Whole-Blood Cellular Responses: A Promising Indicator of SARS-CoV-2 Immunity Compared to Serology
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
2.3. PBMC Stimulation
2.4. Whole Blood Stimulation
2.5. Cytokine Measurement
2.6. Serological Testing
2.7. Statistical Analysis
3. Results
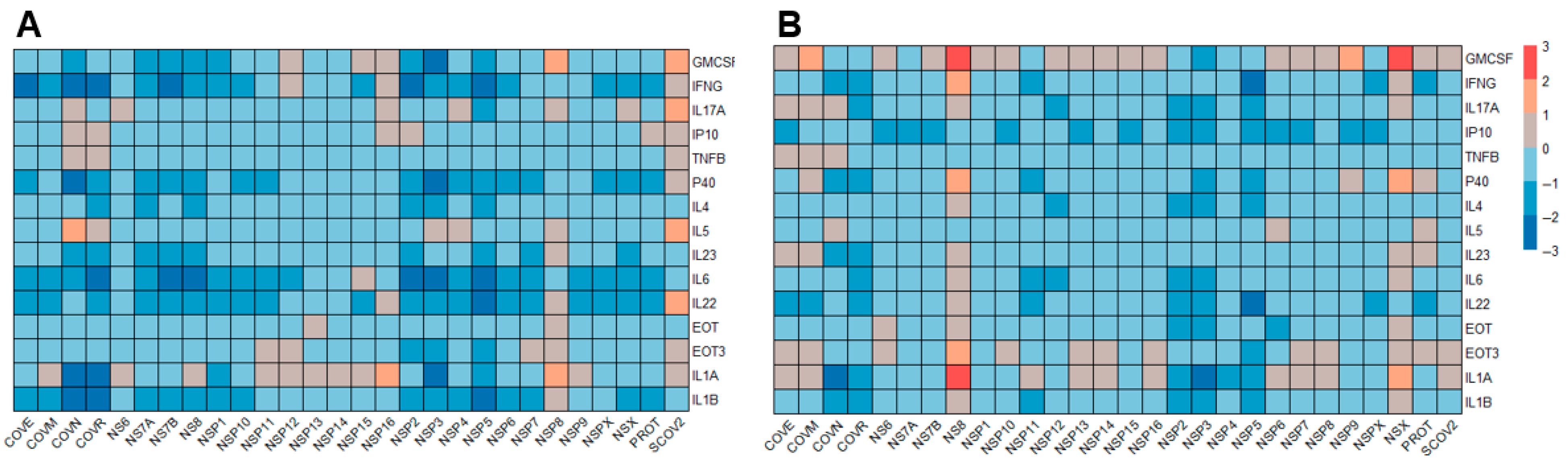
3.1. Cytokine Profiles Induced by SARS-CoV-2 Antigens
3.2. Comparative SARS-CoV-2-Specific Cytokine Profiling of PBMC Vs. Whole Blood
3.3. Identification of Stimulants and Cytokine Readout Method for Highest Accuracy in Determining Previous Exposure
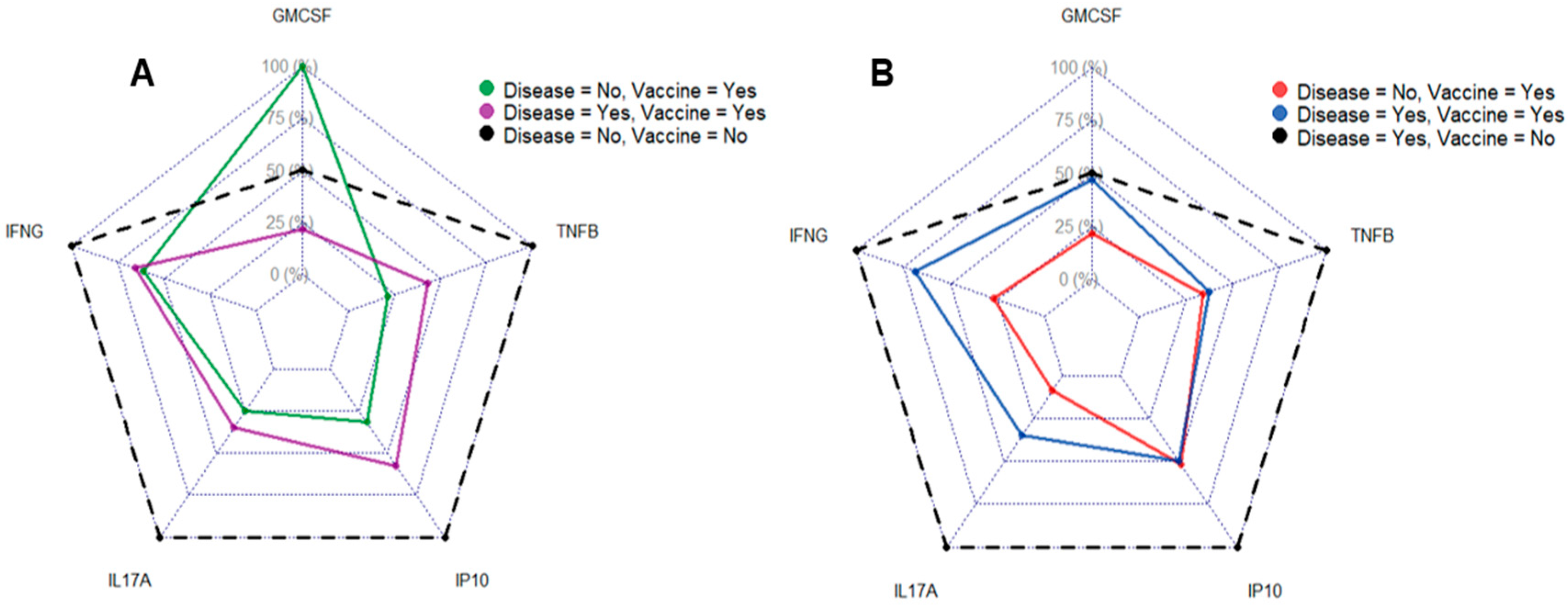
3.4. Vaccination and Disease Status Influence Cytokine Profile
3.5. Distinct Serological Profiles of Donors Based on Self-Reported COVID-19 History
3.6. Cellular and Serological Anti-SARS-CoV-2 Responses Show Only Weak Correlation
3.7. Longitudinal Changes in the Landscape of Epitope-Specific Cellular Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declarations
Disclaimer
Abbreviations
| COVE | SARS-CoV-2 Envelope protein |
| COVID-19 | Coronavirus Disease 2019 |
| COVM | SARS-CoV-2 Membrane protein |
| COVN | SARS-CoV-2 Nucleocapsid |
| FDA | Food and Drug Administration |
| HBSS | Hank’s balanced salt solution |
| IRB | Institutional Review Board |
| LOOCV | Leave-one-out-cross-validation |
| MSD | Mesoscale Diagnostics |
| NHSR | Exempt human use protocol |
| NSP | Nonstructural protein peptide pools |
| PASC | Post-acute sequelae of SARS-CoV-2 infection |
| PBMC | Peripheral blood mononuclear cells |
| PCR | Polymerase chain reaction |
| PROT | SARS-CoV-2 Protease protein |
| RF | Random Forest modeling |
| RUO | Research use only |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SCOV2 | SARS-CoV-2 Spike protein |
References
- Mushtaq, M.Z.; Shakoor, S.; Kanji, A.; Shaheen, N.; Nasir, A.; Ansar, Z.; Ahmed, I.; Mahmood, S.F.; Hasan, R.; Hasan, Z. Discrepancy between PCR based SARS-CoV-2 tests suggests the need to re-evaluate diagnostic assays. BMC Res. Notes 2021, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Dark, C.; Aspden, M.; Cochrane, D.; Competiello, R.; Peltz, M.; Torres, L.; Wrighton-Smith, P.; Dudek, M. Performance of the T-SPOT®. COVID test for detecting SARS-CoV-2-responsive T cells. Int. J. Infect. Dis. 2021, 113, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef]
- Cruz, T.; Mendoza, N.; Lledó, G.M.; Perea, L.; Albacar, N.; Agustí, A.; Sellares, J.; Sibila, O.; Faner, R. Persistence of a SARS-CoV-2 T-cell response in patients with long COVID and lung sequelae after COVID-19. ERJ Open Res. 2023, 9, 00020–02023. [Google Scholar] [CrossRef]
- Webber, J.D.; Przygonska, P.A.; Scalfani, C.R.; Pryal, P.F.; BLeach, B.G.; Abdeen, C.G.; Davey, R.A.; Voell, J.; Sneller, M.; Sereti, I.; et al. Evolution of SARS-CoV-2-Specific T Cell Responses from Acute COVID-19 Infection Through Convalescence. Available online: https://researchfestival.nih.gov/2024/posters/evolution-sars-cov-2-specific-t-cell-responses-acute-covid-19-infection (accessed on 9 August 2025).
- Lehmann, A.A.; Kirchenbaum, G.A.; Zhang, T.; Reche, P.A.; Lehmann, P.V. Deconvoluting the T Cell Response to SARS-CoV-2: Specificity Versus Chance and Cognate Cross-Reactivity. Front. Immunol. 2021, 12, 635942. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.S.; Thaweethai, T.; Kleinman, L.C.; Snowden, J.N.; Rosenzweig, E.B.; Milner, J.D.; Tantisira, K.G.; Rhee, K.E.; Jernigan, T.L.; Kinser, P.A.; et al. Characterizing Long COVID in Children and Adolescents. JAMA 2024, 332, 1174–1188. [Google Scholar] [CrossRef]
- Zang, C.; Guth, D.; Bruno, A.M.; Xu, Z.; Li, H.; Ammar, N.; Chew, R.; Guthe, N.; Hadley, E.; Kaushal, R.; et al. Long COVID after SARS-CoV-2 during pregnancy in the United States. Nat. Commun. 2025, 16, 3005. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Loy, E.X.H.; Tan, W.Z.; Tay, A.T.; Lim, J.T.; Chiew, C.J.; Choolani, M.; Lye, D.C.; Tan, K.B.; Wee, L.E. Long COVID-19 in pregnancy: Increased risk but modest incidence following mild Omicron infection in a boosted obstetric cohort during endemicity. Am. J. Obstet. Gynecol. 2025, 233, 323.e1–323.e12. [Google Scholar] [CrossRef]
- Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Adaptive Biotechnologies T-Detect COVID Test. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-march-9-2021 (accessed on 17 August 2025).
- Jang, J.; Widyasari, K.; Kim, S. Comparative analysis between STANDARD-E Covi-FERON ELISA with pre-existing IFN-γ release assays and determination of the optimum cutoff value for assessment of T-Cell response to SARS-CoV-2. J. Clin. Lab. Anal. 2023, 37, e24882. [Google Scholar] [CrossRef]
- Fonseca Brito, L.; Tödter, S.; Kottlau, J.; Cermann, K.; Spier, A.; Petersen, E.; Schäfer, I.; Twerenbold, R.; Aepfelbacher, M.; Lütgehetmann, M.; et al. Performance of an interferon-γ release assay-based test for cell-mediated immunity to SARS-CoV-2. Front. Immunol. 2023, 14, 1069968. [Google Scholar] [CrossRef]
- Law, J.C.; Watts, T.H. Considerations for Choosing T Cell Assays during a Pandemic. J. Immunol. 2023, 211, 169–174. [Google Scholar] [CrossRef]
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Fernandez, N.D.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022, 13, 80. [Google Scholar] [CrossRef]
- Ogbe, A.; Kronsteiner, B.; Skelly, D.T.; Pace, M.; Brown, A.; Adland, E.; Adair, K.; Akhter, H.D.; Ali, M.; Ali, S.E.; et al. T cell assays differentiate clinical and subclinical SARS-CoV-2 infections from cross-reactive antiviral responses. Nat. Commun. 2021, 12, 2055. [Google Scholar] [CrossRef]
- Farooq, F.; Beck, K.; Paolino, K.M.; Phillips, R.; Waters, N.C.; Regules, J.A.; Bergmann-Leitner, E.S. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Sci. Rep. 2016, 6, 27944. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Lu, P.; Atre, T.; Bolton, J.S.; Duncan, E.H.; Chaudhury, S.; Bergmann-Leitner, E.S. Immunoprofiling Identifies Functional B and T Cell Subsets Induced by an Attenuated Whole Parasite Malaria Vaccine as Correlates of Sterile Immunity. Vaccines 2022, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Hutter, J.; Bolton, J.S.; Hakre, S.; Mose, E.; Wooten, A.; O’Connell, W.; Hudak, J.; Krebs, S.J.; Darden, J.M.; et al. Serological profiles of pan-coronavirus-specific responses in COVID-19 patients using a multiplexed electro-chemiluminescence-based testing platform. PLoS ONE 2021, 16, e0252628. [Google Scholar] [CrossRef]
- Bolton, J.S.; Chaudhury, S.; Dutta, S.; Gregory, S.; Locke, E.; Pierson, T.; Bergmann-Leitner, E.S. Comparison of ELISA with electro-chemiluminescence technology for the qualitative and quantitative assessment of serological responses to vaccination. Malar. J. 2020, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B.; Aleman, S.; Bach, K.; Boribong, B.P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. 2023, 24, 1616–1627. [Google Scholar] [CrossRef]
- Zhang, Y.; Bharathi, V.; Dokoshi, T.; de Anda, J.; Ursery, L.T.; Kulkarni, N.N.; Nakamura, Y.; Chen, J.; Luo, E.W.C.; Wang, L.; et al. Viral afterlife: SARS-CoV-2 as a reservoir of immunomimetic peptides that reassemble into proinflammatory supramolecular complexes. Proc. Natl. Acad. Sci. USA 2024, 121, e2300644120. [Google Scholar] [CrossRef]
- Machkovech, H.M.; Hahn, A.M.; Garonzik Wang, J.; Grubaugh, N.D.; Halfmann, P.J.; Johnson, M.C.; Lemieux, J.E.; O’Connor, D.H.; Piantadosi, A.; Wei, W.; et al. Persistent SARS-CoV-2 infection: Significance and implications. Lancet Infect. Dis. 2024, 24, e453–e462. [Google Scholar] [CrossRef]
- Dorneles, G.P.; da Silva, I.M.; Santos, M.A.; Elsner, V.R.; Fonseca, S.G.; Peres, A.; Romão, P.R.T. Immunoregulation induced by autologous serum collected after acute exercise in obese men: A randomized cross-over trial. Sci. Rep. 2020, 10, 21735. [Google Scholar] [CrossRef]
- Eren, N.; Gerike, S.; Üsekes, B.; Peters, O.; Cosma, N.C.; Hellmann-Regen, J. Effects of autologous serum on TREM2 and APOE in a personalized monocyte-derived macrophage assay of late-onset Alzheimer’s patients. Immun. Ageing 2023, 20, 52. [Google Scholar] [CrossRef]
- Colino, J.; Duke, L.; Snapper, C.M. Autologous albumin enhances the humoral immune response to capsular polysaccharide covalently coattached to bacteria-sized latex beads. Eur. J. Immunol. 2014, 44, 1433–1443. [Google Scholar] [CrossRef]
- Levy, O.; Zarember, K.A.; Roy, R.M.; Cywes, C.; Godowski, P.J.; Wessels, M.R. Selective Impairment of TLR-Mediated Innate Immunity in Human Newborns: Neonatal Blood Plasma Reduces Monocyte TNF-α Induction by Bacterial Lipopeptides, Lipopolysaccharide, and Imiquimod, but Preserves the Response to R-8481. J. Immunol. 2004, 173, 4627–4634. [Google Scholar] [CrossRef]
- Angelone, D.F.; Wessels, M.R.; Coughlin, M.; Suter, E.E.; Valentini, P.; Kalish, L.A.; Levy, O. Innate Immunity of the Human Newborn Is Polarized Toward a High Ratio of IL-6/TNF-α Production In Vitro and In Vivo. Pediatr. Res. 2006, 60, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Morrocchi, E.; van Haren, S.; Palma, P.; Levy, O. Modeling human immune responses to vaccination in vitro. Trends Immunol. 2024, 45, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Ponte, C.G.G.; Hacker, M.A.; Antas, P.R.Z. A whole blood assay as a simple, broad assessment of cytokines and chemokines to evaluate human immune responses to Mycobacterium tuberculosis antigens. Acta Trop. 2013, 127, 75–81. [Google Scholar] [CrossRef]
- He, D.; Yang, C.X.; Sahin, B.; Singh, A.; Shannon, C.P.; Oliveria, J.-P.; Gauvreau, G.M.; Tebbutt, S.J. Whole blood vs. PBMC: Compartmental differences in gene expression profiling exemplified in asthma. Allergy Asthma Clin. Immunol. 2019, 15, 67. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- Müller, S.; Kröger, C.; Schultze, J.L.; Aschenbrenner, A.C. Whole blood stimulation as a tool for studying the human immune system. Eur. J. Immunol. 2024, 54, 2350519. [Google Scholar] [CrossRef]
- Polley, D.J.; Latham, P.; Choi, M.Y.; Buhler, K.A.; Fritzler, M.J.; Fritzler, M.L. Identification of novel clusters of co-expressing cytokines in a diagnostic cytokine multiplex test. Front. Immunol. 2023, 14, 1223817. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002, 23, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Poehlein, E.; Rane, M.S.; Frogel, D.; Kulkarni, S.; Gainus, C.; Profeta, A.; Robertson, M.; Nash, D. Presence of SARS-CoV-2 antibodies following COVID-19 diagnosis: A longitudinal study of patients at a major urgent care provider in New York. Diagn. Microbiol. Infect. Dis. 2022, 103, 115720. [Google Scholar] [CrossRef]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]





| Sample Cohort | Collection Period | Number of Donors |
|---|---|---|
| Pre.COVID | 2012–2019 | 10 |
| Healthy (no documented COVID-19) $ | 2020–2022 | 8 |
| COVID convalescent $ | 2020–2024 | 5 |
| Longitudinal samples $ | 2020, 2021 | 34 |
| Whole blood (healthy donors) | 2023–2024 | 30 |
| Antigen | Source | Concentration in Assay |
|---|---|---|
| SARS-CoV-2 Spike protein (Wuhan HU-1) (SCOV2) SARS-CoV-2 Receptor binding domain (RBD, COVR) HU-1 | WRAIR WRAIR | 5 μg/mL 1.5 μg/mL |
| HuCoV Spike protein 229E HuCoV Spike protein HKU-1 | Sinobiologics Inc. (Houston, TX, USA) Sinobiologics Inc. | 5 μg/mL 5 μg/mL |
| SARS-CoV-2 Nucleocapsid (COVN) | Sinobiologics Inc. | 5 μg/mL |
| SARS-CoV-2 Envelope protein (COVE) | Sinobiologics Inc. | 5 μg/mL |
| SARS-CoV-2 Membrane protein (COVM) | Sinobiologics Inc. | 5 μg/mL |
| SARS-CoV-2 Protease protein (PROT) | Sinobiologics Inc. | 5 μg/mL |
| SARS-CoV-2 ORF3A protein SARS-CoV-2 AP3A peptide pool | JPT Peptides Inc. (Berlin, Germany) JPT Peptides Inc. | 1.5 μg/mL 1.5 μg/mL |
| SARS-CoV-2 Nonstructural protein peptide mixes NSPX megapool (NSP1–NSP16) NSx megapool (NS6, NS7A, NS7B, NS8) | JPT Peptides Inc. JPT Peptides Inc. | 1.5 μg/mL 1.5 μg/mL |
| H1N1 hemagglutinin peptide pool (reference control) | JPT Peptide Inc. | 1.5 μg/mL |
| Anti-CD3 (positive control) | Mabtech Inc. (Cincinnati, OH, USA) | 1:1000 |
| Culture medium (negative control) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.M.; Duncan, E.H.; Boelig, R.C.; Costanzo, M.; Currier, J.R.; Bergmann-Leitner, E.S. Whole-Blood Cellular Responses: A Promising Indicator of SARS-CoV-2 Immunity Compared to Serology. J. Clin. Med. 2025, 14, 6889. https://doi.org/10.3390/jcm14196889
Zhou LM, Duncan EH, Boelig RC, Costanzo M, Currier JR, Bergmann-Leitner ES. Whole-Blood Cellular Responses: A Promising Indicator of SARS-CoV-2 Immunity Compared to Serology. Journal of Clinical Medicine. 2025; 14(19):6889. https://doi.org/10.3390/jcm14196889
Chicago/Turabian StyleZhou, Lucas M., Elizabeth H. Duncan, Rupsa C. Boelig, Margaret Costanzo, Jeffrey R. Currier, and Elke S. Bergmann-Leitner. 2025. "Whole-Blood Cellular Responses: A Promising Indicator of SARS-CoV-2 Immunity Compared to Serology" Journal of Clinical Medicine 14, no. 19: 6889. https://doi.org/10.3390/jcm14196889
APA StyleZhou, L. M., Duncan, E. H., Boelig, R. C., Costanzo, M., Currier, J. R., & Bergmann-Leitner, E. S. (2025). Whole-Blood Cellular Responses: A Promising Indicator of SARS-CoV-2 Immunity Compared to Serology. Journal of Clinical Medicine, 14(19), 6889. https://doi.org/10.3390/jcm14196889







