopenia in hemodialysis patients. BMC Nephrol. 2022, 23, 319. [Google Scholar] [CrossRef]
- Dergaa, I.; Ben Saad, H.; Glenn, J.M.; Ben Aissa, M.M.; Taheri, M.; Swed, S.; Guelmami, N.; Chamari, K. A thorough examination of ChatGPT-3.5 potential applications in medical writing: A preliminary study. Medicine 2024, 103, e39757. [Google Scholar] [CrossRef]
- Dergaa, I.; Fekih-Romdhane, F.; Glenn, J.M.; Saifeddin Fessi, M.; Chamari, K.; Dhahbi, W.; Zghibi, M.; Bragazzi, N.L.; Ben Aissa, M.; Guelmami, N.; et al. Moving beyond the stigma: Understanding and overcoming the resistance to the acceptance and adoption of artificial intelligence chatbots. New Asian J. Med. 2023, 1, 29–36. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 118) | Sarcopenic (n = 50) | Non-Sarcopenic (n = 68) | p-Value | Effect Size (Cohen’s d) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 56.74 ± 14.44 | 65.31 ± 14.19 | 56.15 ± 14.70 | <0.001 | 0.632 |
| Female sex, n (%) | 54 (45.76) | 16 (32.0) | 38 (55.9) | 0.001 | |
| Hemodialysis duration (months) | 99.27 ± 113.45 | 148.47 ± 115.20 | 89.53 ± 98.7 | 0.001 | 0.556 |
| Comorbidities | - | ||||
| Diabetes mellitus, n (%) | 61 (51.7) | 34 (68.0) | 27 (39.7) | 0.01 | - |
| Hypertension, n (%) | 74 (62.7) | 32 (64.0) | 42 (61.8) | 0.32 | - |
| Hospitalization history, n (%) | 40 (33.9) | 28 (56.0) | 12 (17.6) | 0.032 | - |
| BMI (kg/m2) | 24.41 ± 3.59 | 25.25 ± 4.9 | 23.79 ± 3.5 | 0.48 | 0.352 |
| Physical Performance | |||||
| Handgrip strength (kg) | 21.34 ± 8.18 | 15.99 ± 5.39 | 25.27 ± 6.06 | <0.001 | −1.604 |
| Mid-arm circumference (cm) | 24.27 ± 2.03 | 23.12 ± 2.12 | 25.12 ± 2.44 | 0.013 | −0.866 |
| Calf circumference (cm) | 33.21 ± 2.9 | 31.47 ± 2.41 | 34.76 ± 2.34 | 0.002 | −1.388 |
| SPPB score | 9.25 ± 2.43 | 7.56 ± 2.65 | 10.49 ± 1.02 | <0.001 | −1.551 |
| Gait speed (m/s) | 0.83 ± 0.23 | 0.78 ± 0.14 | 0.87 ± 0.27 | 0.01 | −0.401 |
| TUG test (s | 16.66 ± 2.86 | 18.87 ± 2.74 | 15.06 ± 2.51 | <0.001 | 1.460 |
| Quality of Life | |||||
| SF-36 total score | 56.89 ± 10.43 | 32.57 ± 12.03 | 59.45 ± 14.82 | 0.001 | −1.960 |
| Barthel Index | 91.81 ± 10.93 | 86.30 ± 13.69 | 95.85 ± 5.7 | 0.01 | −0.965 |
| Laboratory tests | |||||
| Calcium (mmol/L) | 2.53 ± 0.29 | 2.52 ± 0.23 | 2.54 ± 0.12 | 0.851 | −0.114 |
| Hemoglobin (g/dL) | 8.12 ± 0.76 | 7.76 ± 0.97 | 8.49 ± 0.56 | 0.048 | −0.960 |
| Triglycerides (mmol/L) | 2.36 ± 0.43 | 2.33 ± 0.5 | 2.4 ± 0.36 | 0.364 | −0.165 |
| Albumin (g/L) | 38.87 ± 4.61 | 38.6 ± 4.12 | 39.15 ± 5.1 | 0.119 | −0.117 |
| CRP (mg/L) | 6.88 ± 2.23 | 7.23 ± 2.5 | 6.53 ± 1.97 | 0.571 | 0.317 |
| Ferritin (µg/L) | 457.42 ± 209 | 484.74 ± 220 | 430.11 ± 198 | 0.293 | 0.263 |
| Total n = 118 | Sarcopenic Group n = 50 | Non-Sarcopenic Group n = 68 | p | Effect Size (Cohen’s d) | |
|---|---|---|---|---|---|
| Calories (Kcal/Kg ideal weight/day) | 22.83 ± 19.54 | 24.56 ± 11.23 | 25.34 ± 13.76 | 0.45 | 0.061 |
| Proteins (g/d) | 55.54 ± 5.61 | 52.94 ± 4.23 | 57.59 ± 6.12 | 0.23 | 0.861 |
| Lipids (g/d) | 44.94 ± 7.44 | 40.50 ± 5.19 | 48.49 ± 7.45 | 0.1 | 1.21 |
| Carbohydrates (g/d) | 191.06 ± 39.1 | 173.44 ± 29.4 | 203.29 ± 42.87 | 0.3 | 0.79 |
| Calcium (mg/d) | 403.2 ± 156.21 | 383.29 ± 154.94 | 419.18 ± 156 | 0.211 | 0.231 |
| Magnesium (mg/d) | 174.51 ± 111.83 | 117.76 ± 76.4 | 212.06 ± 114.5 | 0.02 | 0.941 |
| Vitamin D (µg/d) | 2.45 ± 2.43 | 2.0 ± 3.8 | 2.78 ± 0.45 | 0.321 | 0.313 |
| Fibers (g/d) | 21.23 ± 9.67 | 14.11 ± 8.03 | 26.27 ± 5.65 | 0.006 | 1.799 |
| Phosphorus (mg/d) | 795.06 ± 266.25 | 747.74 ± 234 | 831.06 ± 291 | 0.128 | 0.310 |
| Variable | Odds Ratio | IC | p-Value |
|---|---|---|---|
| Longer hemodialysis duration | 1.56 | [1.2, 2.71] | p = 0.028 |
| Diabetes mellitus | 2.14 | [1.3, 3.67] | p < 0.001 |
| SPPB high | 0.482 | [0.26, 0.64] | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Othman, R.; Balti, A.; Boukhris, S.; Ceylan, H.İ.; Jamoussi, H.; Muntean, R.I.; Dergaa, I. Sarcopenia in Hemodialysis Patients: Prevalence, Independent Risk Factors, and Functional Implications—A Multicenter Cross-Sectional Study. J. Clin. Med. 2025, 14, 6893. https://doi.org/10.3390/jcm14196893
Ben Othman R, Balti A, Boukhris S, Ceylan Hİ, Jamoussi H, Muntean RI, Dergaa I. Sarcopenia in Hemodialysis Patients: Prevalence, Independent Risk Factors, and Functional Implications—A Multicenter Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):6893. https://doi.org/10.3390/jcm14196893
Chicago/Turabian StyleBen Othman, Rym, Amani Balti, Sabrine Boukhris, Halil İbrahim Ceylan, Henda Jamoussi, Raul Ioan Muntean, and Ismail Dergaa. 2025. "Sarcopenia in Hemodialysis Patients: Prevalence, Independent Risk Factors, and Functional Implications—A Multicenter Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 6893. https://doi.org/10.3390/jcm14196893
APA StyleBen Othman, R., Balti, A., Boukhris, S., Ceylan, H. İ., Jamoussi, H., Muntean, R. I., & Dergaa, I. (2025). Sarcopenia in Hemodialysis Patients: Prevalence, Independent Risk Factors, and Functional Implications—A Multicenter Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 6893. https://doi.org/10.3390/jcm14196893









