Bruxism as a Biopsychosocial Disorder: An Interdisciplinary Cross-Sectional Study
Abstract
1. Introduction
2. Bruxism
3. Methodology
3.1. Study Design
3.2. Sample Size Calculation
3.3. Bruxism Diagnosis and Functional Orofacial Assessment
3.4. Psychoemotional and Cognitive Evaluation
- DC/TMD Bruxism Symptoms Questionnaire: Assessing subjective severity and frequency of bruxism-related symptoms. It is a self-report instrument derived from the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), designed to evaluate patients’ perception of bruxism behaviors and their clinical relevance [37].
- Stroop Test (105-item version): The Polish version of the Stroop Color and Word Test (SCWT) was used to assess executive function, cognitive inhibition, and selective attention [42,43]. The task included three conditions with 105 randomized stimuli each: (1) word reading (color names in black), (2) color naming (colored squares), and (3) color-word naming (incongruent words and ink colors). In the incongruent condition, participants were required to suppress the automatic reading response in favor of naming the ink color. Reaction times and both corrected and uncorrected errors were recorded. Cognitive interference was calculated by comparing incongruent (CW) performance with word (W) and color (C) conditions. The test is widely validated and frequently applied in both research and clinical assessment of inhibitory control [44,45].
3.5. Control Group Definition
3.6. Statistical Analysis
4. Results
4.1. Participants, Bruxism Diagnosis and Classification
4.2. General
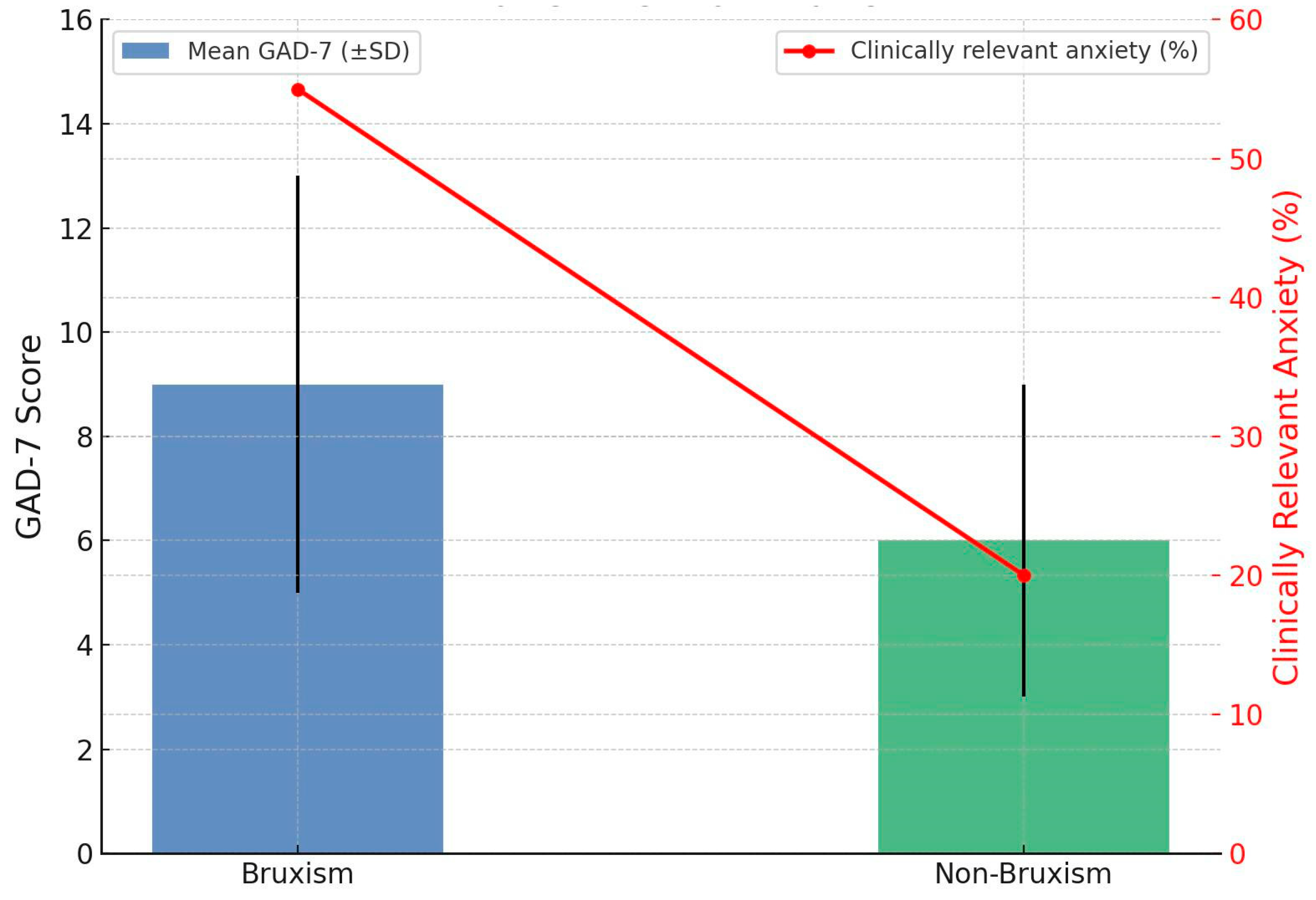
4.2.1. Anxiety Severity in Bruxism
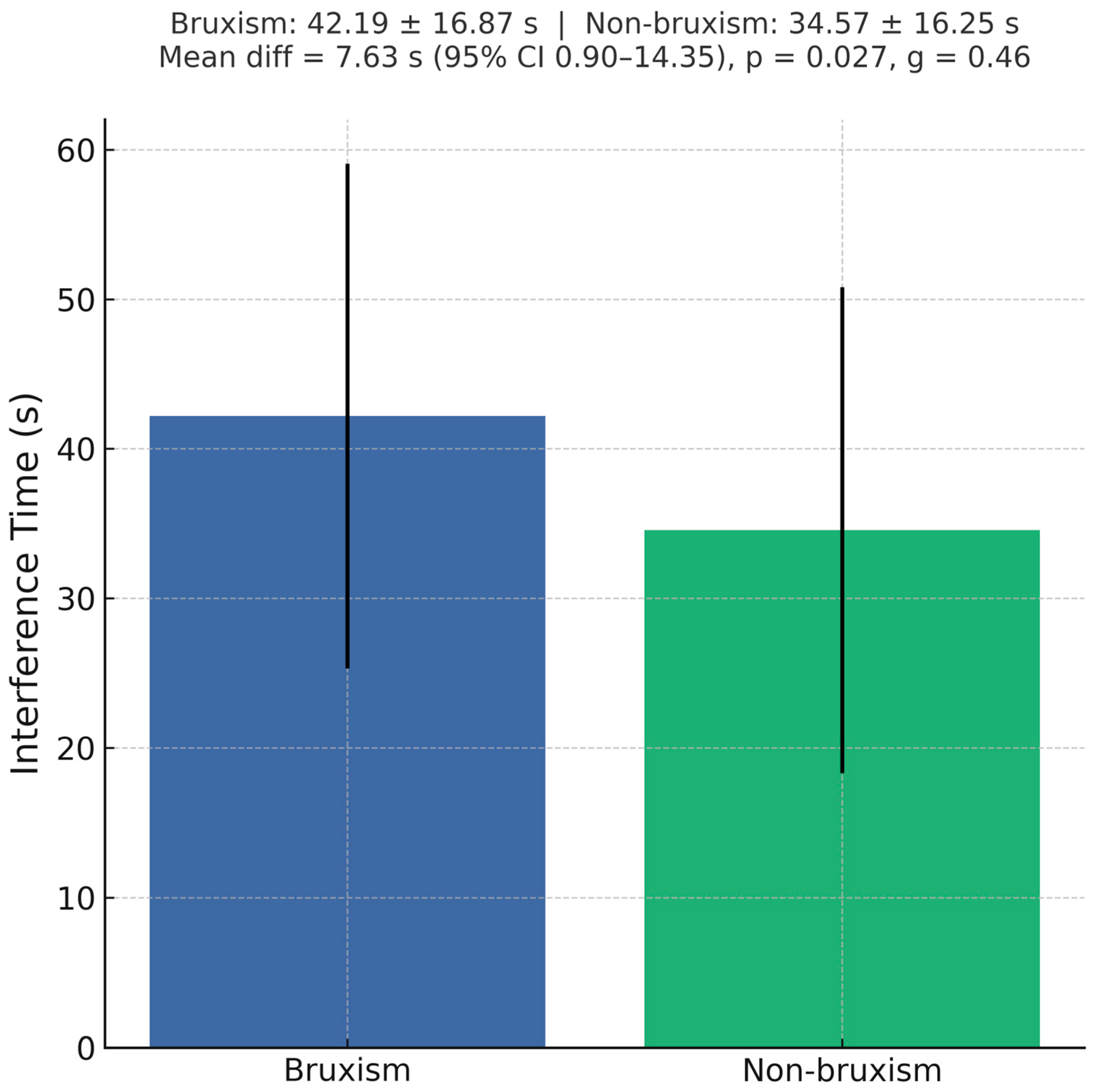
4.2.2. Executive Control (Stroop Interference)
4.2.3. Headache and Anxiety
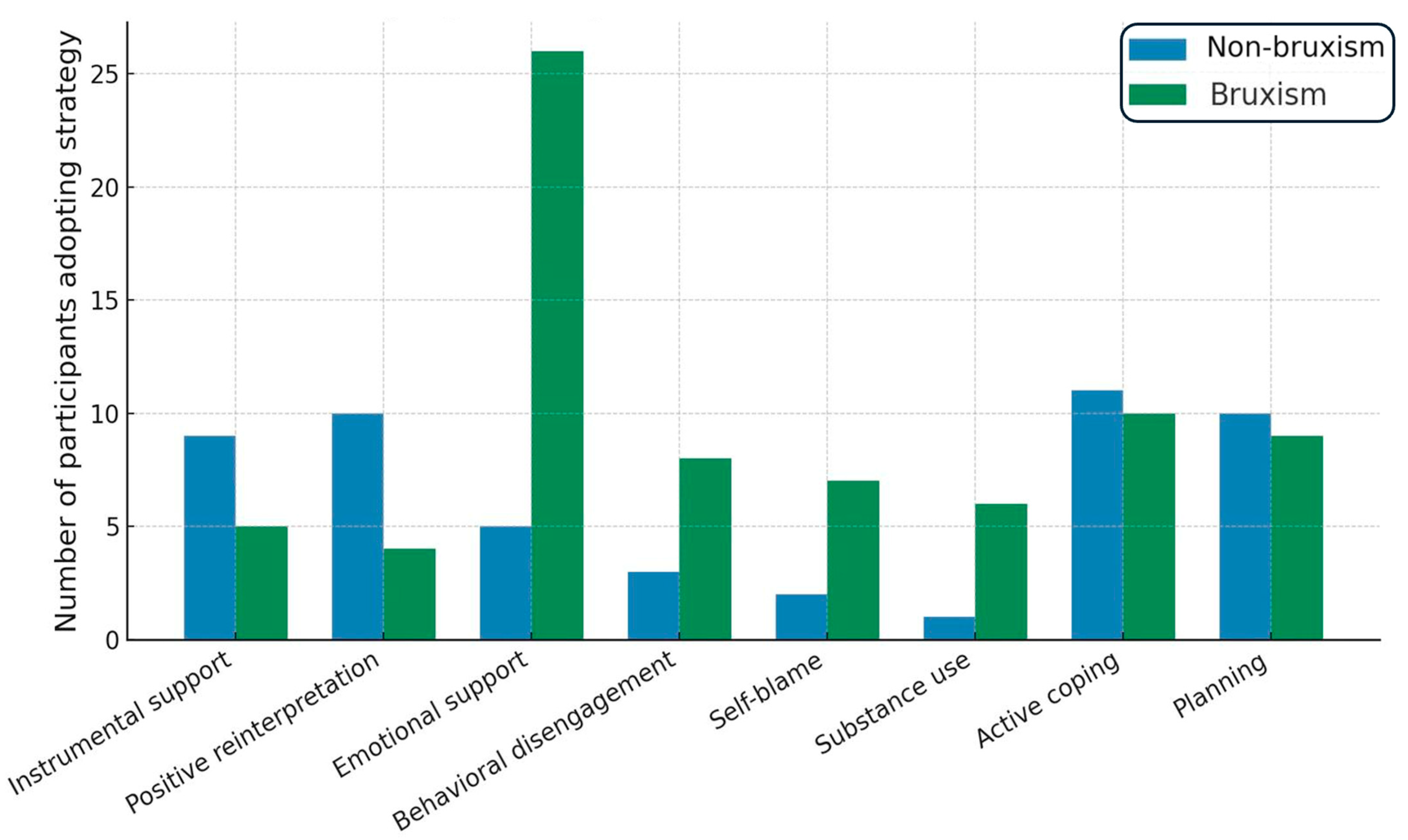
4.3. COPE Inventory
4.4. GAD-7
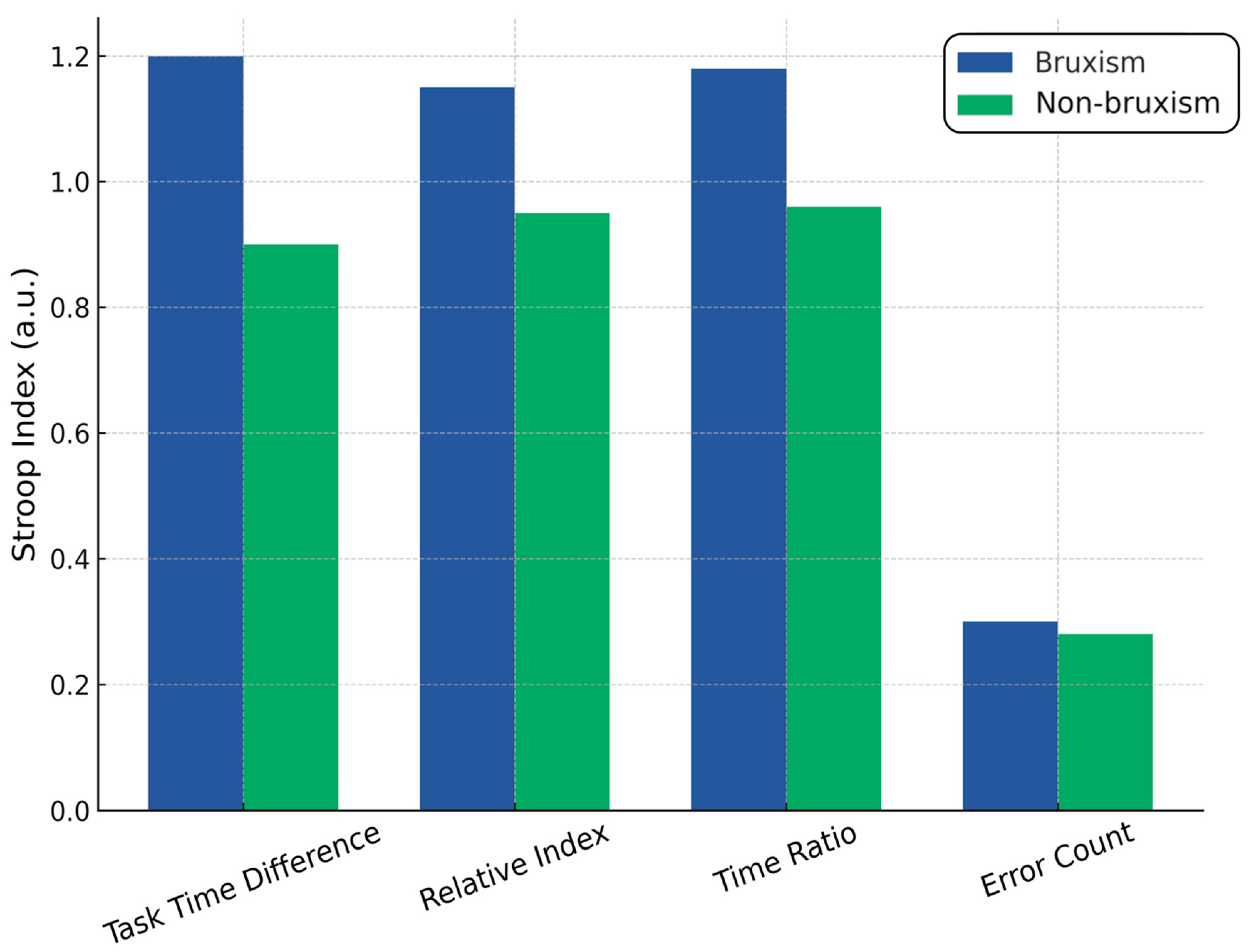
4.5. Stroop Test
4.6. Interpretation
5. Discussion
5.1. The Findings of This Study in the Context of Other Evidence
5.2. Coping Strategies and Pain Severity
5.3. Executive Function Findings and the Role of the DLPFC
5.4. Field of Study as a Modulator of Risk
5.5. Clinical and Research Implications
5.6. Limitations of Our Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minervini, G.; Franco, R.; Di Blasio, M.; Martelli, M.; Gargari, M.; Bollero, P.; Cicciù, M. Prevalence of bruxism in patients affected by epilepsy: A systematic review and meta-analysis. Acta Odontol. Scand. 2025, 84, 155–164. [Google Scholar] [CrossRef]
- Maeda-Iino, A.; Osako, Y.; Nakagawa, S.; Takahashi, K.; Oga, Y.; Furukawa-Sainoki, M.; Harada, M.; Fukushima, M.; Miyawaki, S. Relationship between masseter muscle activity during wakefulness and temporomandibular disorder-related symptoms. J. Oral Rehabil. 2023, 51, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Verhoeff, M.C.; Lobbezoo, F.; Ahlberg, J.; Bender, S.; Bracci, A.; Colonna, A.; Fabbro, C.D.; Durham, J.; Glaros, A.G.; Häggman-Henrikson, B.; et al. Updating the Bruxism Definitions: Report of an International Consensus Meeting. J. Oral Rehabil. 2025, 52, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wieckiewicz, M.; Paradowska-Stolarz, A.; Wieckiewicz, W. Psychosocial aspects of bruxism: The most paramount factor influencing teeth grinding. BioMed Res. Int. 2014, 2014, 469187. [Google Scholar] [CrossRef] [PubMed]
- Koecklin, K.H.U.; Castillo, A.A.-D.; Li, P. The neural substrates of bruxism: Current knowledge and clinical implications. Front. Neurol. 2024, 15, 1451183. [Google Scholar] [CrossRef]
- Flueraşu, M.I.; Bocşan, I.C.; Țig, I.A.; Iacob, S.M.; Popa, D.; Buduru, S. The Epidemiology of bruxism in relation to Psychological Factors. Int. J. Environ. Res. Public Health 2022, 19, 691. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chon, S.; Auh, Q.S.; Verhoeff, M.C.; Lobbezoo, F. Clinical, psychological, and hematological factors predicting sleep bruxism in patients with temporomandibu-lar disorders. Sci. Rep. 2025, 15, 19148. [Google Scholar] [CrossRef]
- AlSahman, L.; AlBagieh, H.; AlSahman, R. Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review. Diagnostics 2024, 14, 1435. [Google Scholar] [CrossRef]
- Soto-Goñi, X.A.; Alen, F.; Buiza-González, L.; Marcolino-Cruz, D.; Sánchez-Sánchez, T.; Ardizone-García, I.; Aneiros-López, F.; Jiménez-Ortega, L. Adaptive stress coping in awake bruxism. Front. Neurol. 2020, 11, 564431. [Google Scholar] [CrossRef]
- Minakuchi, H.; Fujisawa, M.; Abe, Y.; Iida, T.; Oki, K.; Okura, K.; Tanabe, N.; Nishiyama, A. Managements of sleep bruxism in adult: A systematic review. Jpn. Dent. Sci. Rev. 2022, 58, 124–136. [Google Scholar] [CrossRef]
- Saczuk, K.; Lapinska, B.; Wilmont, P.; Pawlak, L.; Lukomska-Szymanska, M. Relationship between Sleep Bruxism, Perceived Stress, and Coping Strategies. Int. J. Environ. Res. Public Heal. 2019, 16, 3193. [Google Scholar] [CrossRef]
- Naber, M.; Vedder, A.; Brown, S.B.R.E.; Nieuwenhuis, S. Speed and lateral inhibition of stimulus processing contribute to individual differences in Stroop-Task performance. Front. Psychol. 2016, 7, 822. [Google Scholar] [CrossRef]
- Mesko, M.E.; Hutton, B.; Skupien, J.A.; Sarkis-Onofre, R.; Moher, D.; Pereira-Cenci, T. Therapies for bruxism: A systematic review and network meta-analysis (protocol). Syst. Rev. 2017, 6, 4. [Google Scholar] [CrossRef]
- Shetty, S.; Pitti, V.; Babu, C.L.S.; Kumar, G.P.S.; Deepthi, B.C. Bruxism: A Literature Review. J. Indian Prosthodont. Soc. 2010, 10, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liang, X.; Lin, X.; Li, W.; Jiang, Y.; Geng, F. Amygdala-Dependent Signaling Pathways and Regulating Circuits in Fear Memory. Synapse 2025, 79, e70026. [Google Scholar] [CrossRef] [PubMed]
- Gizler, M.; Pietrzak, N.; Saczuk, K.; Lukomska-Szymanska, M.; Lapinska, B. Students’ awareness of the bruxism causes, effects and therapies. Heliyon 2023, 10, e23708. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.B.; Da-Cas, C.D.; Valesan, L.F.; Cunha, T.C.A.; Januzzi, E.; Garanhani, R.R.; Canales, G.d.L.T.; de Souza, B.D.M. Prevalence of awake bruxism in the adult population: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 7007–7018. [Google Scholar] [CrossRef]
- Ahlberg, J.; Savolainen, A.; Rantala, M.; Lindholm, H.; Könönen, M. Reported bruxism and biopsychosocial symptoms: A longitudinal study. Community Dent. Oral Epidemiol. 2004, 32, 307–311. [Google Scholar] [CrossRef]
- Mekari, S.; Murphy, R.J.L.; MacKinnon, A.R.S.; Hollohan, Q.; Macdougall, S.C.; Courish, M.K.; Kimmerly, D.S.; Neyedli, H.F. The impact of a short-period head-down tilt on executive function in younger adults. Sci. Rep. 2022, 12, 20888. [Google Scholar] [CrossRef]
- Xu, X.; Deng, Z.-Y.; Huang, Q.; Zhang, W.-X.; Qi, C.-Z.; Huang, J.-A. Prefrontal cortex-mediated executive function as assessed by Stroop task performance associates with weight loss among overweight and obese adolescents and young adults. Behav. Brain Res. 2016, 321, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Colonna, A.; Bracci, A.; Lobbezoo, F. Bruxism: A summary of current knowledge on aetiology, assessment and management. Oral Surg. 2019, 13, 358–370. [Google Scholar] [CrossRef]
- Taravati, S.; Ataeian, F.; Mofradnejad, S.; Rakhshan, V. Risk factors for dental anxiety and cooperativeness in pediatric patients with or without oral habits (bruxism, nail biting, and thumb sucking). BMC Oral Health 2025, 25, 786. [Google Scholar] [CrossRef]
- Famuyiro, K.; Bruxism (Teeth Grinding): Causes, Diagnosis and Treatments—Los Angeles Times. Los Angeles Times, 13 August 2025. Available online: https://www.latimes.com/dentistry/general/preventive/story/bruxism-teeth-grinding-causes-diagnosis-treatments (accessed on 28 August 2025).
- Saricam, E.; Tayman, M.A. Bruxism assessment combining fractal analysis, clinical evaluation, and self-reports: A case-control study. BMC Oral Health 2025, 25, 851. [Google Scholar] [CrossRef]
- Schneider, U.E.M.; Moser, L. Achieving excellence with interdisciplinary approaches in complex orthodontic adult patients. Br. Dent. J. 2024, 237, 349–359. [Google Scholar] [CrossRef]
- Bastos, T.; Pinto, R.; Dias, I.; Leite, I.; Leite, F. Self-care treatment on patients with wakefulness bruxism. Int. J. Orofac. Myol. 2018, 44, 62–74. [Google Scholar] [CrossRef]
- Baron, S. Bruksizm i Jego Powikłania; PZWL: Warsaw, Poland, 2023. [Google Scholar]
- Saracutu, O.I.; Pollis, M.; Bulferetti, L.B.; Kaya, E.; Cagidiaco, E.F.; Ferrari, M.; Colonna, A.; Manfredini, D. Comparison between two different registration protocols for the count of sleep bruxism events in a sample of healthy individuals. Clin. Oral Investig. 2025, 29, 365. [Google Scholar] [CrossRef]
- Colonna, A.; Thomas, D.C.; Do, T.T.; Manfredini, D. Sleep Disorders Affecting Prognosis of Dental Treatment. Dent. Clin. North Am. 2024, 68, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac Pain 2013, 27, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pollis, M.; Lobbezoo, F.; Saracutu, O.I.; Colonna, A.; Manfredini, D. Psychological Distress: A Mediating Factor in the Relationship Between Sleep Bruxism and Tobacco Smoking. J. Oral Rehabil. 2025, 52, 1267–1274. [Google Scholar] [CrossRef]
- Kato, T.; Rompré, P.; Montplaisir, J.Y.; Sessle, B.J.; Lavigne, G.J. Sleep bruxism: An oromotor activity secondary to micro-arousal. J. Dent. Res. 2001, 80, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.; Kato, T.; Kolta, A.; Sessle, B.J. Neurobiological mechanisms involved in sleep bruxism. Crit. Rev. Oral Biol. Med. 2003, 14, 30–46. [Google Scholar] [CrossRef]
- Wieczorek, T.; Jodkowska, A.; Orzeszek, S.; Wieckiewicz, M.; Michalek-Zrabkowska, M.; Mazur, G.; Rymaszewska, J.; Smardz, J.; Wojakowska, A.; Martynowicz, H. Why am I grinding and clenching? Exploration of personality traits, coping strategies, oral parafunctional behaviors, and se-vere sleep bruxism in a polysomnographic study. Front. Psychiatry 2024, 15, 1362429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trindade, M.; Orestes-Cardoso, S.; de Siqueira, T.C. Interdisciplinary treatment of bruxism with an occlusal splint and cognitive behavioral therapy. Gen. Dent. 2015, 63, e1-4. [Google Scholar] [PubMed]
- Osiewicz, M.; Ciapała, B.; Bolt, K.; Kołodziej, P.; Więckiewicz, M.; Ohrbach, R. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): Polish assessment instruments. Dent. Med. Probl. 2024, 61, 5–8. [Google Scholar] [CrossRef]
- Carver, C.S.; Scheier, M.F.; Weintraub, J.K. Assessing coping strategies: A theoretically based approach. J. Personal. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef]
- Juczyński, Z.; Ogińska-Bulik, N. Narzędzia Pomiaru Stresu i Radzenia Sobie ze Stresem; Pracownia Testów Psychologicznych: Szczecin, Poland, 2009. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Basińska, B.A.; Kwissa-Gajewska, Z. Psychometric properties of the Polish version of the Generalized Anxiety Disorder Scale (GAD-7) in a non-clinical sample of employees during pandemic crisis. Int. J. Occup. Med. Environ. Health 2023, 36, 493–504. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Co.: Chicago, IL, USA, 1978. [Google Scholar]
- Cohen, J.D.; Dunbar, K.; McClelland, J.L. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychol. Rev. 1990, 97, 332–361. [Google Scholar] [CrossRef]
- Posner, M.I.; Snyder, C.R. Attention and cognitive control. In Information Processing and Cognition: The Loyola Symposium; Solso, R.L., Ed.; Erlbaum: Hillsdale, NJ, USA, 1975; pp. 55–85. [Google Scholar]
- Chemelo, V.D.S.; Né, Y.G.S.; Frazão, D.R.; de Souza-Rodrigues, R.D.; Fagundes, N.C.F.; Magno, M.B.; da Silva, C.M.T.; Maia, L.C.; Lima, R.R. Is There Association Between Stress and Bruxism? A Systematic Review and Meta-Analysis. Front Neurol. 2020, 11, 590779. [Google Scholar] [CrossRef]
- Ahlberg, J.; Lobbezoo, F.; Ahlberg, K.; Manfredini, D.; Hublin, C.; Sinisalo, J.; Kononen, M.; Savolainen, A. Self-reported bruxism mirrors anxiety and stress in adults. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e7–e11. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Priftis, K.N. Regulation of the Hypothalamic-Pituitary-Adrenal axis. NeuroImmunoModulation 2009, 16, 265–271. [Google Scholar] [CrossRef]
- García, F.E.; Barraza-Peña, C.G.; Wlodarczyk, A.; Alvear-Carrasco, M.; Reyes-Reyes, A. Psychometric properties of the Brief-COPE for the evaluation of coping strategies in the Chilean population. Psicol. Reflex. Crit. 2018, 31, 22. [Google Scholar] [CrossRef]
- Bracci, A.; Lobbezoo, F.; Colonna, A.; Bender, S.; Conti, P.C.R.; Emodi-Perlman, A.; Häggman-Henrikson, B.; Klasser, G.D.; Michelotti, A.; Lavigne, G.J.; et al. Research routes on awake bruxism metrics: Implications of the updated bruxism definition and evaluation strategies. J. Oral Rehabil. 2023, 51, 150–161. [Google Scholar] [CrossRef]
- Weeratunga, E.; Senadheera, C.; Hettiarachchi, M.; Perera, B. Validation of the Sinhalese Version of Brief COPE Scale for patients with cancer in Sri Lanka. BMC Psychol. 2022, 10, 157. [Google Scholar] [CrossRef]
- Perrotta, D.; Bianco, V.; Berchicci, M.; Quinzi, F.; Perri, R.L. Anodal tDCS over the dorsolateral prefrontal cortex reduces Stroop errors. A comparison of different tasks and designs. Behav. Brain Res. 2021, 405, 113215. [Google Scholar] [CrossRef]
- Laskov, O.; Biačková, N.; Stuchlíková, Z.; Kostýlková, L.; Klírová, M. Inhibitory Control in Young Healthy Adults—A tDCS Study. Physiol. Res. 2023, 72, 633–644. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Xu, P.; Liu, N.; Sun, K.; Xiao, W. Initial performance modulates the effects of cathodal transcranial direct current stimulation (tDCS) over the right dorsolateral prefrontal cortex on inhibitory control. Brain Res. 2022, 1774, 147722. [Google Scholar] [CrossRef]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Papakosta, V.K. Neurobiology of bruxism: The impact of stress (Review). Biomed. Rep. 2024, 20, 59. [Google Scholar] [CrossRef]
- Kunzler, A.M.; Helmreich, I.; König, J.; Chmitorz, A.; Wessa, M.; Binder, H.; Lieb, K. Psychological interventions to foster resilience in healthcare students. Cochrane Database Syst. Rev. 2020, 7, CD013684. [Google Scholar] [CrossRef] [PubMed]
- Gallasch, D.; Conlon-Leard, A.; Hardy, M.; Phillips, A.; Van Kessel, G.; Stiller, K. Variable levels of stress and anxiety reported by physiotherapy students during clinical placements: A cohort study. Physiotherapy 2022, 114, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, M.A.; Edwards, R.R.; Becker, W.C.; Kaptchuk, T.J.; Kerns, R.D. Psychological interventions for the treatment of chronic pain in adults. Psychol. Sci. Public Interest 2021, 22, 52–95. [Google Scholar] [CrossRef]
- Bin Rahmah, A.S.; Alsaif, M.I.; Naser, A.Y. The Complex Interplay Between Dental Anxiety, Generalized Anxiety, and Dental Neglect and Oral Health Quality of Life in the General Public. Healthcare 2025, 13, 1382. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Written informed consent to participate | 1. Diagnosed neurological disorders unrelated to bruxism |
| 2. Age between 18 and 60 years | 2. Severe psychiatric conditions |
| 3. Medically stable with no uncontrolled acute or chronic conditions | 3. Current medications affecting central nervous system functions |
| 4. Recent significant dental or orthodontic treatment (within six months prior) | |
| 5. Use of analgesic drugs and/or drugs affecting muscle function | |
| 6. Disease or autoimmune disorder associated with generalized muscular tension |
| Median (Range) | n (%) or M ± SD | Characteristic |
|---|---|---|
| Sex | ||
| 22.5 (18–37) | 74 (66.6%) | Women |
| 21.5 (18–31) | 37 (33.3%) | Men |
| 22 (18–37) | 22.29 ± 3.10 | Age (years) |
| Percentage | Subcategory | Category |
|---|---|---|
| 66.6% | Women | Gender |
| 33.3% | Men | |
| 91.8% | 18–26 | Age Group |
| 6.4% | 27–30 | |
| 1.8% | 31–40 | |
| 17.8% | 19 | Specific Age |
| 16.9% | 23 | |
| 15.7% | 24 | |
| 13.5% | 22 | |
| 12.2% | 20 | |
| 5.1% | 5.1% | 21 |
| 4.1% | 25 | |
| 3.2% | 26 | |
| 5.5% | 27 | |
| 2.4% | 2.4% | 30 |
| 50.9% | Dentistry | Field of Study |
| 40.0% | Biological Renewal | |
| 9.1% | Physiotherapy |
| Clinical Implication | Key Finding | Indication |
|---|---|---|
| High burden in students. Routine screening is justified in university clinics and student health. | Bruxism 63.96% (71/111) | Prevalence |
| Add brief GAD-7 screening to dental and primary care intakes. Early referral to psychology when elevated. | Higher in bruxism vs. controls: 10.63 ± 5.78 vs. 5.80 ± 3.66; mean diff 4.83; g 0.94; p < 0.001. Clinically relevant anxiety 55.4% vs. 19.6% (RR 2.83) | Anxiety (GAD-7) |
| Ask targeted questions about recent headache and TMJ noises to flag anxiety-related risk and bruxism activity. | Temporal headache linked to higher GAD-7; TMJ noises associated with higher GAD-7 | Headache and TMJ noises |
| Consider counseling on stress management and, when available, cognitive training or neurofeedback for inhibitory control. | Slower interference in bruxism: 42.19 ± 16.87 s vs. 34.57 ± 16.25 s; mean diff 7.63 s; g 0.46; p = 0.027; accuracy similar | Cognitive inhibition (Stroop) |
| Use COPE to identify maladaptive strategies. Offer brief coping-skills training or refer for CBT. | Shift toward emotion-focused and avoidance with increasing muscle pain; controls favored active coping and planning | Coping style (COPE) |
| Combine dental pain management with psychoeducation and coping-skills interventions. | Greater maladaptive coping with higher masticatory muscle pain; severe pain linked to behavioral disengagement | Pain severity |
| Prioritize screening and prevention in high-stress programs. Coordinate with program health services. | Physiotherapy and dentistry students reported more muscle pain than biological renewal students | Field of study |
| Acknowledge possible misclassification. Use multimodal confirmation in research and complex cases. | Probable bruxism via DC/TMD exam + self-report. No PSG or EMG | Diagnostic approach |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczyńska-Dragon, K.; Grzybowska-Ganszczyk, D.; Hadzik, P.; Fiegler-Rudol, J.; Dubiel-Holecko, I.; Nitecka-Buchta, A.; Baron, S. Bruxism as a Biopsychosocial Disorder: An Interdisciplinary Cross-Sectional Study. J. Clin. Med. 2025, 14, 6803. https://doi.org/10.3390/jcm14196803
Walczyńska-Dragon K, Grzybowska-Ganszczyk D, Hadzik P, Fiegler-Rudol J, Dubiel-Holecko I, Nitecka-Buchta A, Baron S. Bruxism as a Biopsychosocial Disorder: An Interdisciplinary Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):6803. https://doi.org/10.3390/jcm14196803
Chicago/Turabian StyleWalczyńska-Dragon, Karolina, Dominika Grzybowska-Ganszczyk, Paweł Hadzik, Jakub Fiegler-Rudol, Izabela Dubiel-Holecko, Aleksandra Nitecka-Buchta, and Stefan Baron. 2025. "Bruxism as a Biopsychosocial Disorder: An Interdisciplinary Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 6803. https://doi.org/10.3390/jcm14196803
APA StyleWalczyńska-Dragon, K., Grzybowska-Ganszczyk, D., Hadzik, P., Fiegler-Rudol, J., Dubiel-Holecko, I., Nitecka-Buchta, A., & Baron, S. (2025). Bruxism as a Biopsychosocial Disorder: An Interdisciplinary Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 6803. https://doi.org/10.3390/jcm14196803







