Testing the Stable Unit Treatment Variance Assumption (SUTVA) Within Cochrane Reviews of Antimicrobial-Based Pneumonia Prevention Interventions Among Mechanically Ventilated Patients Using Caterpillar Plots
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Decant
2.2. Contrast-Based Analysis-Method
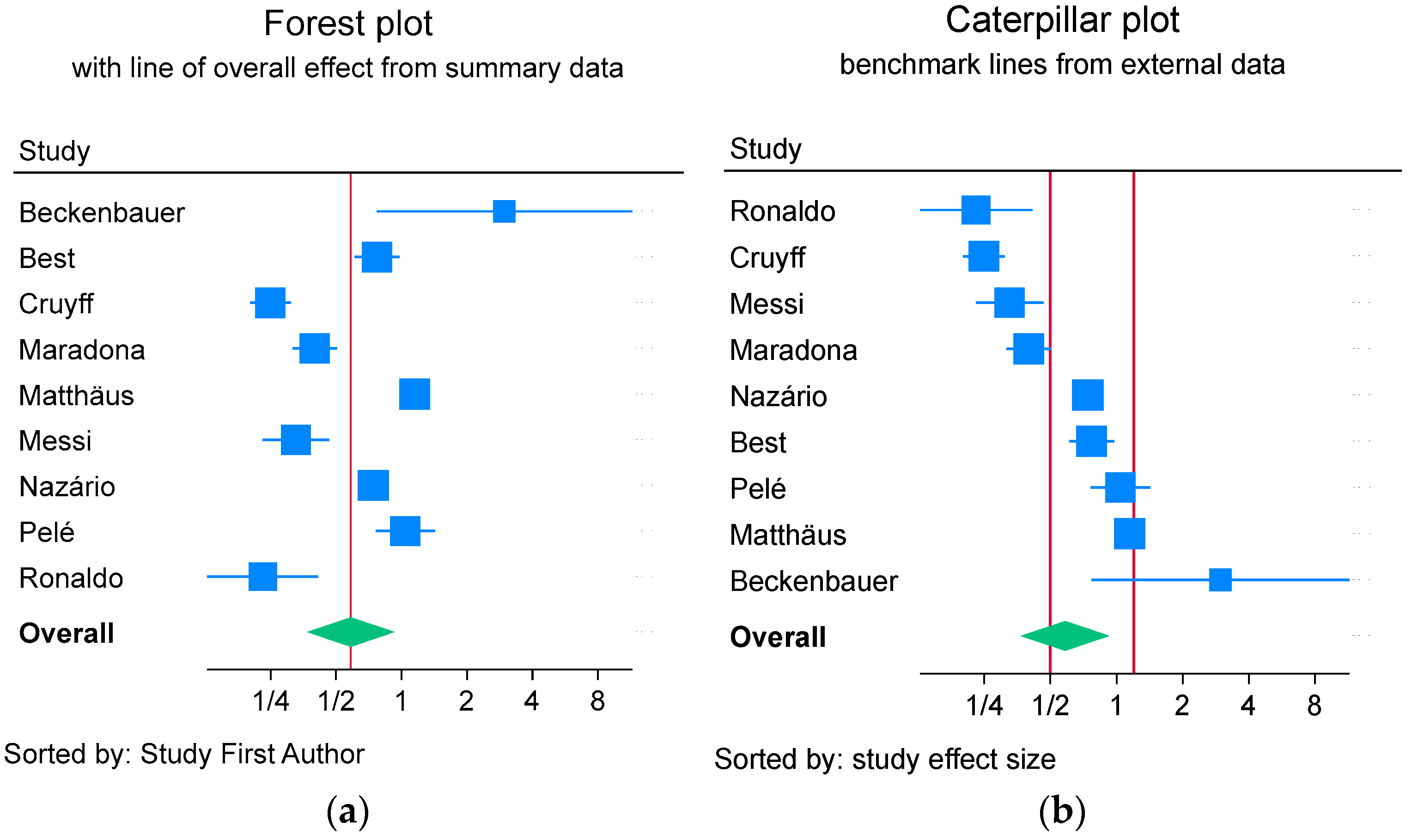
2.3. Arms-Based Analysis and Caterpillar Plots
3. Results
3.1. Characteristics of the Randomized Concurrent Controlled Trials
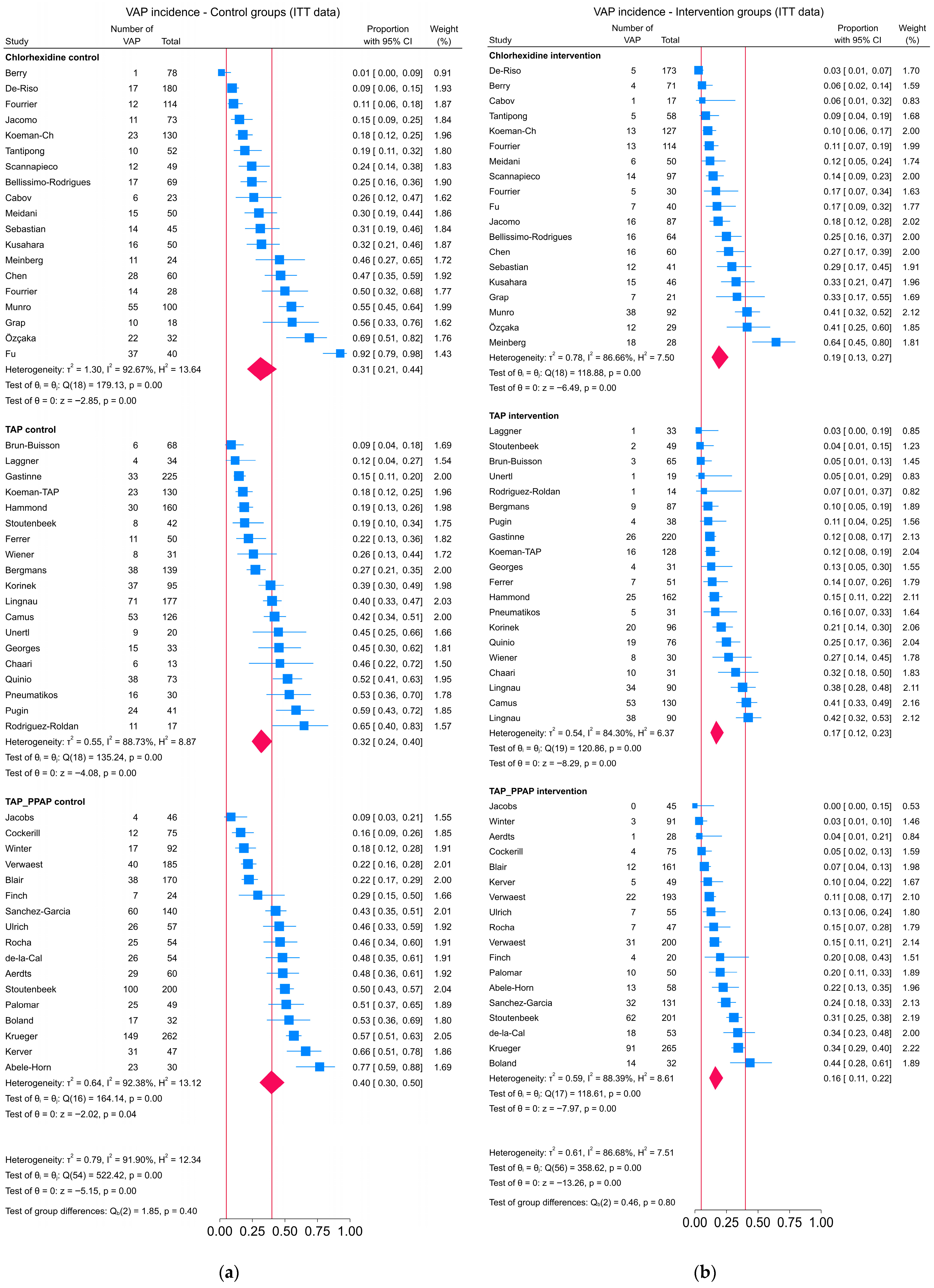
3.2. Contrast-Based Analysis
3.3. Arms-Based Analysis
4. Discussion
4.1. Choice of Benchmark
4.2. SUTVA: Consistency Element
4.3. SUTVA: Spillover Element
4.4. Limitations
4.5. Strengths
4.6. Comparison of Findings
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| ICU | Intensive care unit |
| IQR | Inter-quartile range |
| VAP | Ventilator-associated pneumonia |
| MV | Mechanical ventilation |
| PI | Prediction interval |
| SUTVA | Stable unit treatment variance assumption |
| TAP | Topical antibiotic prophylaxis |
| PPAP | Protocolized parenteral antibiotic prophylaxis |
References
- Bellissimo-Rodrigues, F.; Bellissimo-Rodrigues, W.T.; Viana, J.M.; Teixeira, G.C.; Nicolini, E.; Auxiliadora-Martins, M.; Passos, A.D.C.; Martinez, E.Z.; Basile-Filho, A.; Martinez, R. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infect. Cont. Hosp. Epidemiol. 2009, 30, 952–958. [Google Scholar] [CrossRef]
- Berry, A.M.; Davidson, P.M.; Masters, J.; Rolls, K.; Ollerton, R. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: A randomised control trial. Intern. J. Nurs. Stud. 2011, 48, 681–688. [Google Scholar] [CrossRef]
- Ćabov, T.; Macan, D.; Husedžinović, I.; Škrlin-Šubić, J.; Bošnjak, D.; Šestan-Crnek, S.; Perić, B.; Kovač, Z.; Golubović, V. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: A randomized placebo-controlled study. Wien. Klin. Wochenschr. 2010, 122, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Ye, X.F.; Jiang, Y.Z.; Yan, M.Q. Application of new oral care method to orotracheal intubation. Fujian Med. J. 2008, 30, 155–157. [Google Scholar]
- DeRiso, A.J.; Ladowski, J.S.; Dillon, T.A.; Justice, J.W.; Peterson, A.C. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 1996, 109, 1556–1561. [Google Scholar] [CrossRef]
- Fourrier, F.E.; Cau-Pottier, H.; Boutigny, M.; Roussel-Delvallez, M.; Jourdain Chopin, C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Med. 2000, 26, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, F.; Dubois, D.; Pronnier, P.; Herbecq, P.; Leroy, O.; Desmettre, T.; Roussel-Delvallez, M. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit a double-blind placebo-controlled multicenter study. Crit. Care Med. 2005, 33, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Zhong, Q.; Zheng, C. Bacteriostasis effect of oral administration of chlorhexidine on patients with mechanical ventilation and prevention and treatment of ventilator—Associated pneumonia. Chin. Nurs. Res. 2019, 33, 431–434. [Google Scholar]
- Grap, M.J.; Munro, C.L.; Hamilton, V.A.; Elswick, R.K.J.r.; Sessler, C.N.; Ward, K.R. Early, single chlorhexidine application reduces ventilator-associated pneumonia in trauma patients. Heart Lung 2011, 40, e115–e122. [Google Scholar] [CrossRef]
- Jacomo, A.D.; Carmona, F.; Matsuno, A.K.; Manso, P.H.; Carlotti, A.P. Effect of oral hygiene with 0.12% chlorhexidine gluconate on the incidence of nosocomial pneumonia in children undergoing cardiac surgery. Infect. Cont. Hosp. Epidemiol. 2011, 32, 591–596. [Google Scholar] [CrossRef]
- Koeman, M.; van der Ven, A.J.; Hak, E.; Joore, H.C.; Kaasjager, K.; de Smet, A.G.; Ramsay, G.; Dormans, T.P.; Aarts, L.P.; de Bel, E.E.; et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 1348–1355. [Google Scholar] [CrossRef]
- Kusahara, D.M.; Peterlini, M.A.; Pedreira, M.L. Oral care with 0.12% chlorhexidine for the prevention of ventilator-associated pneumonia in critically ill children: Randomised, controlled and double blind trial. Intern. J. Nurs. Stud. 2012, 49, 1354–1363. [Google Scholar] [CrossRef]
- Meidani, M.; Khorvash, F.; Abbasi, S.; Cheshmavar, M.; Tavakoli, H. Oropharyngeal irrigation to prevent ventilator-associated pneumonia: Comparing potassium permangenate with chlorhexidine. Intern. J. Prev. Med. 2018, 9, 93. [Google Scholar]
- Meinberg, M.C.; Cheade Mde, F.; Miranda, A.L.; Fachini, M.M.; Lobo, S.M. The use of 2% chlorhexidine gel and toothbrushing for oral hygiene of patients receiving mechanical ventilation: Effects on ventilator-associated pneumonia [Uso de clorexidina 2% gel e escovacao mecanica na higiene bucal de pacientes sob ventilacao mecanica: Efeitos na pneumonia associada a ventilador]. Rev. Bras. Ter. Intensiv. 2012, 24, 369–374. [Google Scholar]
- Munro, C.L.; Grap, M.J.; Jones, D.J.; McClish, D.K.; Sessler, C.N. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am. J. Crit. Care 2009, 18, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Özçaka, O.; Basoglu, O.K.; Buduneli, N.; Tasbakan, M.S.; Bacakoglu, F.; Kinane, D.F. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: A randomized clinical trial. J. Periodontal Res. 2012, 47, 584–592. [Google Scholar] [CrossRef]
- Panchabhai, T.S.; Dangayach, N.S.; Krishnan, A.; Kothari, V.M.; Karnad, D.R. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: An open-label randomized trial with 0.01% potassium permanganate as control. Chest 2009, 135, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Yu, J.; Raghavendran, K.; Vacanti, A.; Owens, S.I.; Wood, K.; Mylotte, J.M. A randomized trial of chlorhexidine gluconate on oral bacterial pathogens in mechanically ventilated patients. Crit. Care 2009, 13, R117. [Google Scholar] [CrossRef]
- Sebastian, M.R.; Lodha, R.; Kapil, A.; Kabra, S.K. Oral mucosal decontamination with chlorhexidine for the prevention of ventilator-associated pneumonia in children—A randomized, controlled trial. Pediatr. Crit. Care Med. 2012, 13, e305–e310. [Google Scholar] [CrossRef]
- Tantipong, H.; Morkchareonpong, C.; Jaiyindee, S.; Thamlikitkul, V. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect. Cont. Hosp. Epidemiol. 2008, 29, 131–136. [Google Scholar] [CrossRef]
- Bergmans, D.C.; Bonten, M.J.; Gaillard, C.A.; Paling, J.C.; van der Geeest, S.I.; van Tiel, F.H.; Beysens, A.J.; de Leeuw, P.W.; Stobberingh, E.E. Prevention of ventilator-associated pneumonia by oral decontamination: A prospective, randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2001, 164, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Brun-Buisson, C.; Legrand, P.; Rauss, A.; Richard, C.; Montravers, F.; Besbes, M.; Meakins, J.L.; Soussy, C.J.; Lemaire, F. Intestinal decontamination for control of nosocomial multiresistant Gram-negative bacilli. Ann. Intern. Med. 1989, 110, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Camus, C.; Bellissant, E.; Sebille, V.; Perrotin, D.; Garo, B.; Legras, A.; Renault, A.; Le Corre, P.; Donnio, P.Y.; Gacouin, A. Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit. Care Med. 2005, 33, 307–314. [Google Scholar] [CrossRef]
- Gastinne, H.M.; Wolff, M.; Delatour, F.; Faurisson, F.; Chevret, S. A controlled trial in intensive care units of selective decontamination of the digestive tract with nonabsorbable antibiotics. N. Engl. J. Med. 1992, 326, 594–599. [Google Scholar] [CrossRef]
- Georges, B.; Mazerolles, M.; Decun, J.-F.; Rouge, P.; Pomies, S.; Cougot, P.; Andrieu, P. Décontamination digestive sélective résultats d’une étude chez le polytraumatisé. Réanimation Soins Intensifs Médecin D’Urgence 1994, 3, 621–627. [Google Scholar] [CrossRef]
- Korinek, A.M.; Laisne, M.J.; Nicolas, M.H.; Raskine, L.; Deroin, V.; Sanson-lepors, M.J. Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: A double-blind, randomized, placebo-controlled study. Crit. Care Med. 1993, 21, 1466–1473. [Google Scholar] [CrossRef]
- Pneumatikos, I.; Koulouras, V.; Nathanail, C.; Goe, D.; Nakos, G. Selective decontamination of subgloottic area in mechanically ventilated patients with multiple trauma. Intensive Care Med. 2002, 28, 432–437. [Google Scholar] [CrossRef]
- Pugin, J.; Auckenthaler, R.; Lew, D.P.; Suter, P.M. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia. JAMA 1991, 265, 2704–2710. [Google Scholar] [CrossRef]
- Quinio, B.; Albanese, J.; Bues-Charbit, M.; Viviand, X.; Martin, C. Selective decontamination of the digestive tract in multiple trauma patients. A prospective double-blind, randomized, placebo-controlled study. Chest 1996, 109, 765–772. [Google Scholar] [CrossRef]
- Rodríguez-Roldán, J.M.; Altuna-Cuesta, A.; López, A.; Carrillo, A.; Garcia, J.; León, J.; Martínez-Pellús, A.J. Prevention of nosocomial lung infection in ventilated patients: Use of an antimicrobial pharyngeal nonabsorbable paste. Crit. Care Med. 1990, 18, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Unertl, K.; Ruckdeschel, G.; Selbmann, H.K.; Jensen, U.; Forst, H.; Lenhart, F.P.; Peter, K. Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med. 1987, 13, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Itokazu, G.; Nathan, C.; Kabins, S.A.; Weinstein, R.A. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination in a medical-surgical intensive care unit. Clin. Infect. Dis. 1995, 20, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Chaari, A.; Zribi, E.; Dammak, H.; Ghadoun, H.; Chtara, K.; Sfar, S.; Bahloul, M.; Bouaziz, M. Does selective digestive decontamination prevent ventilatorassociated pneumonia in trauma patients? Am. J. Ther. 2014, 21, 470–476. [Google Scholar] [CrossRef]
- Ferrer, M.; Torres, A.; Gonzalez, J.; Puig de la Bellacasa, J.; el-Ebiary, M.; Roca, M.; Gatell, J.M.; Rodriguez-Roisin, R. Utility of selective digestive decontamination in mechanically ventilated patients. Ann. Intern. Med. 1994, 120, 389–395. [Google Scholar] [CrossRef]
- Hammond, J.M.J.; Potgieter, P.D.; Saunders, G.L.; Forder, A.A. Double blind study of selective decontamination of the digestive tract in intensive care. Lancet 1992, 340, 5–9. [Google Scholar] [CrossRef]
- Laggner, A.N.; Tryba, M.; Georgopoulos, A.; Lenz, K.; Grimm, G.; Graninger, W.; Schneeweiss, B.; Druml, W. Oropharyngeal decontamination with gentamicin for long-term ventilated patients on stress ulcer prophylaxis with sucralfate? Wien. Klin. Wochenschr. 1994, 106, 15–19. [Google Scholar] [PubMed]
- Lingnau, W.; Berger, J.; Javorsky, F.; Lejeune, P.; Mutz, N.; Benzer, H. Selective intestinal decontamination in multiple trauma patients: Prospective.; controlled trials. J. Trauma 1997, 42, 687–694. [Google Scholar] [CrossRef]
- Stoutenbeek, C.P.; Van Saene, H.K.F.; Zandstra, D.F. Prevention of multiple organ failure by selective decontamination of the digestive tract in multiple trauma patients. In Immune Consequences of Trauma, Shock and Sepsis. Mechanisms and Therapeutic Approaches; Faist, E., Baue, A.E., Schildberg, F.W., Eds.; Pabst Science Publishers: Berlin, Germany, 1996; Volume 2, pp. 1055–1066. [Google Scholar]
- Abele-Horn, M.; Dauber, A.; Bauernfeind, A.; Russwurm, W.; Seyfarth-Metzger, I.; Gleich, P.; Ruckdeschel, G. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD). Intensive Care Med. 1997, 23, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aerdts, S.J.; van Dalen, R.; Clasener, H.A.; Festen, J.; van Lier, H.J.; Vollaard, E.J. Antibiotic prophylaxis of respiratory tract infection in mechanically ventilated patients. A prospective, blinded, randomized trial of the effect of a novel regimen. Chest 1991, 100, 783–791. [Google Scholar] [CrossRef]
- Blair, P.; Rowlands, B.J.; Lowry, K.; Webb, H.; Armstrong, P.; Smilie, J. Selective decontamination of the digestive tract: A stratified, randomized, prospective study in a mixed intensive care unit. Surgery 1991, 110, 303–309. [Google Scholar]
- Boland, J.P.; Sadler, D.L.; Stewart, W.; Wood, D.J.; Zerick, W.; Snodgrass, K.R. Reduction of nosocomial respiratory tract infections in the multiple trauma patients requiring mechanical ventilation by selective parenteral and enteral antisepsis regimen (SPEAR) in the intensive care. In Proceedings of the XVII Congress of Chemotherapy, Berlin, Germany, 23–28 June 1991. [Google Scholar]
- Cockerill, F.R.; Muller, S.R.; Anhalt, J.P.; Marsh, H.M.; Farnell, M.B.; Mucha, P.; Gillespie, D.J.; Ilstrup, D.M.; Larson-Keller, J.J.; Thompson, R.L. Prevention of infection in critically ill patients by selective decontamination of the digestive tract. Ann. Intern. Med. 1992, 117, 545–553. [Google Scholar] [CrossRef] [PubMed]
- de La Cal, M.A.; Cerdá, E.; Garcıa-Hierro, P.; Van Saene HKGómez-Santos, D.; Negro, E.; Lorente, J.A. Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: A randomized, placebo controlled, double-blind trial. Ann. Surg. 2005, 241, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Finch, R.G.; Tomlinson, P.; Holliday, M.; Sole, K.; Stack, C.; Rocker, G. Selective decontamination of the digestive tract (SDD) in the prevention of secondary sepsis in a medical/surgical intensive care unit. In Proceedings of the XVII International Congress Chemotherapy, Berlin, Germany, 23–28 June 1991. [Google Scholar]
- Jacobs, S.; Foweraker, J.E.; Roberts, S.E. Effectiveness of selective decontamination of the digestive tract (SDD) in an ICU with a policy encouraging a low gastric pH. Clin. Intensive Med. 1992, 3, 52–58. [Google Scholar]
- Kerver, A.J.H.; Rommes, J.H.; Mevissen-Verhage, E.A.E.; Hulstaert, P.F.; Vos, A.; Verhoef, J.; Wittebol, P. Prevention of colonization and infection in critically ill patients: A prospective randomized study. Crit. Care Med. 1988, 16, 1087. [Google Scholar] [CrossRef]
- Krueger, W.A.; Lenhart, F.P.; Neeser, G.; Ruckdeschel, G.; Schreckhase, H.; Eissner, H.J.; Forst, H.; Eckart, J.; Peter, K.; Unertl, K.E. Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions and mortality in critically ill surgical patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1029–1037. [Google Scholar] [CrossRef]
- Palomar, M.; Alvarez-Lerma, F.; Jorda, R.; Bermejo, B.; Catalan Study Group of Nosocomial Pneumonia Prevention. Prevention of nosocomial infection in mechanically ventilated patients: Selective digestive decontamination versus sucralfate. Clin. Intensive Care 1997, 8, 228–235. [Google Scholar] [CrossRef]
- Rocha, L.A.; Martin, M.J.; Pita, S.; Paz, J.; Seco, C.; Margusino, L.; Villanueva, R.; Duran, M.T. Prevention of nosocomial infection in critically ill patients by selective decontamination of the digestive tract. A randomized, double blind, placebo-controlled study. Intensive Care Med. 1992, 18, 398–404. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Cambronero, J.A.; Lopez-Diaz, J.; Gomez Aguinaga, M.A.; Onoro Canaveral, J.J.; Sacristan del Castillo, J. Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled multicenter trial. Am. Rev. Respir. Dis. 1998, 158, 908–916. [Google Scholar] [CrossRef]
- Stoutenbeek, C.P.; van Saene, H.K.F.; Little, R.A.; Whitehead, A. The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: A multicenter randomized controlled trial. Intensive Care Med. 2007, 33, 261–270. [Google Scholar] [CrossRef]
- Ulrich, C.; Harinck-deWeerd, J.E.; Bakker, N.C.; Jacz, K.; Doornbos, L.; De Ridder, V.A. Selective decontamination of the digestive tract with norfloxacin in the prevention of ICU-acquired infections: A prospective randomized study. Intensive Care Med. 1989, 15, 424–431. [Google Scholar] [CrossRef]
- Verwaest, C.; Verhaegen, J.; Ferdinande, P.; Schetz, M.; Van den Berghe, G.; Verbist, L.; Lauwers, P. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit. Care Med. 1997, 25, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.; Humphreys, H.; Pick, A.; MacGowan, A.P.; Willatts, S.M.; Speller, D.C. A controlled trial of selective decontamination of the digestive tract in intensive care and its effect on nosocomial infection. J. Antimicrob. Chemother. 1992, 30, 73–87. [Google Scholar] [CrossRef]
- Tuon, F.F.; Gavrilko, O.; Almeida, S.; Sumi, E.R.; Alberto, T.; Rocha, J.L.; Rosa, E.A. Prospective, randomised, controlled study evaluating early modification of oral microbiota following admission to the intensive care unit and oral hygiene with chlorhexidine. J. Glob. Antimicrob. Resist. 2017, 8, 159–163. [Google Scholar] [CrossRef]
- De Smet, A.M.; Kluytmans, J.A.; Cooper, B.S.; Mascini, E.M.; Benus, R.F.; Van der Werf, T.S.; Van der Hoeven, J.G.; Pickkers, P.; Bogaers-Hofman, D.; Van Der Meer, N.J.; et al. Decontamination of the digestive tract and oropharynx in ICU patients. N. Engl. J. Med. 2009, 360, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Xie, H.; Worthington, H.V.; Furness, S.; Zhang, Q.; Li, C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2016, 10, CD008367. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, X.; Zhang, Q.; Li, C.; Worthington, H.V.; Hua, F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2020, 12, CD008367. [Google Scholar] [CrossRef]
- D’Amico, R.; Pifferi, S.; Leonetti, C.; Torri, V.; Tinazzi, A.; Liberati, A. Effectiveness of antibiotic prophylaxis in critically ill adult patients: Systematic review of randomised controlled trials. BMJ 1998, 316, 1275–1285. [Google Scholar] [CrossRef]
- Liberati, A.; D’Amico, R.; Pifferi, S.; Torri, V.; Brazzi, L.; Parmelli, E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care (Review). Cochrane Database Syst. Rev. 2009, 2009, CD000022. [Google Scholar]
- Minozzi, S.; Pifferi, S.; Brazzi, L.; Pecoraro, V.; Montrucchio, G.; D’Amico, R. Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst. Rev. 2021, 1, CD000022. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Comment: Which ifs have causal answers. J. Am. Stat. Ass. 1986, 81, 961–962. [Google Scholar] [CrossRef]
- Holland, P.W. Statistics and causal inference. J. Am. Stat. Ass. 1986, 81, 945–970. [Google Scholar] [CrossRef]
- Stone, R. The assumptions on which causal inferences rest. J. R. Stat. Soc. Ser. B Stat. Methodol. 1993, 55, 455–466. [Google Scholar] [CrossRef]
- Hurley, J. Spillover effects on mortality within randomized concurrent controlled trials of antimicrobial-based infection prevention interventions among the mechanically ventilated patient population. A reappraisal of Cochrane review data. J. Hosp. Infect. 2025, 163, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Impact of selective digestive decontamination on respiratory tract Candida among patients with suspected ventilator-associated pneumonia. A meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1121–1135. [Google Scholar] [CrossRef]
- StataCorp. Stata 18 Meta-Analysis Reference Manual; Stata Press: College Station, TX, USA, 2023. [Google Scholar]
- Hurley, J.C. Forrest plots or caterpillar plots? J. Clin. Epidemiol. 2020, 121, 109–110. [Google Scholar] [CrossRef]
- Álvarez-Lerma, F.; Palomar-Martínez, M.; Sánchez-García, M.; Martínez-Alonso, M.; Álvarez-Rodríguez, J.; Lorente, L.; Arias-Rivera, S.; García, R.; Gordo, F.; Añón, J.M.; et al. Prevention of ventilator-associated pneumonia: The multimodal approach of the Spanish ICU “Pneumonia Zero” Program. Crit. Care Med. 2018, 46, 181–188. [Google Scholar] [CrossRef]
- Apostolopoulou, E.; Bakakos, P.; Katostaras, T.; Gregorakos, L. Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens. Respir. Care 2003, 48, 681–688. [Google Scholar]
- Edwards, J.R.; Peterson, K.D.; Mu, Y.; Banerjee, S.; Allen-Bridson, K.; Morrell, G.; Dudeck, M.A.; Pollock, D.A.; Horan, T.C. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am. J. Infect. Control 2009, 37, 783–805. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Yang, Z.; Wang, J.; Jin, A.; Wang, W.; Chen, R.; Zhan, S. Incidence, temporal trend and factors associated with ventilatorassociated pneumonia in mainland China: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 468. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Xiao, W.; Song, T.; Wang, S. Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: A meta-analysis. Neurocrit. Care 2020, 32, 272–285. [Google Scholar] [CrossRef]
- Fagon, J.Y.; Chastre, J.; Vuagnat, A.; Trouillet, J.L.; Novara, A.; Gibert, C. Nosocomial pneumonia and mortality among patients in Intensive care unit. JAMA 1996, 275, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Chevret, S.; Hemmer, M.; Carlet, J.; Langer, M. Incidence and risk factors of pneumonia acquired in intensive care units. Results from a multicenter prospective study on 996 patients. European Cooperative Group on Nosocomial Pneumonia. JAMA 1996, 275, 866–869. [Google Scholar]
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed]
- Reignier, J.; Mercier, E.; Le Gouge, A.; Boulain, T.; Desachy, A.; Bellec, F.; Clavel, M.; Frat, J.-P.; Plantefeve, G.; Quenot, J.-P.; et al. Effect of not monitoring residual gastric volume on risk of ventilatorassociated pneumonia in adults receiving mechanical ventilation and early enteral feeding: A randomized controlled trial. JAMA 2013, 309, 249–256. [Google Scholar] [CrossRef]
- Seguin, P.; Laviolle, B.; Dahyot-Fizelier, C.; Dumont, R.; Veber, B.; Gergaud, S.; Asehnoune, K.; Mimoz, O.; Donnio, P.-Y.; Bellissant, E.; et al. Effect of oropharyngeal povidoneiodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: A multicenter, randomized controlled trial. Crit. Care Med. 2014, 42, 1–8. [Google Scholar] [CrossRef]
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Haley, R.W.; Hooton, T.M.; Culver, D.H.; Stanley, R.C.; Emori, T.G.; Hardison, C.D.; Quade, D.; Shachtman, R.H.; Schaberg, D.R.; Shah, B.V.; et al. Nosocomial infections in US hospitals, 1975–1976: Estimated frequency by selected characteristics of patients. Am. J. Med. 1981, 70, 947–959. [Google Scholar] [CrossRef]
- Pennington, J.E. Nosocomial respiratory infection. In Principles and Practice of Infectious Diseases; Mandell, G.L., Douglas, R.G., Jr., Bennet, J.E., Eds.; Churchill Livingstone: St. Louis, MO, USA, 1990; pp. 2199–2205. [Google Scholar]
- Chastre, J.; Fagon, J.Y. Pneumonia in the ventilator-dependent patient. In Principles and Practice of Mechanical Ventilation; Tobin, M.J., Ed.; McGraw-Hill: New York, NY, USA, 1994; pp. 857–890. [Google Scholar]
- Centers for Disease Control and Prevention. Monitoring hospital- acquired infections to promote patient safety: United States, 1990–1999. MMWR Morb. Mortal. Wkly. Rep. 2000, 49, 149–153. [Google Scholar]
- National Nosocomial Infections Surveillance (NNIS) System. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am. J. Infect. Control 1999, 27, 520–532. [Google Scholar]
- Kollef, M.H.; Chastre, J.; Fagon, J.Y.; François, B.; Niederman, M.S.; Rello, J.; Torres, A.; Vincent, J.L.; Wunderink, R.G.; Go, K.W.; et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014, 42, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Visualizing and diagnosing spillover within randomized concurrent controlled trials through the application of diagnostic test assessment methods. BMC Med. Res. Methodol. 2024, 24, 182. [Google Scholar] [CrossRef]
- Van Nieuwenhoven, C.A.; Buskens, E.; Van Tiel, F.H.; Bonten, M.J. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA 2001, 286, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Stoutenbeek, C.P.; Van Saene, H.K.; Miranda, D.R.; Zandstra, D.F. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984, 10, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, E.J.; Clasener, H.A. Colonization resistance. Antimicrob. Agents Chemother. 1994, 38, 409–414. [Google Scholar] [CrossRef] [PubMed]
- The SuDDICU Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group. Effect of selective decontamination of the digestive tract on hospital mortality in critically ill patients receiving mechanical ventilation: A randomized clinical trial. JAMA 2022, 328, 1911–1921. [Google Scholar] [CrossRef]
- Wittekamp, B.H.; Plantinga, N.L.; Cooper, B.S.; Lopez-Contreras, J.; Coll, P.; Mancebo, J.; Wise, M.P.; Morgan, M.P.; Depuydt, P.; Boelens, J.; et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: A randomized clinical trial. JAMA 2018, 320, 2087–2098. [Google Scholar] [CrossRef]
- Dale, C.M.; Rose, L.; Carbone, S.; Pinto, R.; Smith, O.M.; Burry, L.; Fan, E.; Amaral, A.C.; McCredie, V.A.; Scales, D.C.; et al. Effect of oral chlorhexidine de-adoption and implementation of an oral care bundle on mortality for mechanically ventilated patients in the intensive care unit (CHORAL): A multi-center stepped wedge cluster-randomized controlled trial. Intensive Care Med. 2021, 47, 1295–1302. [Google Scholar] [CrossRef]
- Hammond, N.E.; Myburgh, J.; Seppelt, I.; Garside, T.; Vlok, R.; Mahendran, S.; Adigbli, D.; Finfer, S.; Gao, Y.; Goodman, F.; et al. Association between selective decontamination of the digestive tract and in-hospital mortality in intensive care unit patients receiving mechanical ventilation: A systematic review and meta-analysis. JAMA 2022, 328, 1922–1934. [Google Scholar] [CrossRef]
- Hurley, J. Estimating the herd effects of anti-microbial-based decontamination (ABD) interventions on intensive care unit (ICU) acquired bloodstream infections: A deductive meta-analysis. BMJ Open 2024, 14, e092030. [Google Scholar] [CrossRef]
- Hurley, J.C. Estimating the herd effects of antimicrobial prevention interventions on ventilator-associated pneumonia within ICU populations: A cluster randomized trial emulation using data from Cochrane reviews. J. Antimicrob. Chemother. 2025, 80, 1047–1058. [Google Scholar] [CrossRef]
- Hurley, J.C. Establishing the safety of Selective Digestive Decontamination within the ICU population. A bridge too far? Trials 2023, 24, 337. [Google Scholar] [CrossRef] [PubMed]
| Patients (n) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Author | Year | Ref. | QS b | Study Intervention c | n | N | n | N |
| Chlorhexidine studies | ||||||||
| Bellissimo-Rodrigues | 2009 | [1] | 2 | Chlx solution | 16 | 64 | 17 | 69 |
| Berry | 2011 | [2] | 2 | Chlx solution + TB | 4 | 71 | 1 | 78 |
| Cabov | 2010 | [3] | 2 | Chlx gel | 1 | 17 | 6 | 23 |
| Chen | 2008 | [4] | 1 | Chlx solution | 16 | 60 | 28 | 60 |
| De Riso d | 1996 | [5] | 2 | Chlx solution | 5 | 173 | 17 | 180 |
| Fourrier | 2000 | [6] | 2 | Chlx gel | 5 | 30 | 14 | 28 |
| Fourrier | 2005 | [7] | 2 | Chlx gel | 13 | 114 | 12 | 114 |
| Fu e | 2019 | [8] | 1 | Chlx solution | 7 | 40 | 37 | 40 |
| Grap | 2011 | [9] | 1 | Chlx solution | 7 | 21 | 10 | 18 |
| Jacomo f | 2011 | [10] | 2 | Chlx solution | 16 | 87 | 11 | 73 |
| Koeman-Ch | 2006 | [11] | 2 | Chlx gel | 13 | 127 | 23 | 130 |
| Kusahara f | 2012 | [12] | 2 | Chlx gel + TB | 15 | 46 | 16 | 50 |
| Meidani e | 2018 | [13] | 1 | Chlx solution | 6 | 50 | 15 | 50 |
| Meinberg g | 2012 | [14] | 2 | Chlx gel + TB | 18 | 28 | 11 | 24 |
| Munro | 2009 | [15] | 1 | Chlx solution ± TB | 38 | 92 | 55 | 100 |
| Özçaka | 2012 | [16] | 2 | Chlx solution | 12 | 29 | 22 | 32 |
| Panchabhai h | 2009 | [17] | 1 | Chlx solution (+PP) | 14 | 88 | 15 | 83 |
| Scannapieco | 2009 | [18] | 2 | Chlx solution + TB | 14 | 97 | 12 | 49 |
| Sebastian | 2012 | [19] | 2 | Chlx gel | 12 | 41 | 14 | 45 |
| Tantipong | 2008 | [20] | 1 | Chlx solution + TB | 5 | 58 | 10 | 52 |
| Patients (n) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Author | Year | Ref. | QS b | Study Intervention c | n | N | n | N |
| TAP studies | ||||||||
| Bergmans | 2001 | [21] | 2 | PGV | 9 | 87 | 38 | 139 |
| Brun-Buisson | 1989 | [22] | 2 | PNeNa | 3 | 65 | 6 | 68 |
| Camus | 2005 | [23] | 1 | PT | 53 | 130 | 53 | 126 |
| Gastinne | 1992 | [24] | 1 | PTA | 26 | 220 | 33 | 225 |
| Georges d | 1994 | [25] | 2 | PNeA | 4 | 31 | 15 | 33 |
| Koeman-TAP e | 2006 | [11] | 2 | PChlx | 16 | 128 | 23 | 130 |
| Korinek | 1993 | [26] | 2 | PTAV | 20 | 96 | 37 | 95 |
| Pneumatikos d | 2002 | [27] | 1 | PTA | 5 | 31 | 16 | 30 |
| Pugin | 1991 | [28] | 2 | PNeV | 4 | 38 | 24 | 41 |
| Quinio d | 1995 | [29] | 2 | PGA | 19 | 76 | 38 | 73 |
| Rodriguez-Roldan | 1990 | [30] | 1 | PTNeA | 1 | 14 | 11 | 17 |
| Unertl | 1987 | [31] | 2 | PGA | 1 | 19 | 9 | 20 |
| Wiener | 1995 | [32] | 2 | PGNy | 8 | 30 | 8 | 31 |
| Duplex TAP studies | ||||||||
| Chaari d,e | 2014 | [33] | 2 | 10 | 31 | 6 | 13 | |
| Ferrer | 1994 | [34] | 2 | PTA-Ctx + -Ctx | 7 | 51 | 11 | 50 |
| Hammond | 1992 | [35] | 2 | PTA-Ctx + -Ctx | 25 | 162 | 30 | 160 |
| Laggner | 1994 | [36] | 1 | GA + Aug | 1 | 33 | 4 | 34 |
| Lingnau d,f | 1997 | [37] | 2 | PTA-Cip + -Cip | 38 | 90 | 71 | 177 |
| Lingnau d,f | 1997 | [37] | 2 | PCipA + -Cip | 34 | 90 | 71 | 177 |
| Stoutenbeek d | 1996 | [38] | 2 | PTA-C + -C | 2 | 49 | 8 | 42 |
| Patients (n) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Author | Year | Ref. | QS b | Study Intervention c | n | N | n | N |
| TAP + PPAP studies | ||||||||
| Abele-Horn d | 1997 | [39] | 2 | PTA-C | 13 | 58 | 23 | 30 |
| Aerdts | 1991 | [40] | 2 | PNoA-Ctx | 1 | 28 | 29 | 60 |
| Blair | 1991 | [41] | 1 | PTA-Ctx | 12 | 161 | 38 | 170 |
| Boland d | 1991 | [42] | 2 | PTNy-Ctx | 14 | 32 | 17 | 32 |
| Cockerill | 1992 | [43] | 2 | NyPG-Ctx | 4 | 75 | 12 | 75 |
| de la Cal d,e | 2005 | [44] | 2 | PTA-C | 18 | 53 | 26 | 54 |
| Finch | 1991 | [45] | 2 | PGA-C | 4 | 20 | 7 | 24 |
| Jacobs | 1992 | [46] | 2 | PTA-Ctx | 0 | 45 | 4 | 46 |
| Kerver | 1988 | [47] | 2 | PTA-Ctx | 5 | 49 | 31 | 47 |
| Krueger | 2002 | [48] | 2 | PG-Cip | 91 | 265 | 149 | 262 |
| Palomar | 1997 | [49] | 2 | PTA-Ctx | 10 | 50 | 25 | 49 |
| Rocha d | 1992 | [50] | 2 | PTA-Ctx | 7 | 47 | 25 | 54 |
| Sanchez-Garcia | 1998 | [51] | 2 | PTA-Ctx | 32 | 131 | 60 | 140 |
| Stoutenbeek d | 2007 | [52] | 2 | PTA-Ctx | 62 | 201 | 100 | 200 |
| Ulrich | 1989 | [53] | 1 | PNoA-Tr | 7 | 55 | 26 | 57 |
| Verwaest f | 1997 | [54] | 2 | PTA-C | 22 | 193 | 40 | 185 |
| Verwaest f | 1997 | [54] | 2 | OA-O | 31 | 200 | 40 | 185 |
| Winter | 1992 | [55] | 2 | PTA-Ctz | 3 | 91 | 17 | 92 |
| Chlorhexidine | TAP/Duplex a | TAP + PPAP | |
|---|---|---|---|
| Number of studies b | 19 | 20 | 18 |
| Year (median) | 2010 | 1994 | 1992 |
| range (min–max) | 1996–2019 | 1987–2014 | 1988–2007 |
| IQR c | 2008–2012 | 1992–2001 | 1991–1998 |
| Majority quality score d | 13 | 15 | 16 |
| Study results | |||
| VAP prevention effect size | 0.53 | 0.46 | 0.30 |
| 95% CI (n) | 0.35–0.8 | 0.33–0.64 | 0.22–0.42 |
| I2 | 70.7 | 66.7 | 65.2 |
| Control group results | |||
| Group VAP—incidence | |||
| • >40% | 7 | 8 | 11 |
| • ≥5% to ≤40% | 11 | 11 | 6 |
| • <5% | 1 | 0 | 0 |
| Total | 19 | 19 | 17 |
| Group size | |||
| Median | 50 | 50 | 57 |
| IQR | 32–78 | 31–130 | 47–140 |
| VAP incidence | |||
| Mean | 31 | 32 | 40 |
| 95% CI e | 21–44 (19) | 24–40 | 30–50 |
| I2 | 92.7 | 88.7 | 92.4 |
| 90% PI f | 6–78 | 11–64 | 13–74 |
| Intervention group results | |||
| Group VAP—incidence | |||
| • >40% | 3 | 2 | 1 |
| • ≥5% to ≤40% | 15 | 16 | 14 |
| • <5% | 1 | 2 | 3 |
| Total | 19 | 20 | 18 |
| Group size | |||
| Median | 58 | 58 | 56 |
| IQR | 30–92 | 31–93 | 47–161 |
| VAP incidence | |||
| Mean | 19 | 17 | 16 |
| 95% CI e | 13–27 | 12–23 | 11–22 |
| I2 | 86.7 | 84.3 | 88.4 |
| 90% PI f | 5–53 | 5–43 | 5–44 |
| Citation in Source | Ref. | Original Source |
|---|---|---|
| “…with a reported prevalence ranging between 6% and 52%…” | [58], p. 6 | [71,72] |
| “…VAP is a relatively common nosocomial infection in critically ill patients, with pooled incidence from 23.8% to 36.0% in recent systematic reviews…” | [59], p. 9 | [73,74] |
| “…incidence of pneumonia in such patients ranges between 7% and 40%…” | [60], p. 1276 | [75] |
| “The incidence of pneumonia has been reported to vary from 7% to more than 40% in ICU patients” | [61], p. 2 | [76,77,78] |
| “…ventilator-associated pneumonia (VAP) has been estimated to affect 5% to 40% of patients treated with mechanical ventilation for at least 48 h.” | [62], p. 8 | [79,80,81] |
| “…ventilator-associated pneumonia (VAP) continues to complicate the course of 8 to 28%…” | [80], p. 867 (and abstract) | [81,82,83,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurley, J.C. Testing the Stable Unit Treatment Variance Assumption (SUTVA) Within Cochrane Reviews of Antimicrobial-Based Pneumonia Prevention Interventions Among Mechanically Ventilated Patients Using Caterpillar Plots. J. Clin. Med. 2025, 14, 6841. https://doi.org/10.3390/jcm14196841
Hurley JC. Testing the Stable Unit Treatment Variance Assumption (SUTVA) Within Cochrane Reviews of Antimicrobial-Based Pneumonia Prevention Interventions Among Mechanically Ventilated Patients Using Caterpillar Plots. Journal of Clinical Medicine. 2025; 14(19):6841. https://doi.org/10.3390/jcm14196841
Chicago/Turabian StyleHurley, James C. 2025. "Testing the Stable Unit Treatment Variance Assumption (SUTVA) Within Cochrane Reviews of Antimicrobial-Based Pneumonia Prevention Interventions Among Mechanically Ventilated Patients Using Caterpillar Plots" Journal of Clinical Medicine 14, no. 19: 6841. https://doi.org/10.3390/jcm14196841
APA StyleHurley, J. C. (2025). Testing the Stable Unit Treatment Variance Assumption (SUTVA) Within Cochrane Reviews of Antimicrobial-Based Pneumonia Prevention Interventions Among Mechanically Ventilated Patients Using Caterpillar Plots. Journal of Clinical Medicine, 14(19), 6841. https://doi.org/10.3390/jcm14196841






