Increased Eplet Mismatch Load and Reduced Immunosuppressive Exposure Elevate the Risk of Baseline Lung Allograft Dysfunction
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
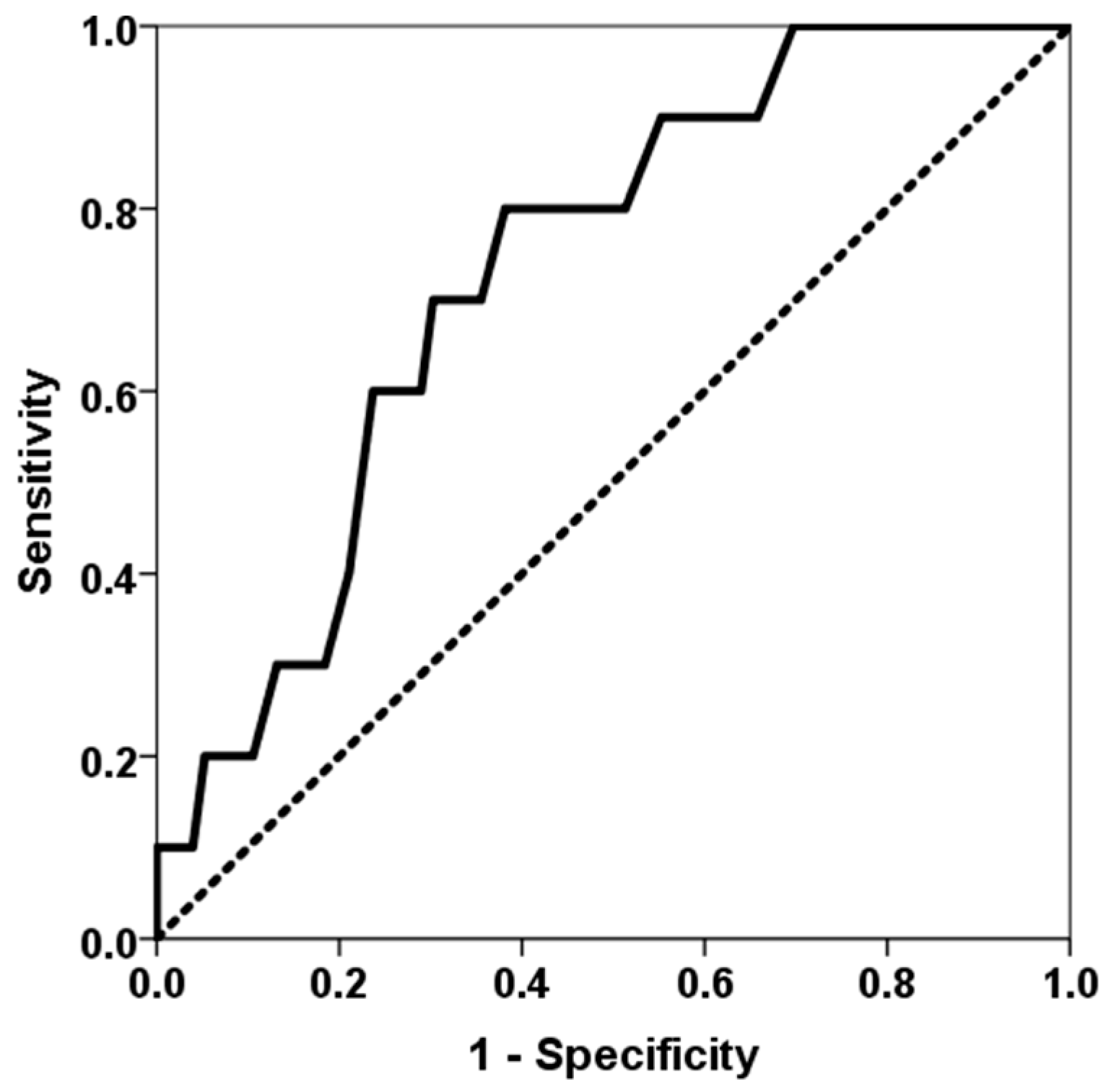
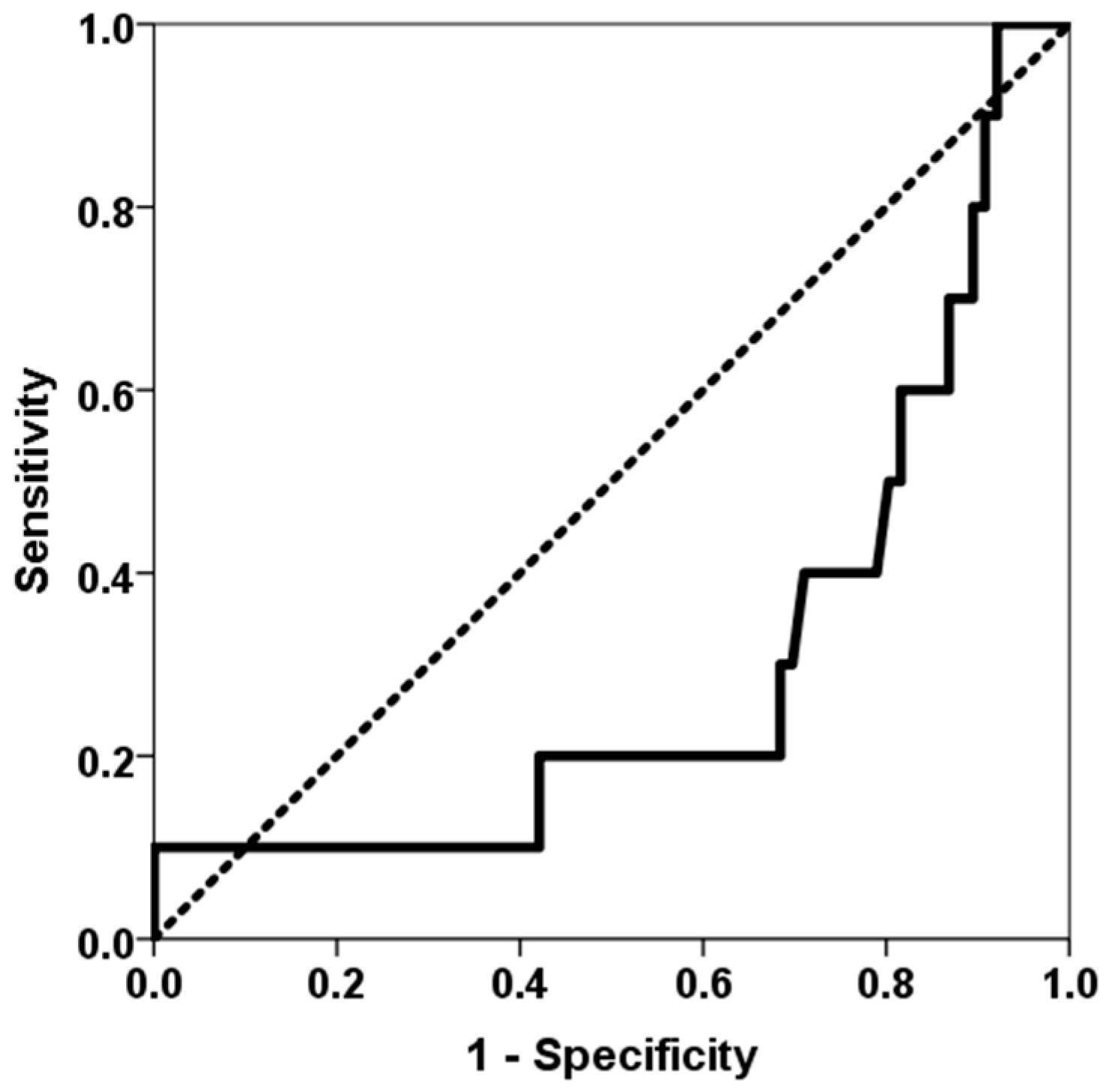
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLAD | Baseline lung allograft dysfunction |
| BMI | Body mass index |
| CLAD | Chronic lung allograft dysfunction |
| COPD | Chronic obstructive pulmonary disease |
| CV | Coefficient of variability |
| DSA | Donor-specific antibody |
| Ep | Eplet |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| ICU | Intensive care unit |
| ILD | Interstitial lung disease |
| IQR | Interquartile range |
| LAS | Lung allocation score |
| LungTx | Lung transplant |
| MM | Mismatch |
| MPA | Mycophenolic acid |
| MPA AUC0–12 | Mycophenolic acid area under the curve |
| PGD | Primary graft dysfunction |
| TTR | Time in therapeutic range |
References
- Mohanka, M.R.; Kanade, R.; Garcia, H.; Mahan, L.; Bollineni, S.; Mullins, J.; Joerns, J.; Kaza, V.; Torres, F.; Zhang, S.; et al. Significance of Best Spirometry in the First Year After Bilateral Lung Transplantation: Association with 3-Year Outcomes. Transplantation 2020, 104, 1712–1719. [Google Scholar] [CrossRef]
- Hage, R.; Steinack, C.; Schuurmans, M.M. Baseline Lung Allograft Dysfunction: Real-World Data for an Emerging Early Posttransplant Clinical Phenotype. Exp. Clin. Transplant. 2025, 23, 409–414. [Google Scholar] [CrossRef]
- Bando, K.O.; Paradis, I.L.; Keenan, R.J.; Yousem, S.A.; Komatsu, K.; Konishi, H.; Guilinger, R.A.; Masciangelo, T.N.; Pham, S.M.; Armitage, J.M. Comparison of outcomes after single and bilateral lung transplantation for obstructive lung disease. J. Heart Lung Transplant. 1995, 14, 692–698. [Google Scholar] [PubMed]
- Liu, J.; Jackson, K.; Weinkauf, J.; Kapasi, A.; Hirji, A.; Meyer, S.; Mullen, J.; Nagendran, J.; Lien, D.; Halloran, K. Baseline lung allograft dysfunction is associated with impaired survival after double-lung transplantation. J. Heart Lung Transplant. 2018, 37, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Verleden, G.M.; Bos, S. The ABC of transplant: ALAD, BLAD, and CLAD: Definition and significance. Curr. Opin. Pulm. Med. 2025, 31, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.B.; Sun, J.; Alnababteh, M.; Ponor, L.; Shah, P.D.; Mathew, J.; Kong, H.; Charya, A.; Luikart, H.; Aryal, S.; et al. Baseline Lung Allograft Dysfunction After Bilateral Lung Transplantation Is Associated with an Increased Risk of Death: Results from a Multicenter Cohort Study. Transplant. Direct 2024, 10, e1669. [Google Scholar] [CrossRef]
- Li, D.; Weinkauf, J.; Kapasi, A.; Hirji, A.; Varughese, R.; Lien, D.; Nagendran, J.; Halloran, K. Baseline lung allograft dysfunction in primary graft dysfunction survivors after lung transplantation. Respir. Med. 2021, 188, 106617. [Google Scholar] [CrossRef]
- Yoon, B.R.; Park, J.E.; Kim, C.Y.; Park, M.S.; Kim, Y.S.; Chung, K.S.; Song, J.H.; Paik, H.C.; Lee, J.G.; Kim, S.Y. Factors Associated with Lung Function Recovery at the First Year after Lung Transplantation. Yonsei Med. J. 2018, 59, 1088–1095. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kawashima, M.; Muraoka, T.; Yamaya, T.; Cong, Y.; Nakao, K.; Nagano, M.; Konoeda, C.; Kage, H.; Sato, M. Baseline lung allograft dysfunction after bilateral deceased-donor lung transplantation: A single-center experience in Japan. Respir. Investig. 2024, 62, 838–843. [Google Scholar] [CrossRef]
- Li, D.; Weinkauf, J.; Hirji, A.; Weatherald, J.; Varughese, R.; van den Bosch, L.; Lien, D.; Nagendran, J.; Halloran, K. Lung Transplantation from Donors with a History of Substance Use. Clin. Transplant. 2025, 39, e70162. [Google Scholar] [CrossRef]
- Rullay, A.; Kaur, K.; Holman, J.; van den Bosch, L.C.; Weinkauf, J.G.; Nagendran, J.; Varughese, R.A.; Hirji, A.S.; Lien, D.C.; Weatherald, J.C.; et al. Health-related Quality of Life and Exercise Capacity in Double Lung Transplant Recipients with Baseline Lung Allograft Dysfunction. Transplant. Direct 2025, 11, e1751. [Google Scholar] [CrossRef]
- Gallagher, H.M.; Sarwar, G.; Tse, T.; Sladden, T.M.; Hii, E.; Yerkovich, S.T.; Hopkins, P.M.; Chambers, D.C. Erratic tacrolimus exposure, assessed using the standard deviation of trough blood levels, predicts chronic lung allograft dysfunction and survival. J. Heart Lung Transplant. 2015, 34, 1442–1448. [Google Scholar] [CrossRef]
- Ensor, C.R.; Iasella, C.J.; Harrigan, K.M.; Morrell, M.R.; Moore, C.A.; Shigemura, N.; Zeevi, A.; McDyer, J.F.; Venkataramanan, R. Increasing tacrolimus time-in-therapeutic range is associated with superior one-year outcomes in lung transplant recipients. Am. J. Transpl. 2018, 18, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, H.; Matsuda, Y.; Watanabe, T.; Eba, S.; Hoshi, F.; Hirama, T.; Oishi, H.; Sado, T.; Noda, M.; Sakurada, A.; et al. Plasma mycophenolic acid concentration and the clinical outcome after lung transplantation. Clin. Transplant. 2020, 34, e14088. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Yerkovich, S.; Hopkins, P.M.; Leisfield, T.; Winks, L.; Chambers, D.C.; Divithotawela, C. Erratic tacrolimus levels at 6 to 12 months post-lung transplant predicts poor outcomes. JHLT Open 2023, 3, 100043. [Google Scholar] [CrossRef] [PubMed]
- González-López, E.; Mora-Cuesta, V.M.; Roa-Bautista, A.; Comins-Boo, A.; Renaldo, A.; Irure-Ventura, J.; Iturbe-Fernández, D.; Tello-Mena, S.; San Segundo, D.; Cifrián-Martínez, J.; et al. DQA1 Eplet Mismatch Load As an Independent Risk Factor of CLAD After Lung Transplantation. Transplant. Direct 2023, 9, e1513. [Google Scholar] [CrossRef]
- Alnababteh, M.; Sun, J.; Meda, R.; Ponor, L.; Shah, P.; Mathew, J.; Kong, H.; Charya, A.; Luikart, R.H.; Aryal, S.; et al. Impact of donor specific antibodies on longitudinal lung function and baseline lung allograft dysfunction. J. Heart Lung Transplant. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Zajacova, A.; Alkhouri, M.; Ferrao, G.; Guney, M.; Rezac, D.; Vyskocilova, K.; Kotowski, T.; Dutkova, A.; Dvorackova, E.; Lischke, R.; et al. Early post-lung transplant cell-free DNA levels are associated with baseline lung allograft function. Transpl. Immunol. 2025, 92, 102245. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Christie, J.D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction. part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef]
- Rodrigo, E.; San Segundo, D.; Fernandez-Fresnedo, G.; Lopez-Hoyos, M.; Benito, A.; Ruiz, J.C.; de Cos, M.A.; Arias, M. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation 2016, 100, 2479–2485. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Colmenero, J.; González, A.; Gastaca, M.; Curell, A.; Caballero-Marcos, A.; Sánchez-Martínez, A.; Di Maira, T.; Herrero, J.I.; Almohalla, C.; et al. Chronic immunosuppression, cancer Spanish consortium. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am. J. Transplant. 2022, 22, 1671–1682. [Google Scholar] [CrossRef]
- Pawinski, T.; Luszczynska, P.; Durlik, M.; Majchrzak, J.; Baczkowska, T.; Chrzanowska, M.; Sobiak, J.; Glyda, M.; Kuriata-Kordek, M.; Kaminska, D.; et al. Development and validation of limited sampling strategies for the estimation of mycophenolic acid area under the curve in adult kidney and liver transplant recipients receiving concomitant enteric-coated mycophenolate sodium and tacrolimus. Ther. Drug Monit. 2013, 35, 760–769. [Google Scholar] [CrossRef]
- Van Gelder, T.; Silva, H.T.; de Fijter, J.W.; Budde, K.; Kuypers, D.; Arns, W.; Soulillou, J.P.; Kanellis, J.; Zelvys, A.; Ekberg, H.; et al. Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid. Transplantation 2010, 89, 595–599. [Google Scholar] [CrossRef]
- Mora-Cuesta, V.M.; Murillo-Brito, D.A.; Argos-Vélez, P.J.; Iturbe-Fernández, D.; Naranjo-Gozalo, S.; Sánchez-Moreno, L.; Tello-Mena, S.; Berjón-de la Vega, L.; Izquierdo-Cuervo, S.; Poo-Fernández, C.; et al. Impact of lung resections on lung transplant grafts due to size discrepancy. Heart Lung 2025, 74, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Poo-Fernández, C.; Iturbe-Fernández, D.; Tello-Mena, S.; Izquierdo-Cuervo, S.; Sánchez-Moreno, L.; Murillo-Brito, D.A.; Naranjo-Gozalo, S.; Mons-Lera, R.; Alonso-Lecue, P.; Cifrián-Martínez, J.M.; et al. The impact of donor-recipient size mismatch on lung transplant outcomes. J. Thorac. Dis. 2025, 17, 4621–4632. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, Q.; Weinkauf, J.; Kapasi, A.; Varughese, R.; Hirji, A.; Lien, D.; Meyer, S.; Laing, B.; Nagendran, J.; et al. Azithromycin prophylaxis after lung transplantation is associated with improved overall survival. J. Heart Lung Transplant. 2020, 39, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Cristeto Porras, M.; Mora Cuesta, V.M.; Iturbe Fernandez, D.; Tello Mena, S.; Alonso Lecue, P.; Sanchez Moreno, L.; Minambres Garcia, E.; Naranjo Gozalo, S.; Izquierdo Cuervo, S.; Cifrian Martinez, J.M. Early onset of azithromycin to prevent CLAD in lung transplantation: Promising results of a retrospective single centre experience. Clin. Transplant. 2023, 37, e14832. [Google Scholar] [CrossRef]
| No BLAD = 76 | BLAD = 10 | p-Value | |
|---|---|---|---|
| Recipient age (years) | 63 (IQR 8) | 56 (IQR 21) | 0.021 |
| Female recipient | 44.7% | 80.0% | 0.036 |
| BMI (kg/m2) | 24.5 (IQR 4.3) | 25.4 (IQR 6.0) | 0.903 |
| Recipient height (cm) | 165 (IQR 14) | 163 (IQR 8) | 0.594 |
| Recipient weight (kg) | 67 (IQR 16) | 64 (IQR 17) | 0.666 |
| Native lung disease: ILD | 47.4% | 50.0% | 0.876 |
| Native lung disease: COPD | 43.4% | 30.0% | 0.419 |
| LAS | 33.5 (IQR 2.6) | 33.9 (IQR 4.5) | 0.657 |
| Ventilation length (days) | 1.0 (IQR 0.1) | 2.0 (IQR 11.0) | 0.028 |
| ICU stay length (days) | 3.0 (IQR 2.0) | 10.0 (IQR 28.0) | 0.007 |
| Hospitalization stay length (days) | 21.0 (IQR 9.0) | 32.0 (IQR 45.0) | 0.027 |
| PGD grade 3 at 0–72 h | 9.2% | 10.0% | 0.936 |
| Ischemic time (min) | 373 (IQR 95) | 396 (IQR 104) | 0.220 |
| Donor age | 55 (IQR 19) | 59 (IQR 6) | 0.505 |
| Female donor | 43.4% | 80.0% | 0.029 |
| Donor smoking history | 68.5% | 50.0% | 0.246 |
| Donor height (cm) | 168 (IQR 15) | 166 (IQR 10) | 0.746 |
| Donor weight (kg) | 73 (IQR 20) | 70 (IQR 22) | 0.622 |
| All class-I Ep MM | 29.0 (IQR 15.0) | 35.0 (IQR 16.0) | 0.054 |
| All DRB1 Ep MM | 14.0 (IQR 14.0) | 17.5 (IQR 20.0) | 0.151 |
| All DQ Ep MM | 10.0 (IQR 8.0) | 13.0 (IQR 11.0) | 0.113 |
| All DR and DQ EpMM | 25.2 (IQR 19.5) | 28.0 (IQR 19.0) | 0.133 |
| All class-I and II Ep MM | 55 (IQR 22) | 67 (IQR 20) | 0.018 |
| Cellular rejection | 35.5% | 20.0% | 0.329 |
| Third-month dd-cfDNA * | 0.39 (IQR 0.74) | 0.51 (IQR 4.16) | 0.499 |
| Third-month dd-cfDNA > 1% * | 22.2% | 40.0% | 0.371 |
| Six-month FVC (ml) | 3190 (IQR 1525) | 2110 (IQR 1005) | <0.001 |
| Six-month predicted FVC% | 90.5 (IQR 22.0) | 59.0 (IQR 11.0) | <0.001 |
| Six-month FEV1 (ml) | 2490 (IQR 1253) | 1655 (IQR 735) | <0.001 |
| Six-month predicted FEV1% | 92.5 (IQR 26.0) | 60.0 (IQR 11.0) | <0.001 |
| Third-month % time in therapeutic range | 39.6 (IQR 24.3) | 26.6 (IQR 14.0) | 0.039 |
| Third-month % time in therapeutic range below first quartile | 21.1% | 60.0% | 0.008 |
| Total tacrolimus exposure at third month | 1096 (IQR 140) | 1075 (IQR 234) | 0.649 |
| Third-month tacrolimus CV | 42.0 (IQR 8.7) | 43.0 (IQR 7.4) | 0.957 |
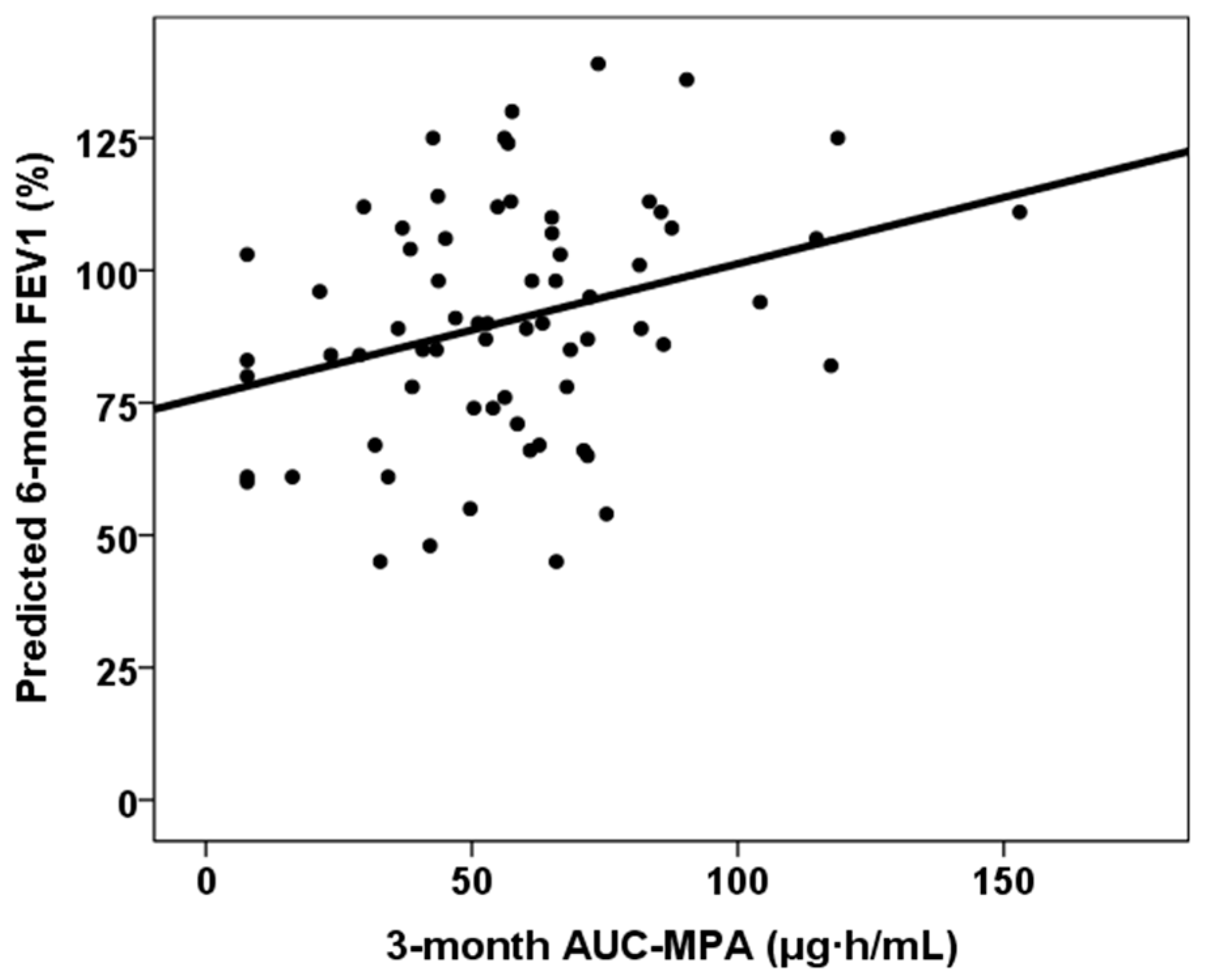
| Third-month MPA AUC0–12 ** | 56.8 (IQR 29.9) | 32.8 (IQR 44.7) | 0.069 |
| Third-month MPA AUC0–12 > 30 mg × h/L ** | 89.2% | 57.1% | 0.020 |
| Six-Month Predicted FVC% | Six-Month Predicted FEV1% | |
|---|---|---|
| Recipient age (years) | rho = 0.125, p = 0.262 | rho = 0.093, p = 0.406 |
| BMI (kg/m2) | rho = −0.081, p = 0.469 | rho = −0.171, p = 0.124 |
| Recipient height (cm) | rho = 0.131, p = 0.240 | rho = 0.036, p = 0.751 |
| Recipient weight (kg) | rho = 0.019, p = 0.869 | rho = −0.112, p = 0.317 |
| LAS | rho = −0.115, p = 0.305 | rho = −0.258, p = 0.020 |
| Ventilation length (days) | rho = −0.360, p = 0.001 | rho = −0.324, p = 0.004 |
| ICU stay length (days) | rho = −0.204, p = 0.066 | rho = −0.263, p = 0.017 |
| Hospitalization stay length (days) | rho = −0.285, p = 0.009 | rho = −0.215, p = 0.053 |
| Ischemic time (min) | rho = −0.294, p = 0.007 | rho = −0.189, p = 0.088 |
| Donor age | rho = 0.094, p = 0.402 | rho = −0.120, p = 0.283 |
| Donor height (cm) | rho = 0.177, p = 0.112 | rho = 0.050, p = 0.654 |
| Donor weight (kg) | rho = 0.051, p = 0.652 | rho = −0.078, p = 0.484 |
| All class-I Ep MM | rho = −0.062, p = 0.577 | rho = 0.019, p = 0.868 |
| All DR Ep MM | rho = −0.281, p = 0.010 | rho = −0.303, p = 0.006 |
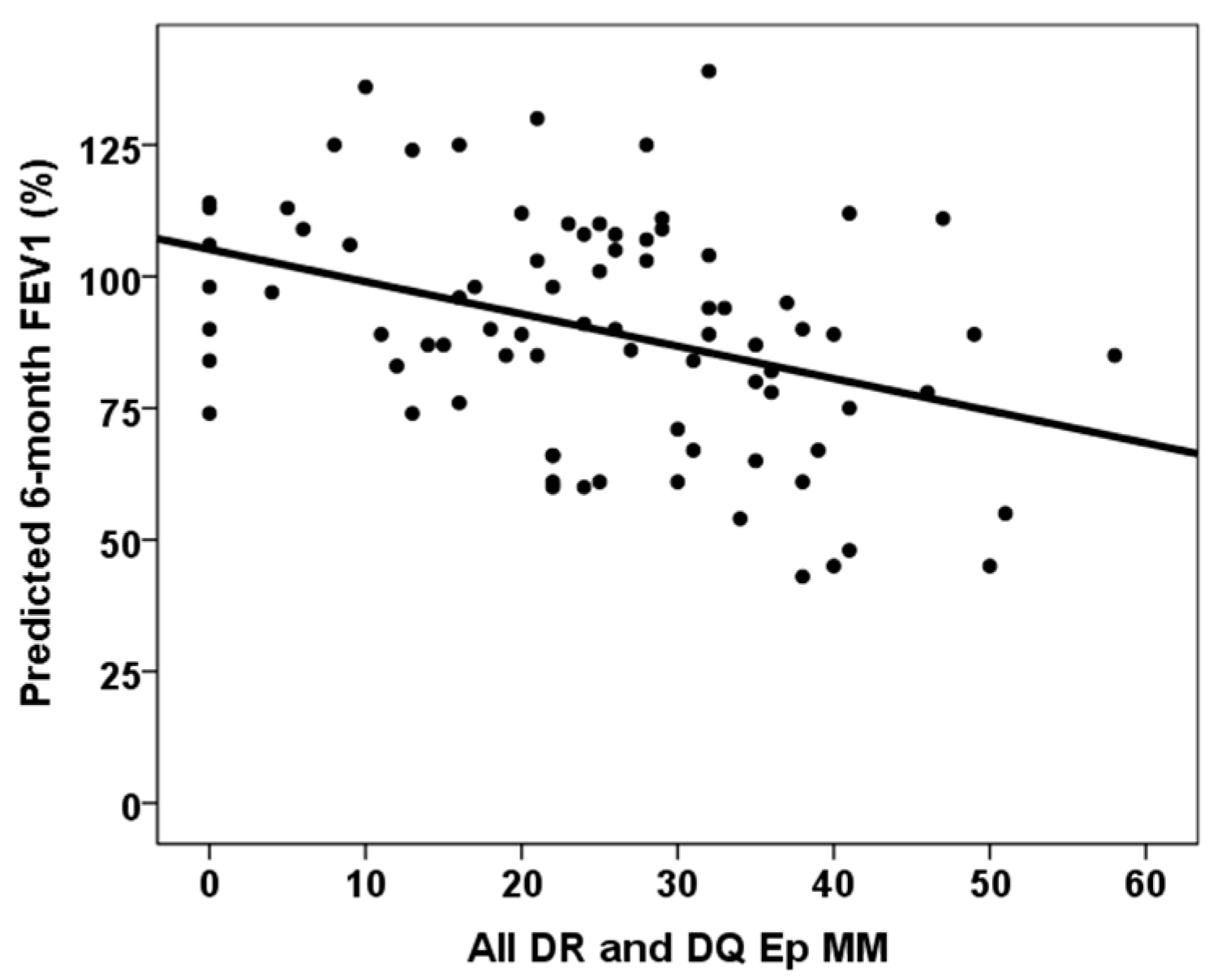
| All DQ Ep MM | rho = −0.265, p = 0.016 | rho = −0.338, p = 0.002 |
| All DR and DQ Ep MM | rho = −0.320, p = 0.003 | rho = −0.369, p = 0.001 |
| All classes-I and -II Ep MM | rho = −0.308, p = 0.005 | rho = −0.315, p = 0.004 |
| Third-month dd-cfDNA | rho = −0.108, p = 0.429 | rho = −0.069, p = 0.615 |
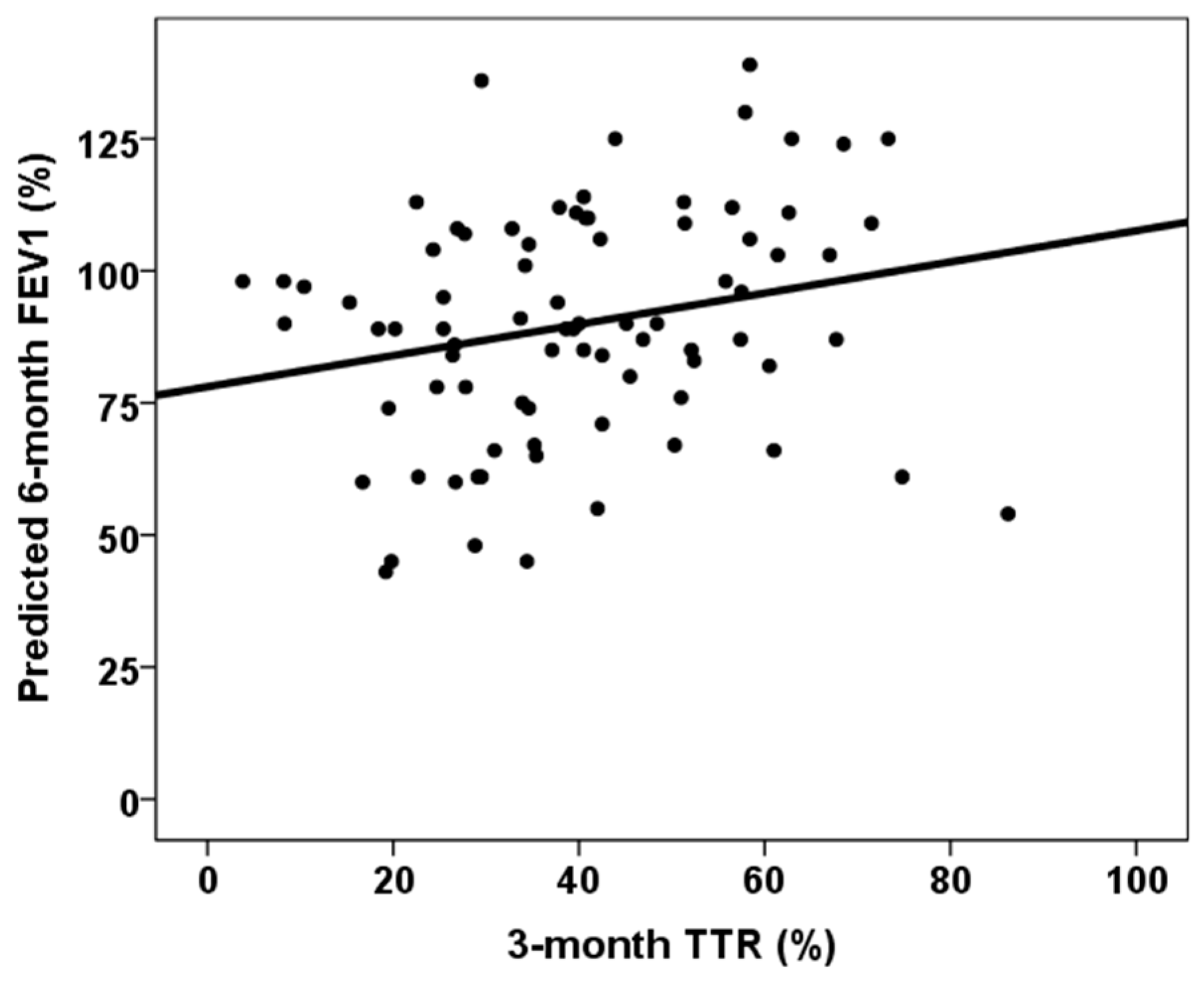
| Third-month % time in therapeutic range | rho = 0.383, p < 0.001 | rho = 0.251, p = 0.023 |
| Total tacrolimus exposure at third month | rho = 0.062, p = 0.585 | rho = 0.055, p = 0.630 |
| Third-month tacrolimus CV | rho = −0.083, p = 0.460 | rho = −0.013, p = 0.905 |
| Third-month MPA AUC0–12 | rho = 0.306, p = 0.011 | rho = 0.292, p = 0.016 |
| Six-Month Predicted FVC% | Six-Month Predicted FVC% | Six-Month Predicted FEV1% | Six-Month Predicted FEV1% | |
|---|---|---|---|---|
| Female recipient | β = −0.327, p = 0.019 | β = −0.288, p = 0.065 | - | - |
| ILD | - | - | β = −0.249, p = 0.020 | β = −0.282, p = 0.011 |
| Recipient height (cm) | β = −0.171, p = 0.225 | β = −0.085, p = 0.589 | β = −0.005, p = 0.960 | β = 0.031, p = 0.779 |
| Ventilation length (days) | β = −0.327, p = 0.002 | β = −0.276, p = 0.012 | β = −0.242, p = 0.023 | β = −0.164, p = 0.134 |
| All DR and DQ EpMM | β = −0.338, p = 0.001 | β = −0.340, p = 0.002 | β = −0.311, p = 0.004 | β = −0.348, p = 0.002 |
| Third-month % TTR | β = 0.172, p = 0.090 | β = 0.093, p = 0.395 | β = 0.067, p = 0.530 | β =- 0.003, p = 0.981 |
| Third-month MPA AUC0–12 | - | β = 0.254, p = 0.018 | - | β = 0.285, p = 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, V.M.; Rodrigo, E.; González-López, E.; Ocejo-Vinyals, J.G.; San Segundo, D.; Iturbe-Fernández, D.; Izquierdo, S.; Tello, S.; López-Hoyos, M.; García-Saiz, M.M.; et al. Increased Eplet Mismatch Load and Reduced Immunosuppressive Exposure Elevate the Risk of Baseline Lung Allograft Dysfunction. J. Clin. Med. 2025, 14, 6864. https://doi.org/10.3390/jcm14196864
Mora VM, Rodrigo E, González-López E, Ocejo-Vinyals JG, San Segundo D, Iturbe-Fernández D, Izquierdo S, Tello S, López-Hoyos M, García-Saiz MM, et al. Increased Eplet Mismatch Load and Reduced Immunosuppressive Exposure Elevate the Risk of Baseline Lung Allograft Dysfunction. Journal of Clinical Medicine. 2025; 14(19):6864. https://doi.org/10.3390/jcm14196864
Chicago/Turabian StyleMora, Victor M., Emilio Rodrigo, Elena González-López, Javier Gonzalo Ocejo-Vinyals, David San Segundo, David Iturbe-Fernández, Sheila Izquierdo, Sandra Tello, Marcos López-Hoyos, Maria Mar García-Saiz, and et al. 2025. "Increased Eplet Mismatch Load and Reduced Immunosuppressive Exposure Elevate the Risk of Baseline Lung Allograft Dysfunction" Journal of Clinical Medicine 14, no. 19: 6864. https://doi.org/10.3390/jcm14196864
APA StyleMora, V. M., Rodrigo, E., González-López, E., Ocejo-Vinyals, J. G., San Segundo, D., Iturbe-Fernández, D., Izquierdo, S., Tello, S., López-Hoyos, M., García-Saiz, M. M., García-Berbel, P., & Cifrián, J. M. (2025). Increased Eplet Mismatch Load and Reduced Immunosuppressive Exposure Elevate the Risk of Baseline Lung Allograft Dysfunction. Journal of Clinical Medicine, 14(19), 6864. https://doi.org/10.3390/jcm14196864








