Neutrophil-to-Lymphocyte Ratio (NLR)—Independent Prognostic Marker of Renal Function Decline in Chronic Kidney Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
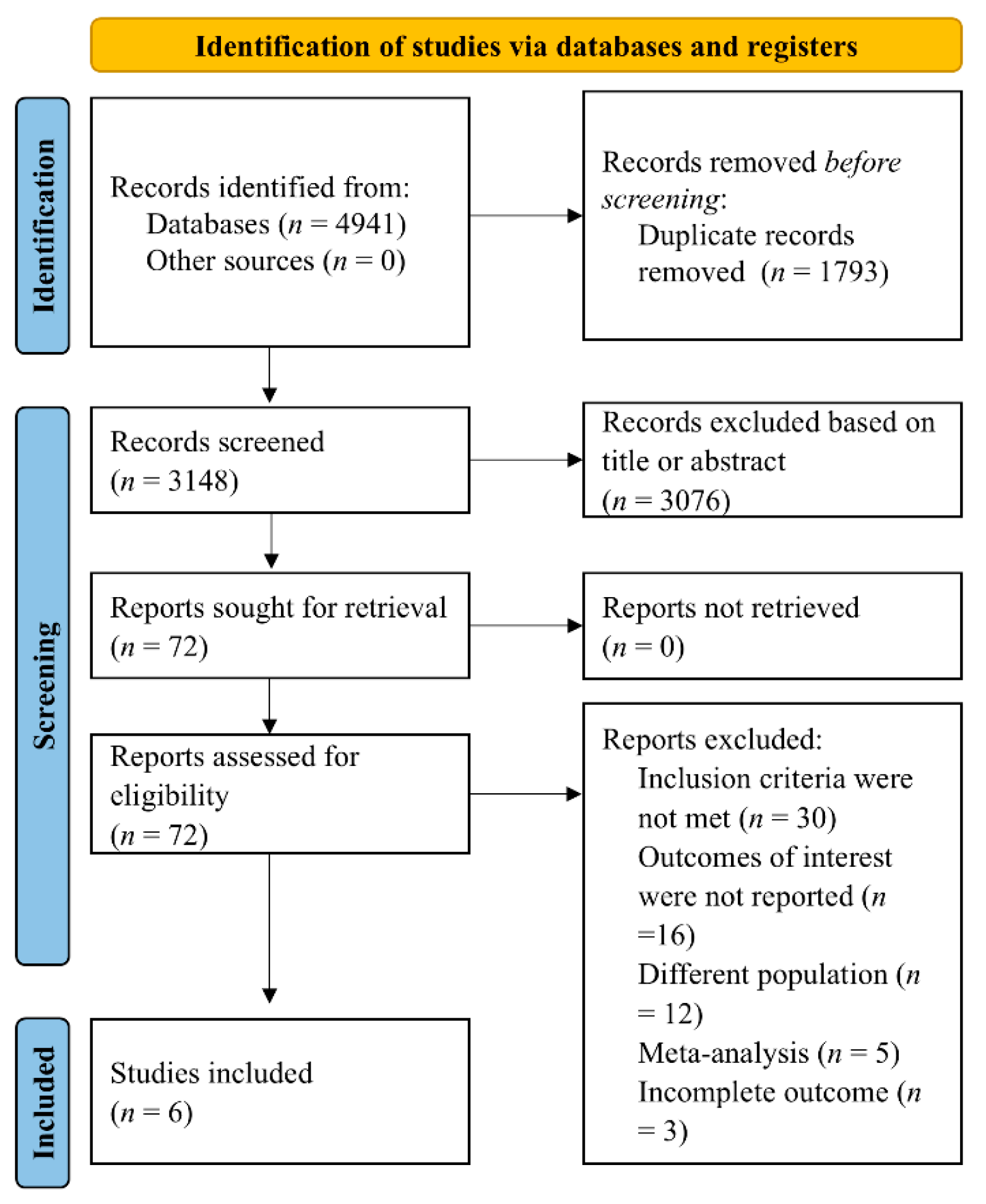
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria and Outcomes
2.3. Data Extraction and Synthesis
2.4. Quality Assessment
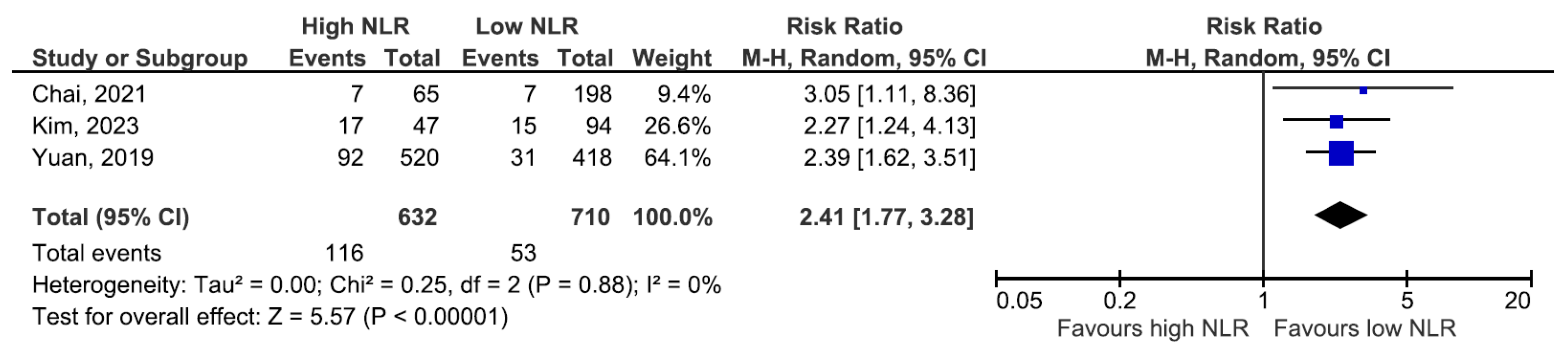
3. Results
- (a)
- Progression to ESKD/RRT
- (b)
- Baseline kidney function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| CV | Cardiovascular |
| eGFR | Estimated glomerular filtration rate |
| ESKD | End-stage kidney disease |
| hs-CRP | High-sensitivity C-reactive protein |
| IgAN | IgA nephropathy |
| IL-6 | Interleukin-6 |
| NLR | Neutrophil-to-lymphocyte ratio |
| NOS | Newcastle–Ottawa Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RRT | Renal replacement therapy |
| SMD | Standardized mean difference |
References
- Yuan, Q.; Wang, J.; Peng, Z.; Zhou, Q.; Xiao, X.; Xie, Y.; Wang, W.; Huang, L.; Tang, W.; Sun, D.; et al. Neutrophil-to-Lymphocyte Ratio and Incident End-Stage Renal Disease in Chinese Patients with Chronic Kidney Disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-Stride). J. Transl. Med. 2019, 17, 86. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High Neutrophil/Lymphocyte Ratio Is Associated with Poor Renal Outcomes in Japanese Patients with Chronic Kidney Disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Wang, G. The Neutrophil–Lymphocyte Ratio Is Associated with All-Cause and Cardiovascular Mortality in Cardiovascular Patients. Sci. Rep. 2024, 14, 26692. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, R.; Wang, X.; Zhang, W.; Li, M.; Ni, T.; Weng, W.; Li, Q. The Neutrophil-to-Lymphocyte Ratio Is Associated with All-Cause and Cardiovascular Mortality among Individuals with Hypertension. Cardiovasc. Diabetol. 2024, 23, 117. [Google Scholar] [CrossRef]
- Ge, H.M.; Zhang, L.M.; Zhang, W.M.; Yuan, Q.; Xiao, X. Neutrophil-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Chronic Kidney Disease–Related Pulmonary Hypertension: A Retrospective Study. Medicine 2024, 103, e40161. [Google Scholar] [CrossRef]
- Altunoren, O.; Akkus, G.; Sezal, D.T.; Ciftcioglu, M.; Guzel, F.B.; Isiktas, S.; Torun, G.I.; Uyan, M.; Sokmen, M.F.; Sevim, H.A.; et al. Does Neutrophyl to Lymphocyte Ratio Really Predict Chronic Kidney Disease Progression? Int. Urol. Nephrol. 2019, 51, 129–137. [Google Scholar] [CrossRef]
- Chai, L.; Cai, K.; Wang, K.; Luo, Q. Relationship between Blood Neutrophil-Lymphocyte Ratio and Renal Tubular Atrophy/Interstitial Fibrosis in Iga Nephropathy Patients. J. Clin. Lab. Anal. 2021, 35, e23774. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Pei, G.; Jiang, Z.; Qin, A.; Tan, J.; Tang, Y.; Qin, W. High Neutrophil-to-Lymphocyte Ratio Is an Independent Risk Factor for End Stage Renal Diseases in Iga Nephropathy. Front. Immunol. 2021, 12, 700224. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.H.; Oh, T.R.; Suh, S.H.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Kim, S.W.; Bae, E.H. Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Patients with Chronic Kidney Disease. Korean J. Intern. Med. 2023, 38, 725–733. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Paganelli, R.; Di Iorio, A. The Neutrophil-to-Lymphocyte Ratio in Aging and Immunosenescence. Explor. Immunol. 2025, 5, 1003200. [Google Scholar] [CrossRef]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, Nlrp3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef]
- Carollo, C.; Sorce, A.; Cirafici, E.; Ciuppa, M.E.; Mulè, G.; Caimi, G. Silent Inflammation, Loud Consequences: Decoding Nlr across Renal, Cardiovascular and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 8256. [Google Scholar] [CrossRef] [PubMed]
- Okyay, G.U.; İnal, S.; Öneç, K.; Er, R.E.; Paşaoğlu, Ö.; Paşaoğlu, H.; Derici, Ü.; Erten, Y. Neutrophil to Lymphocyte Ratio in Evaluation of Inflammation in Patients with Chronic Kidney Disease. Ren. Fail. 2012, 35, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, I.; Eroglu, E.; Unal, A.; Sipahioglu, M.H.; Tokgoz, B.; Oymak, O.; Utas, C. Role of Neutrophil/Lymphocyte Ratio in Prediction of Disease Progression in Patients with Stage–4 Chronic Kidney Disease. J. Nephrol. 2012, 26, 358–365. [Google Scholar] [CrossRef]
- Zhao, W.M.; Tao, S.M.; Liu, G.L. Neutrophil-to-Lymphocyte Ratio in Relation to the Risk of All-Cause Mortality and Cardiovascular Events in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Ren. Fail 2020, 42, 1059–1066. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of Oxidative Stress in Chronic Kidney Disease: A Review on Current Concepts and Therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Frąk, W.; Kućmierz, J.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. New Insights into Molecular Mechanisms of Chronic Kidney Disease. Biomedicines 2022, 10, 2846. [Google Scholar] [CrossRef]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with Ckd. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells. Toxins 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.B.; Stinghen, A.E.M.; Kato, S.; Bucharles, S.; Aita, C.; Yuzawa, Y.; Pecoits–Filho, R. Characteris Tics and Causes of Immune Dysfunction Related to Uremia and Dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2008, 28, 183–187. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Mai, X.; Zhang, M. Prognostic Value of Neutrophil-to-Lymphocyte Ratio for the Clinical Outcomes of Chronic Kidney Diseases: An Update Systematic Review and Meta-Analysis. BMC Nephrol. 2025, 26, 419. [Google Scholar] [CrossRef]

| Research Question | Is an elevated neutrophil-to-lymphocyte ratio (NLR) associated with increased risk of adverse renal outcomes, including progression to ESRD and an eGFR decline, in adults with CKD stages 1–4? | |
| Structured research question (PICO Format) | Population (P) | Adults with chronic kidney disease (CKD) stages 1–4, not on dialysis. |
| Intervention/Exposure (I) | Elevated neutrophil-to-lymphocyte ratio (NLR), measured at baseline. | |
| Comparator (C) | Lower neutrophil-to-lymphocyte ratio (NLR), or within-study comparison groups stratified by NLR tertiles/quartiles. | |
| Outcomes (O) | Primary: progression to end-stage kidney disease (ESRD) or renal replacement therapy (RRT) initiation. Secondary: estimated glomerular filtration rate (eGFR). | |
| Author, Year | Design | Patients, No | Age, Years | eGFR, mL/min/1.73 m2 | Setting | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|---|
| Altunoren, 2019 [6] | Observational, retrospective | 740 | 62.8 ± 0.57 | 40.56 ± 0.45 | Patients with CKD stage 2–4 | Progression to stage 5 CKD or start of RRT | 51.2 ± 30 months |
| Chai, 2021 [7] | Observational, retrospective | 263 | 41.15 ± 12.64 | 86.68 ± 29.44 | Patients with IgAN | (a) Renal tubular atrophy or interstitial fibrosis (b) Progression to ESKD | 2 years |
| Kim, 2023 [9] | Observational, retrospective | 141 | 56.47 ± 10.35 | 49.40 ± 29.76 | Patients with non-dialysis CKD | (a) ≥50% eGFR decline or initiation of RRT (b) all-cause mortality | 5.45 ± 2.11 years |
| Wang, 2021 [8] | Observational, retrospective | 966 | 35 | Median 96.23 (low NLR) and 87.05 (high NLR) | Patients with biopsy-proven IgAN | Progression to ESKD or start of RRT | 58.67 months |
| Yoshitomi, 2019 [2] | Observational, prospective | 350 | 68 (55–77) | 33.6 (22.6–56.8) | Consecutive patients with CKD stage 1–4 | ESKD requiring dialysis or death | 31.8 months |
| Yuan, 2019 [1] | Observational, retrospective | 938 | 52.8 ± 14.14 | 57.22 ± 32.67 | Patients with CKD stage 1–4 | (a) ESKD requiring RRT (b) CV events (c) all-cause death | 4.55 years |
| Author, Year | Outcomes | Results | p-Value |
|---|---|---|---|
| Altunoren, 2019 [6] | Progression to stage 5 CKD or start of RRT | Patients with high NLR had lower eGFR values (39.4 ± 12.0 vs. 41.6 ± 12.0 mL/min/1.73 m2) | p = 0.03 |
| High NLR was associated with increased annual eGFR decline (−4.96 ± 8.13 vs. −3.59 ± 6.15 mL/min/1.73 m2/year) | p = 0.04 | ||
| Baseline NLR was similar between patients who reached the endpoint and those who did not (3.54 ± 2.85 vs. 3.14 ± 1.99) | p = 0.09 | ||
| Baseline NLR was higher in patients with faster eGFR decline compared to those without (3.41 ± 1.82 vs. 3.13 ± 2.34) | p = 0.01 | ||
| Chai, 2021 [7] | Progression to ESKD | Over 2 years, 14 patients developed ESRD (7 in highest quartile, 1 in lowest; renal survival rate 87.04% vs. 98.11%) | p = 0.029 |
| Tubular atrophy/ interstitial fibrosis | Higher NLR was associated with a greater proportion of tubular atrophy/interstitial fibrosis (T1/T2: 40% in highest NLR quartile vs. 22.73% in lowest) | p = 0.033 | |
| NLR was an independent predictor of tubular atrophy/interstitial fibrosis (β = 1.230, 95% CI 0.081–2.379) | p = 0.036 | ||
| Kim, 2023 [9] | Composite renal outcome | Highest NLR tertile (T3) had more events than lowest (T1): 55.3% vs. 17.0%; adjusted HR 3.33 (95% CI 1.43–7.76) | p = 0.005 |
| ≥50% eGFR decline | Highest NLR tertile vs. lowest tertile: 51.1% vs. 12.8%; adjusted HR 3.12 (95% CI 1.23–7.91) | p = 0.017 | |
| Initiation of RRT | Highest NLR tertile vs. lowest tertile: 36.2% vs. 8.5%; adjusted HR 2.87 (95% CI 0.89–9.25) | p = 0.078 | |
| All-cause mortality | Highest NLR tertile vs. lowest tertile: 10.6% vs. 4.3% | p = 0.213 | |
| Wang, 2021 [8] | ESKD or start of RRT | High NLR associated with greater ESKD risk in univariate analysis (HR 2.68, 95% CI 1.60–4.50, p < 0.001) and remained an independent risk factor after multivariate adjustment (HR 1.74, 95% CI 0.98–3.05) | p = 0.043 |
| Yoshitomi, 2019 [2] | ESKD requiring dialysis or death | Composite endpoint occurred in 83 patients, 54 in high NLR group vs. 29 in low NLR group; adjusted HR 1.67 (95% CI 1.02–2.77) | |
| Yuan, 2019 [1] | ESKD requiring RRT | Higher NLR levels were associated with an increased incidence of ESKD events | p < 0.001 |
| In CKD stage 4 patients, baseline NLR remained an independent predictor of ESKD after multivariable adjustment, with an HR of 2.12 (95% CI 1.10–4.10) compared with lower NLR. | p = 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlacu, A.; Namolovan, C.A.; Brinza, C.; Covic, A.; Floria, M.; Voroneanu, L.; Covic, A. Neutrophil-to-Lymphocyte Ratio (NLR)—Independent Prognostic Marker of Renal Function Decline in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6822. https://doi.org/10.3390/jcm14196822
Burlacu A, Namolovan CA, Brinza C, Covic A, Floria M, Voroneanu L, Covic A. Neutrophil-to-Lymphocyte Ratio (NLR)—Independent Prognostic Marker of Renal Function Decline in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(19):6822. https://doi.org/10.3390/jcm14196822
Chicago/Turabian StyleBurlacu, Alexandru, Calin Andrei Namolovan, Crischentian Brinza, Andreea Covic, Mariana Floria, Luminita Voroneanu, and Adrian Covic. 2025. "Neutrophil-to-Lymphocyte Ratio (NLR)—Independent Prognostic Marker of Renal Function Decline in Chronic Kidney Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 19: 6822. https://doi.org/10.3390/jcm14196822
APA StyleBurlacu, A., Namolovan, C. A., Brinza, C., Covic, A., Floria, M., Voroneanu, L., & Covic, A. (2025). Neutrophil-to-Lymphocyte Ratio (NLR)—Independent Prognostic Marker of Renal Function Decline in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(19), 6822. https://doi.org/10.3390/jcm14196822