Early Open Kinetic Chain Hamstring Exercise After ACL Reconstruction: A Retrospective Safety and Efficacy Study
Abstract
1. Introduction
2. Materials and Methods
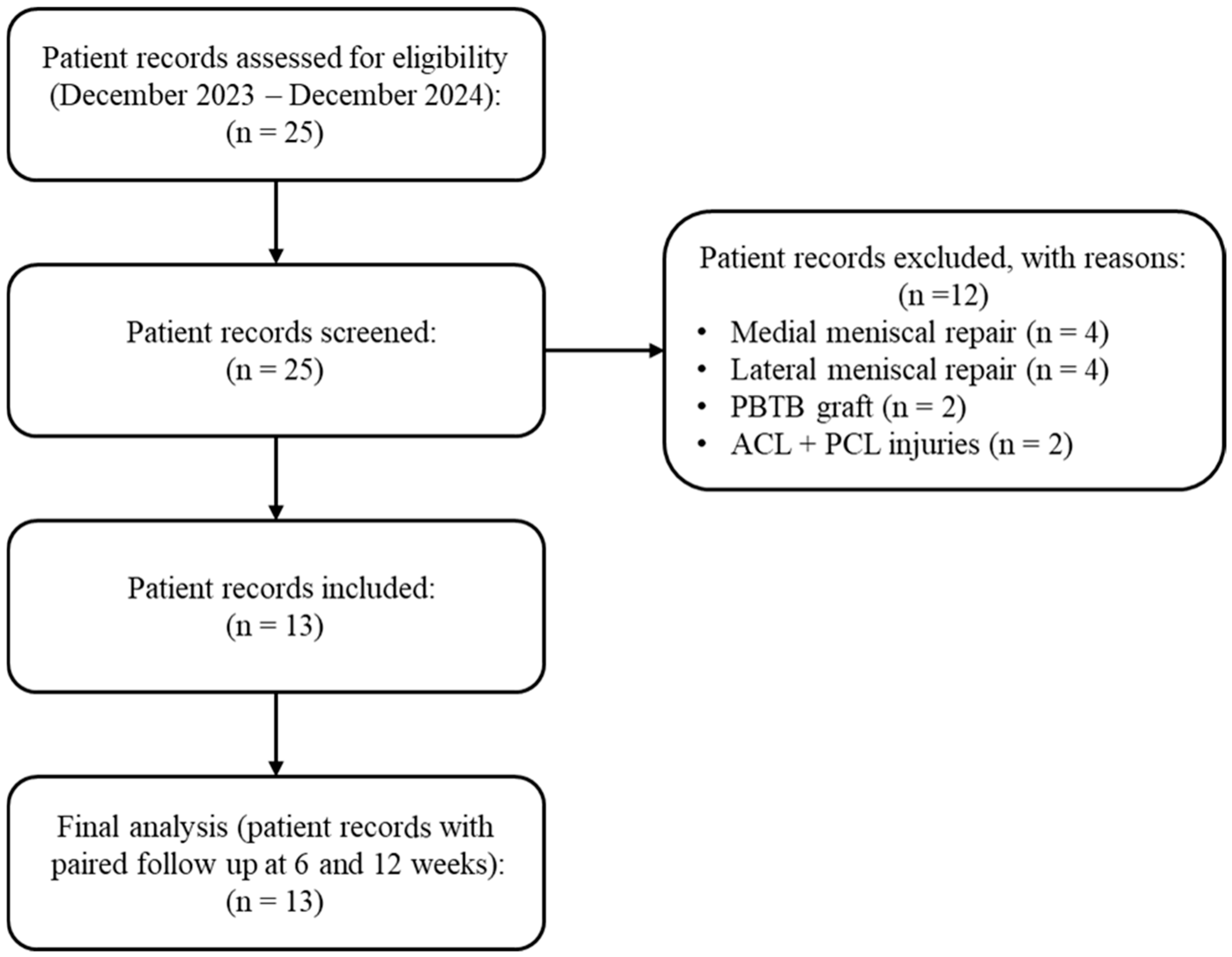
2.1. Study Design and Participants
- Age 18–35 years;
- Tegner Activity Scale ≥ 5;
- Isolated ACLR or combined with partial meniscectomy or lateral extra-articular tenodesis;
- Baseline knee range of motion ≥ 90° [40];
- Resting knee pain ≤ 3/10 on the Numeric Pain Rating Scale (NPRS);
- Absence of clinical signs of effusion or acute inflammatory flare (joint swelling < Trace on Stroke test compared to contralateral side, no redness or local heat or any other warning signs) [41].
- Meniscal repair;
- Concomitant Posterior Cruciate Ligament or posterolateral corner injuries;
- Use of grafts other than hamstring tendons;
- ACL revision surgery;
- Additional ligament injuries requiring protection beyond standard ACLR rehab;
- Cartilage procedures requiring protected loading (e.g., microfracture);
- Clinically relevant postoperative complications before week 3 (e.g., infection, arthrofibrosis).
2.2. Rehabilitation Protocol
- Two quadriceps-focused exercises (leg extension and leg press),
- Two calf exercises (bent-knee and straight-knee calf raises),
- Two core stabilization exercises (plank and side plank),
- Two hip-focused exercises targeting the adductors and abductors (Copenhagen plank and lateral walk with a mini-band positioned at the ankles),
- One single leg balance exercise with eye closed.
2.3. Outcome Measures
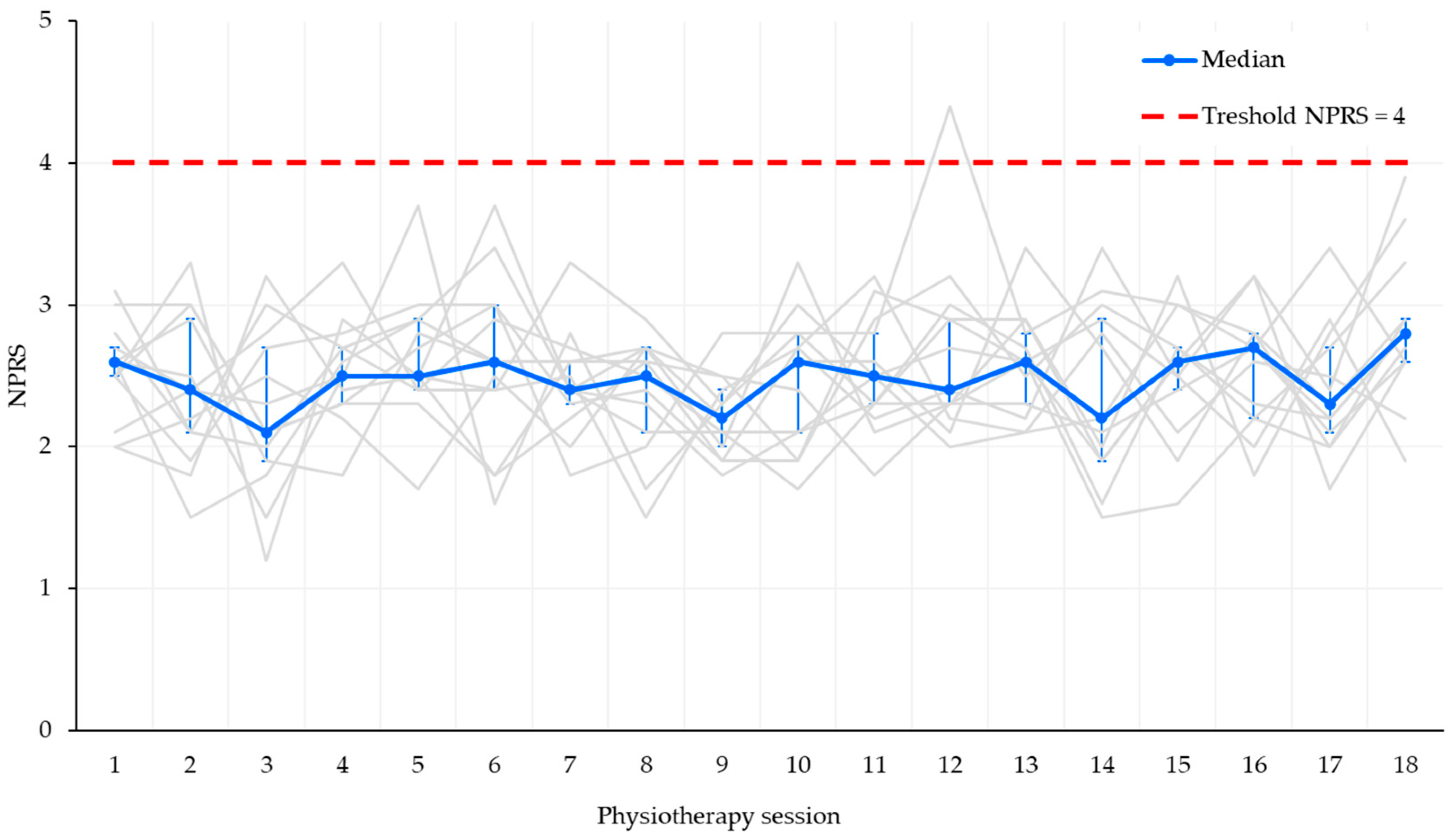
- Pain rated > 4/10 on the NPRS.
- Hematoma in the posterior thigh or at the graft-harvest site, described on inspection or palpation and, when necessary, confirmed by musculoskeletal ultrasound.
- Clinical signs of hamstring-muscle strain, identified by acute localized pain in the posterior thigh or tendon, pain exacerbated by stretching or palpation, and, when suspected, confirmed by magnetic-resonance imaging [44].
2.4. Statistical Analysis
3. Results
3.1. Safety
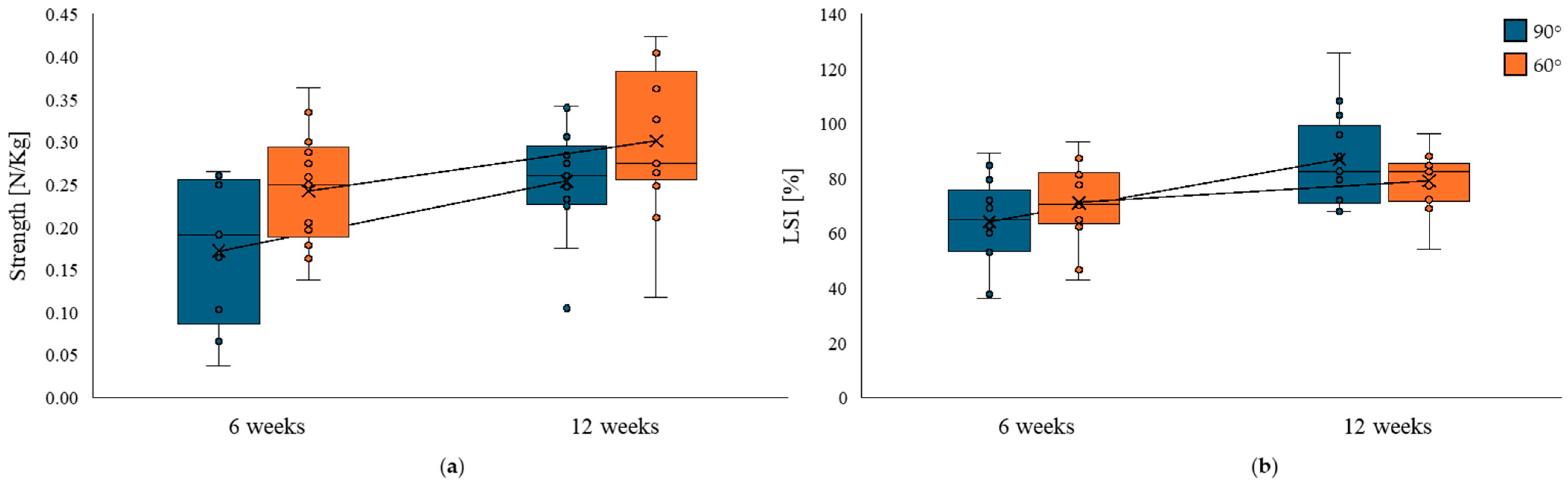
3.2. Strength Recovery
4. Discussion
4.1. Clinical Implications
4.2. Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farinelli, L.; Abermann, E.; Meena, A.; Ueblacker, P.; Hahne, J.; Fink, C. Return to Play and Pattern of Injury After ACL Rupture in a Consecutive Series of Elite UEFA Soccer Players. Orthop. J. Sports Med. 2023, 11, 23259671231153629. [Google Scholar] [CrossRef]
- Manojlovic, M.; Ninkovic, S.; Matic, R.; Versic, S.; Modric, T.; Sekulic, D.; Drid, P. Return to Play and Performance After Anterior Cruciate Ligament Reconstruction in Soccer Players: A Systematic Review of Recent Evidence. Sports Med. 2024, 54, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Niederer, D.; Engeroff, T.; Wilke, J.; Vogt, L.; Banzer, W. Return to Play, Performance, and Career Duration after Anterior Cruciate Ligament Rupture: A Case-Control Study in the Five Biggest Football Nations in Europe. Scand. J. Med. Sci. Sports 2018, 28, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.H.; Ardern, C.L.; Feller, J.A.; Webster, K.E. Eighty-Three per Cent of Elite Athletes Return to Preinjury Sport after Anterior Cruciate Ligament Reconstruction: A Systematic Review with Meta-Analysis of Return to Sport Rates, Graft Rupture Rates and Performance Outcomes. Br. J. Sports Med. 2018, 52, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Longstaffe, R.; Leiter, J.; Gurney-Dunlop, T.; McCormack, R.; MacDonald, P. Return to Play and Career Length After Anterior Cruciate Ligament Reconstruction Among Canadian Professional Football Players. Am. J. Sports Med. 2020, 48, 1682–1688. [Google Scholar] [CrossRef]
- Van Melick, N.; Van Cingel, R.E.H.; Brooijmans, F.; Neeter, C.; Van Tienen, T.; Hullegie, W.; Nijhuis-Van Der Sanden, M.W.G. Evidence-Based Clinical Practice Update: Practice Guidelines for Anterior Cruciate Ligament Rehabilitation Based on a Systematic Review and Multidisciplinary Consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef]
- Leathers, M.P.; Merz, A.; Wong, J.; Scott, T.; Wang, J.C.; Hame, S.L. Trends and Demographics in Anterior Cruciate Ligament Reconstruction in the United States. J. Knee Surg. 2015, 28, 390–394. [Google Scholar] [CrossRef]
- Pinheiro, V.H.; Borque, K.A.; Laughlin, M.S.; Jones, M.; Balendra, G.; Kent, M.R.; Ajgaonkar, R.; Williams, A. Determinants of Performance in Professional Soccer Players at 2 and 5 Years After ACL Reconstruction. Am. J. Sports Med. 2023, 51, 3649–3657. [Google Scholar] [CrossRef]
- Ricupito, R.; Grassi, A.; Mourad, F.; Di Filippo, L.; Gobbo, M.; Maselli, F. Anterior Cruciate Ligament Return to Play: “A Framework for Decision Making”. J. Clin. Med. 2025, 14, 2146. [Google Scholar] [CrossRef]
- Briem, K.; Zebis, M.K.; Haraldsson, B.Þ.; Bencke, J.; Fernandes, L. Rehabilitation Guidelines after Autograft Anterior Cruciate Ligament Reconstruction Need More Graft-Specific Exercise Recommendations—A Scoping Review. Knee Surg. Sports Traumatol. Arthrosc. 2025. [Google Scholar] [CrossRef]
- Persson, A.; Fjeldsgaard, K.; Gjertsen, J.E.; Kjellsen, A.B.; Engebretsen, L.; Hole, R.M.; Fevang, J.M. Increased Risk of Revision with Hamstring Tendon Grafts Compared with Patellar Tendon Grafts after Anterior Cruciate Ligament Reconstruction: A Study of 12,643 Patients from the Norwegian Cruciate Ligament Registry, 2004–2012. Am. J. Sports Med. 2014, 42, 285–291. [Google Scholar] [CrossRef]
- Yang, J.S.; Prentice, H.A.; Reyes, C.E.; Lehman, C.R.; Maletis, G.B. Risk of Revision and Reoperation After Quadriceps Tendon Autograft ACL Reconstruction Compared with Patellar Tendon and Hamstring Autografts in a US Cohort of 21,973 Patients. Am. J. Sports Med. 2024, 52, 670–681. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Carrozzo, A.; Saithna, A.; Monaco, E.; Vieira, T.D.; Musahl, V.; Getgood, A.; Helito, C.P.; Bedi, A.; Bryant, D.; et al. Surgical Treatment and Complications of Lateral Extra-Articular Procedures in the ACL Reconstructed Knee: Part II of An International Consensus Statement. Arthrosc. J. Arthrosc. Relat. Surg. 2025, 41, 3313–3321. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Carrozzo, A.; Saithna, A.; Monaco, E.; Vieira, T.D.; Musahl, V.; Getgood, A.; Helito, C.P.; Bedi, A.; Bryant, D.; et al. Indications for Lateral Extra-Articular Procedures in the ACL Reconstructed Knee: Part I of An International Consensus Statement. Arthrosc. J. Arthrosc. Relat. Surg. 2025, 41, 3303–3312. [Google Scholar] [CrossRef]
- Sundberg, A.; Senorski, R.H.; Högberg, J.; Piussi, R.; Samuelsson, K.; Thomeé, R.; Senorski, E.H. Persistent Isokinetic Knee Flexion Strength Deficits at the Time of Return to Sport Are Not Associated with a Second ACL Injury. Knee Surg. Sports Traumatol. Arthrosc. 2025, 333, 2971–2983. [Google Scholar] [CrossRef]
- Draganich, L.F.; Vahey, J.W. An in Vitro Study of Anterior Cruciate Ligament Strain Induced by Quadriceps and Hamstrings Forces. J. Orthop. Res. 1990, 8, 57–63. [Google Scholar] [CrossRef] [PubMed]
- More, R.C.; Karras, B.T.; Neiman, R.; Fritschy, D.; Woo, S.L.Y.; Daniel, D.M. Hamstrings—An Anterior Cruciate Ligament Protagonist: An in Vitro Study. Am. J. Sports Med. 1993, 21, 231–237. [Google Scholar] [CrossRef]
- Scanlan, S.F.; Chaudhari, A.M.W.; Dyrby, C.O.; Andriacchi, T.P. Differences in Tibial Rotation during Walking in ACL Reconstructed and Healthy Contralateral Knees. J. Biomech. 2010, 43, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Verrall, G.M.; Slavotinek, J.P.; Barnes, P.G.; Fon, G.T.; Spriggins, A.J. Clinical Risk Factors for Hamstring Muscle Strain Injury: A Prospective Study with Correlation of Injury by Magnetic Resonance Imaging. Br. J. Sports Med. 2001, 35, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.N.; Bruder, A.M.; Mentiplay, B.F.; Carey, D.L.; Patterson, B.E.; Crossley, K.M. Eccentric Knee Flexor Weakness in Elite Female Footballers 1–10 Years Following Anterior Cruciate Ligament Reconstruction. Phys. Ther. Sport 2019, 37, 144–149. [Google Scholar] [CrossRef]
- Messer, D.J.; Shield, A.J.; Williams, M.D.; Timmins, R.G.; Bourne, M.N. Hamstring Muscle Activation and Morphology Are Significantly Altered 1–6 Years after Anterior Cruciate Ligament Reconstruction with Semitendinosus Graft. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 733–741. [Google Scholar] [CrossRef]
- Bouzekraoui Alaoui, I.; Moiroux-Sahraoui, A.; Mazeas, J.; Kakavas, G.; Biały, M.; Douryang, M.; Forelli, F. Impact of Hamstring Graft on Hamstring Peak Torque and Maximum Effective Angle After Anterior Cruciate Ligament Reconstruction: An Exploratory and Preliminary Study. Bioengineering 2025, 12, 465. [Google Scholar] [CrossRef]
- Girdwood, M.; Culvenor, A.G.; Rio, E.K.; Patterson, B.E.; Haberfield, M.; Couch, J.; Mentiplay, B.; Hedger, M.; Crossley, K.M. Tale of Quadriceps and Hamstring Muscle Strength after ACL Reconstruction: A Systematic Review with Longitudinal and Multivariate Meta-Analysis. Br. J. Sports Med. 2025, 59, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, C.; Welling, W.; Hoppe, M.W.; Freiwald, J.; Gokeler, A. Angle-Specific Analysis of Isokinetic Quadriceps and Hamstring Torques and Ratios in Patients after ACL-Reconstruction. BMC Sports Sci. Med. Rehabil. 2018, 10, 23. [Google Scholar] [CrossRef]
- Williams, G.N.; Snyder-Mackler, L.; Barrance, P.J.; Axe, M.J.; Buchanan, T.S. Muscle and Tendon Morphology after Reconstruction of the Anterior Cruciate Ligament with Autologous Semitendinosus-Gracilis Graft. J. Bone Jt. Surg. 2004, 86, 1936–1946. [Google Scholar] [CrossRef]
- Irie, K.; Tomatsu, T. Atrophy of Semitendinosus and Gracilis and Flexor Mechanism Function after Hamstring Tendon Harvest for Anterior Cruciate Ligament Reconstruction. Orthopedics 2002, 25, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.J.; Wilcox, J.J.; Burks, R.T.; Greis, P.E. Evaluation of Muscle Size and Fatty Infiltration with MRI Nine to Eleven Years Following Hamstring Harvest for ACL Reconstruction. J. Bone Jt. Surg. 2012, 94, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Biały, M.; Wilczyński, B.; Forelli, F.; Hewett, T.E.; Gnat, R. Functional Deficits in Non-Elite Soccer (Football) Players: A Strength, Balance, and Movement Quality Assessment After Anterior Cruciate Lig Ament Reconstruction. Cureus 2024, 16, e75846. [Google Scholar] [CrossRef]
- Stergiou, M.; Calvo, A.L.; Forelli, F. Effectiveness of Neuromuscular Training in Preventing Lower Limb Soccer Injuries: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1714. [Google Scholar] [CrossRef]
- Forelli, F.; Traulle, M.; Bechaud, N.; Sansonnet, C.; Marine, P.; Vandebrouck, A.; Duffiet, P.; Mazeas, J. Predict Anterior Cruciate Ligament Injury in Elite Male Soccer Players? Focus On the Five Factors Maximum Model. Int. J. Physiother. 2021, 8, 211–216. [Google Scholar] [CrossRef]
- Buckthorpe, M.; Della Villa, F. Optimising the ‘Mid-Stage’ Training and Testing Process After ACL Reconstruction. Sports Med. 2020, 50, 657–678. [Google Scholar] [CrossRef]
- Saketh, A.S.P.V.S.; Subramanyam, K. Anterior Cruciate Ligament Rehabilitation Dilemma: Finding the Optimal Path between Conservative and Accelerated Methods. J. Telangana Orthop. Surg. Assoc. 2023, 1, 25. [Google Scholar] [CrossRef]
- Hiranyakumar, S.; Karthik, M.N. Functional Outcome of Accelerated Rehabilitation in Arthroscopic Anterior Cruciate Ligament Reconstruction with Semitendinosis and Gracilis Graft. Int. J. Sci. Study 2015, 3, 40–44. [Google Scholar] [CrossRef]
- Carofino, B.; Fulkerson, J. Medial Hamstring Tendon Regeneration Following Harvest for Anterior Cruciate Ligament Reconstruction: Fact, Myth, and Clinical Implication. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 1257–1265. [Google Scholar] [CrossRef]
- Escamilla, R.F.; MacLeod, T.D.; Wilk, K.E.; Paulos, L.; Andrews, J.R. Anterior Cruciate Ligament Strain and Tensile Forces for Weight-Bearing and Non-Weight-Bearing Exercises: A Guide to Exercise Selectiona. J. Orthop. Sports Phys. Ther. 2012, 42, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Ristanis, S.; Tsepis, E.; Giotis, D.; Stergiou, N.; Cerulli, G.; Georgoulis, A.D. Electromechanical Delay of the Knee Flexor Muscles Is Impaired after Harvesting Hamstring Tendons for Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2009, 37, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Buckthorpe, M.; Danelon, F.; La Rosa, G.; Nanni, G.; Stride, M.; Della Villa, F. Recommendations for Hamstring Function Recovery After ACL Reconstruction. Sports Med. 2021, 51, 607–624. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kobayashi, M.; Arai, R.; Tamaki, A.; Nakamura, T.; Moritani, T. Effect of Early Implementation of Electrical Muscle Stimulation to Prevent Muscle Atrophy and Weakness in Patients after Anterior Cruciate Ligament Reconstruction. J. Electromyogr. Kinesiol. 2011, 21, 622–630. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Forelli, F.; Mazeas, J.; Korakakis, V.; Ramtoola, H.; Vandebrouck, A.; Duffiet, P.; Ratte, L.; Kakavas, G.; Bouzekaroui Alaoui, I.; Douryang, M.; et al. Criteria-Based Decision Making for Introducing Open Kinetic Chain Exercise after-ACL Reconstruction: A Scoping Review. Sports Med. Open 2025, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, L.P.; Snyder-Mackler, L.; Manal, T.J.; Axe, M.J. Interrater Reliability of a Clinical Scale to Assess Knee Joint Effusion. J. Orthop. Sports Phys. Ther. 2009, 39, 845–849. [Google Scholar] [CrossRef]
- Medeiros, D.M.; de Quadros Robaina, B.; Rigotti, V.V.W.; Baroni, B.M. Resistance Training with Linear Periodization Is Superior to the ‘3 × 10 Reps Protocol’ after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. Phys. Ther. Sport 2025, 74, 75–82. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine Position Stand. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Martin, R.R.L.; Cibulka, M.T.; Bolgla, L.A.; Koc, T.A.; Loudon, J.K.; Manske, R.C.; Weiss, L.; Christoforetti, J.J.; Heiderscheit, B.C. Hamstring Strain Injury in Athletes. J. Orthop. Sports Phys. Ther. 2022, 52, CPG1–CPG44. [Google Scholar] [CrossRef]
- Högberg, J.; Piussi, R.; Simonsson, R.; Wernbom, M.; Samuelsson, K.; Thomeé, R.; Hamrin Senorski, E. The NordBord Test Reveals Persistent Knee Flexor Strength Asymmetry When Assessed Two and Five Years after ACL Reconstruction with Hamstring Tendon Autograft. Phys. Ther. Sport 2024, 66, 53–60. [Google Scholar] [CrossRef]
- Manchado, I.; Motta, L.M.; Blanco, G.; González, J.; Garcés, G.L. Isometric Knee Muscle Strength and Patient-Reported Measures Five Years after Anterior Cruciate Ligament Reconstruction: Comparison of Single versus Dual Autograft Hamstring Tendon Harvesting. J. Clin. Med. 2022, 11, 5682. [Google Scholar] [CrossRef]
- Harput, G.; Erkan Kilinc, H.; Ozer, H.; Baltaci, G.; Mattacola, C.G. Quadriceps and Hamstring Strength Recovery during Early Neuromuscular Rehabilitation after ACL Hamstring-Tendon Autograft Reconstruction. J. Sport Rehabil. 2015, 24, 398–404. [Google Scholar] [CrossRef] [PubMed]
- van Melick, N.; van der Weegen, W.; van der Horst, N. Quadriceps and Hamstrings Strength Reference Values for Athletes With and Without Anterior Cruciate Ligament Reconstruction Who Play Popular Pivoting Sports, Including Soccer, Basketball, and Handball: A Scoping Review. J. Orthop. Sports Phys. Ther. 2022, 52, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Lesnak, J.; Anderson, D.; Farmer, B.; Katsavelis, D.; Grindstaff, T.L. Validity of Hand-Held Dynamometry in Measuring Quadriceps Strength and Rate of Torque Development. Int. J. Sports Phys. Ther. 2019, 14, 180–187. [Google Scholar] [CrossRef]
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Hamstring Strength Recovery After Hamstring Tendon Harvest for Anterior Cruciate Ligament Reconstruction: A Comparison Between Graft Types. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ardern, C.; Webster, K. Knee Flexor Strength Recovery Following Hamstring Tendon Harvest for Anterior Cruciate Ligament Reconstruction: A Systematic Review. Orthop. Rev. 2009, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Ochi, M.; Uchio, Y.; Sakai, Y.; Kuriwaka, M.; Fujihara, A. Harvesting Hamstring Tendons for ACL Reconstruction Influences Postoperative Hamstring Muscle Performance. Arch. Orthop. Trauma Surg. 2003, 123, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Belloir, M.; Mazeas, J.; Traullé, M.; Vandebrouck, A.; Duffiet, P.; Ratte, L.; Forelli, F. Influence of the Open Kinetic Chain on the Distension of the Transplant After Anterior Cruciate Ligament Surgery with Hamstring Graft: Search for Risk Factors. Int. J. Physiother. 2020, 7. [Google Scholar] [CrossRef]
- Moiroux-Sahraoui, A.; Forelli, F.; Mazeas, J.; Rambaud, A.J.; Bjerregaard, A.; Riera, J. Quadriceps Activation After Anterior Cruciate Ligament Reconstruction: The Early Bird Gets the Worm! Int. J. Sports Phys. Ther. 2024, 19, 1044. [Google Scholar] [CrossRef]
- Moiroux-Sahraoui, A.; Mazeas, J.; Gold, M.; Kakavas, G.; Forelli, F. Neuromuscular Control Deficits After Anterior Cruciate Ligament Reconstruction: A Pilot Study Using Single-Leg Functional Tests and Electromyography. J. Funct. Morphol. Kinesiol. 2025, 10, 98. [Google Scholar] [CrossRef]
- Forelli, F.; Moiroux-Sahraoui, A.; Mazeas, J.; Dugernier, J.; Cerrito, A. Rethinking the Assessment of Arthrogenic Muscle Inhibition After ACL Reconstruction: Implications for Return-to-Sport Decision-Making—A Narrative Review. J. Clin. Med. 2025, 14, 2633. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Hopper, G.P.; Gousopoulos, L.; Vieira, T.D.; Thaunat, M.; Fayard, J.M.; Freychet, B.; Ouanezar, H.; Cavaignac, E.; Saithna, A. Arthrogenic Muscle Inhibition Following Knee Injury or Surgery: Pathophysiology, Classification, and Treatment. Video J. Sports Med. 2022, 2. [Google Scholar] [CrossRef]
| Day 1 | Day 2 | Day 3 |
|---|---|---|
| 10′ bike | 10′ bike | 10′ bike |
| Seated hamstring isometric curl at 90° | Horizontal single leg press | Seated hamstring isometric curl at 90° |
| Seated hamstring isometric curl at 60° | Seated hamstring isometric curl at 60° | |
| Isometric long lever glute bridge | Seated leg isometric leg extensions | Isometric long lever glute bridge |
| Horizontal single leg press | Standing calf raise | Horizontal single leg press |
| Seated leg isometric leg extensions | Bent knee calf raise | Seated leg isometric leg extensions |
| Standing calf raise | Lateral walk with mini band | Standing calf raise |
| Bent knee calf raise | Adductor plank | Bent knee calf raise |
| Lateral walk with mini band | Single leg balance exercise with eyes closed | Lateral walk with mini band |
| Adductor plank | Adductor plank | |
| Single leg balance exercise with eyes closed | Single leg balance exercise with eyes closed |
| Day 1 | Day 2 | Day 3 |
|---|---|---|
| 10′ bike | 10′ bike | 10′ bike |
| Seated isotonic leg curl | Horizontal single leg press | Seated isotonic leg curl |
| Isotonic long lever glute bridge | Seated leg isometric leg extensions | Isotonic long lever glute bridge |
| Horizontal single leg press | Standing calf raise | Horizontal single leg press |
| Seated leg isometric leg extensions | Bent knee calf raise | Seated leg isometric leg extensions |
| Standing calf raise | Lateral walk with mini band | Standing calf raise |
| Bent knee calf raise | Adductor plank | Bent knee calf raise |
| Lateral walk with mini band | Single leg balance exercise with eyes closed | Lateral walk with mini band |
| Adductor plank | Adductor plank | |
| Single leg balance exercise with eyes closed | Single leg balance exercise with eyes closed |
| Characteristics | ACL Patients (n = 13) |
|---|---|
| Age 1 | 27 (25–30) |
| Gender | |
| Male, n (%) | 9 (69.2) |
| Female, n (%) | 4 (30.8) |
| Weight (Kg) 1 | 71 (68–79) |
| Height (cm) 1 | 178 (172–183) |
| Graft type | |
| R HT/G, n (%) | 6 |
| L HT/G, n (%) | 7 |
| Time from surgery (weeks) | 21 (19–22) |
| Isometric Hamstring Muscle Test | LSI at 6 Weeks Median [IQR] | LSI at 12 Weeks Median [IQR] | Δ LSI | Z | p-Value | r | 95% Confidence Interval |
|---|---|---|---|---|---|---|---|
| Knee flexion = 60° | 70.4% [42.9–93.4] | 82.4% [54.2–96.3] | +9.5% | −1.64 | 0.108 | −0.52 | −0.83 to 0.05 |
| Knee flexion = 90° | 65% [36.4–89.2] | 82.7% [67.9–126] | +19.5% | −2.62 | 0.006 | −0.82 | −0.95 to −0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricupito, R.; Castellucci, R.; Maselli, F.; Bravi, M.; Santacaterina, F.; Guarise, R.; Forelli, F. Early Open Kinetic Chain Hamstring Exercise After ACL Reconstruction: A Retrospective Safety and Efficacy Study. J. Clin. Med. 2025, 14, 6871. https://doi.org/10.3390/jcm14196871
Ricupito R, Castellucci R, Maselli F, Bravi M, Santacaterina F, Guarise R, Forelli F. Early Open Kinetic Chain Hamstring Exercise After ACL Reconstruction: A Retrospective Safety and Efficacy Study. Journal of Clinical Medicine. 2025; 14(19):6871. https://doi.org/10.3390/jcm14196871
Chicago/Turabian StyleRicupito, Roberto, Rosalba Castellucci, Filippo Maselli, Marco Bravi, Fabio Santacaterina, Riccardo Guarise, and Florian Forelli. 2025. "Early Open Kinetic Chain Hamstring Exercise After ACL Reconstruction: A Retrospective Safety and Efficacy Study" Journal of Clinical Medicine 14, no. 19: 6871. https://doi.org/10.3390/jcm14196871
APA StyleRicupito, R., Castellucci, R., Maselli, F., Bravi, M., Santacaterina, F., Guarise, R., & Forelli, F. (2025). Early Open Kinetic Chain Hamstring Exercise After ACL Reconstruction: A Retrospective Safety and Efficacy Study. Journal of Clinical Medicine, 14(19), 6871. https://doi.org/10.3390/jcm14196871









