Impact of Smoking on Outcomes in HPV-Positive Oropharyngeal Squamous Cell Carcinoma in a Chinese Cohort Under AJCC 8th Edition Staging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Demographic and Clinical Data
2.3. Assessment of HPV Status
2.4. Definitive Treatment
2.5. Patient Follow-Up and Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes Based on HPV Status
3.3. Sensitivity Analysis
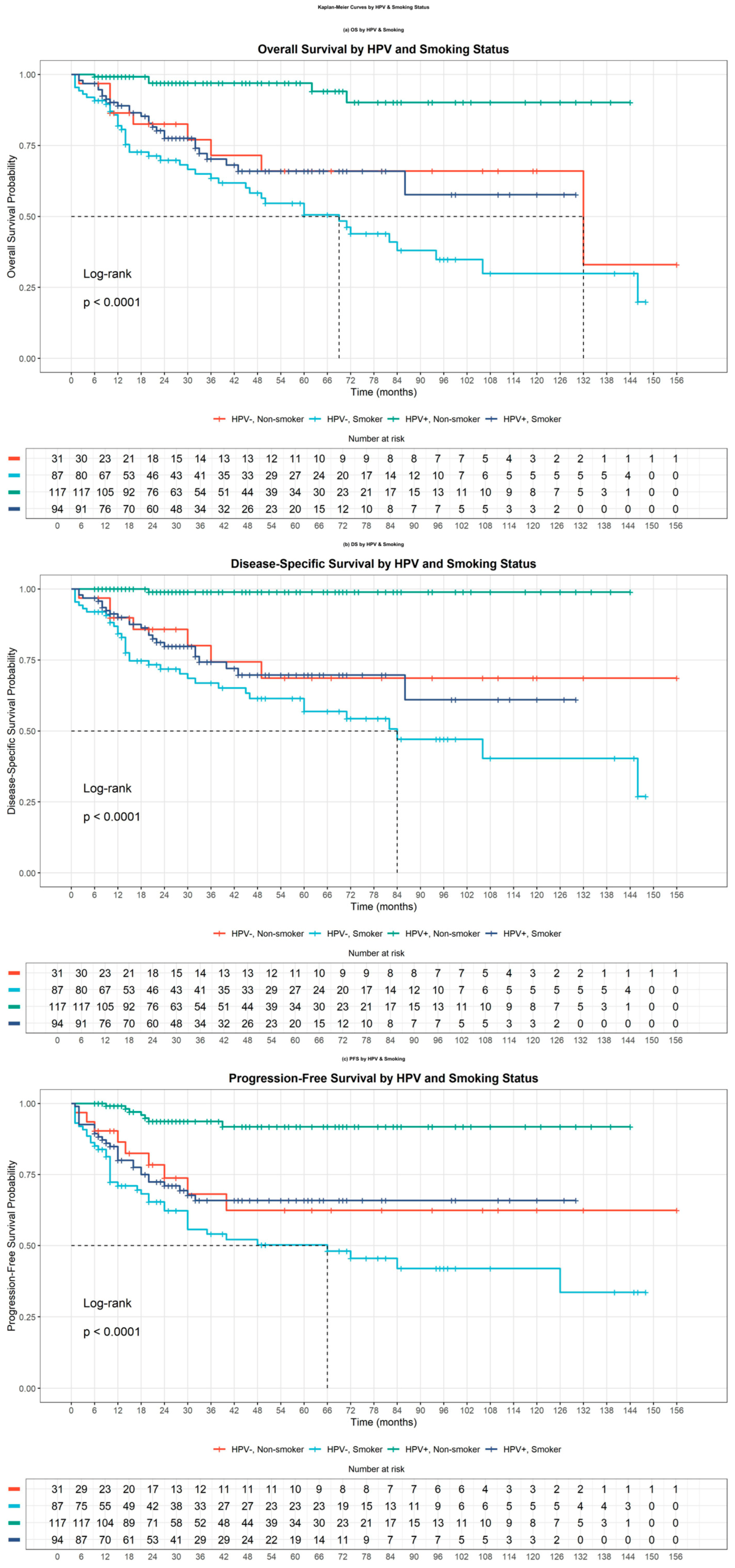
3.4. Interaction Analysis of Survival Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, K.R.; Calzada, G.; Hanby, J.D.; Garden, A.S.; Glisson, B.S.; Li, G.; Roberts, D.B.; Weber, R.S.; Sturgis, E.M. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: A staging system in need of repair. Cancer 2013, 119, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.A.; Weinstein, G.S.; O’Malley, B.W., Jr.; Feldman, M.; Quon, H. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck 2011, 33, 573–580. [Google Scholar] [CrossRef]
- Garden, A.S.; Kies, M.S.; Morrison, W.H.; Weber, R.S.; Frank, S.J.; Glisson, B.S.; Gunn, G.B.; Beadle, B.M.; Ang, K.K.; Rosenthal, D.I.; et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat. Oncol. 2013, 8, 21. [Google Scholar] [CrossRef]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Johansson, M.; Waterboer, T.; Kaaks, R.; Chang-Claude, J.; Drogen, D.; Tjønneland, A.; Overvad, K.; Quirós, J.R.; González, C.; et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J. Clin. Oncol. 2013, 31, 2708–2715. [Google Scholar] [CrossRef]
- Lang Kuhs, K.A.; Anantharaman, D.; Waterboer, T.; Johansson, M.; Brennan, P.; Michel, A.; Willhauck-Fleckenstein, M.; Purdue, M.P.; Holcátová, I.; Ahrens, W.; et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: A pooled analysis. Cancer Epidemiol. Biomark. Prev. 2015, 24, 683–689. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Salesky, M.; Benjamin, T.; El-Sayed, I.H.; George, J.R.; Ha, P.K.; Ryan, W.R.; Heaton, C.M. Impact of Smoking and Primary Tumor Subsite on Recurrence in HPV-Associated Oropharyngeal Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2022, 166, 704–711. [Google Scholar] [CrossRef]
- Haigentz, M., Jr.; Suarez, C.; Strojan, P.; Rodrigo, J.P.; Rinaldo, A.; Bradford, C.R.; Corry, J.; Takes, R.P.; Ferlito, A. Understanding interactions of smoking on prognosis of HPV-associated oropharyngeal cancers. Adv. Ther. 2018, 35, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wurdemann, N.; Wagner, S.; Sharma, S.J.; Prigge, E.S.; Reuschenbach, M.; Gattenlöhner, S.; Klussmann, J.P.; Wittekindt, C. Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front. Oncol. 2017, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Lee, N.; Riaz, N.; Pei, X.; Koyfman, S.A.; et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the international collaboration on oropharyngeal cancer network for staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar] [CrossRef]
- Geltzeiler, M.; Bertolet, M.; Albergotti, W.; Gleysteen, J.; Olson, B.; Persky, M.; Gross, N.; Li, R.; Andersen, P.; Kim, S.; et al. Staging HPV-related oropharyngeal cancer: Validation of AJCC-8 in a surgical cohort. Oral Oncol. 2018, 84, 82–87. [Google Scholar] [CrossRef]
- Nauta, I.H.; Rietbergen, M.M.; van Bokhoven, A.A.J.D.; Bloemena, E.; Lissenberg-Witte, B.I.; Heideman, D.A.M.; Baatenburg de Jong, R.J.; Brakenhoff, R.H.; Leemans, C.R. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann. Oncol. 2018, 29, 1273–1279. [Google Scholar] [CrossRef]
- Malm, I.J.; Fan, C.J.; Yin, L.X.; Li, D.X.; Koch, W.M.; Gourin, C.G.; Pitman, K.T.; Richmon, J.D.; Westra, W.H.; Kang, H.; et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer 2017, 123, 1768–1777. [Google Scholar] [CrossRef]
- Husain, Z.A.; Chen, T.; Corso, C.D.; Wang, Z.; Park, H.; Judson, B.; Yarbrough, W.; Deshpande, H.; Mehra, S.; Kuo, P.; et al. A comparison of prognostic ability of staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. JAMA Oncol. 2017, 3, 358–365. [Google Scholar] [CrossRef]
- van Gysen, K.; Stevens, M.; Guo, L.; Jayamanne, D.; Veivers, D.; Wignall, A.; Pang, L.; Guminski, A.; Lee, A.; Hruby, G.; et al. Validation of the 8(th) edition UICC/AJCC TNM staging system for HPV associated oropharyngeal cancer patients managed with contemporary chemo-radiotherapy. BMC Cancer 2019, 19, 674. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Wang, S.J.; van Zante, A.; Zhang, Y.; Rettig, E.; Yin, L.X.; Ryan, W.R.; Ha, P.K.; Wentz, A.; et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer 2017, 123, 1566–1575. [Google Scholar] [CrossRef]
- ISO 20387:2018; Biotechnology-Biobanking-General Requirements for Biobanking [EB/OL]. Available online: https://www.iso.org/standard/67888.html (accessed on 1 June 2025).
- Chidambaram, S.; Nakken, E.R.; Kennedy, W.; Thorstad, W.L.; Chen, S.Y.; Pipkorn, P.; Zevallos, J.P.; Mazul, A.L. Prognostic Significance of Smoking in Human Papillomavirus-Positive Oropharyngeal Cancer Under American Joint Committee on Cancer Eighth Edition Stage. Laryngoscope 2020, 130, 1961–1966. [Google Scholar] [PubMed]
- Chen, P.; Chen, X.H.; Huang, Z.G. Matched-pair analysis of survival in patients with poorly differentiated versus well-differentiated glottic squamous cell carcinoma. Oncotarget 2017, 8, 14770–14776. [Google Scholar] [CrossRef]
- Nesic, V.S.; Petrovic, Z.M.; Sipetic, S.B.; Jesic, S.D.; Soldatovic, I.A.; Kastratovic, D.A. Comparison of the adult comorbidity evaluation 27 and the charlson comorbidity indices in patients with laryngeal squamous cell carcinoma. J. Laryngol. Otol. 2012, 126, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Pham, Y.; Ward, M.C.; Houston, N.; Reddy, C.A.; Joshi, N.P.; Greskovich, J.F., Jr.; Woody, N.M.; Chute, D.J.; Lamarre, E.D.; et al. Impact of active smoking on outcomes in HPV+ oropharyngeal cancer. Head Neck 2020, 42, 269–280. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; Fernandez, L.; et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef]
- Wyss, A.; Hashibe, M.; Chuang, S.C.; Lee, Y.C.; Zhang, Z.F.; Yu, G.P.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013, 178, 679–690. [Google Scholar] [CrossRef]
- Chen, S.Y.; Massa, S.; Mazul, A.L.; Kallogjeri, D.; Yaeger, L.; Jackson, R.S.; Zevallos, J.; Pipkorn, P. The association of smoking and outcomes in HPV-positive oropharyngeal cancer: A systematic review. Am. J. Otolaryngol. 2020, 41, 102592. [Google Scholar] [CrossRef]
- Ference, R.; Liao, D.; Gao, Q.; Mehta, V. Impact of smoking on survival outcomes in HPV-related oropharyngeal carcinoma: A meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 1114–1122. [Google Scholar] [CrossRef]
- Anantharaman, D.; Muller, D.C.; Lagiou, P.; Ahrens, W.; Holcátová, I.; Merletti, F.; Kjærheim, K.; Polesel, J.; Simonato, L.; Canova, C.; et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int. J. Epidemiol. 2016, 45, 752–761. [Google Scholar] [CrossRef]
- Skoulakis, A.; Tsea, M.; Koltsidopoulos, P.; Lachanas, V.; Hajiioannou, J.; Petinaki, E.; Bizakis, J.; Skoulakis, C. Do smoking and human papilloma virus have a synergistic role in the development of head and neck cancer? A systematic review and meta-analysis. J. BUON 2020, 25, 1107–1115. [Google Scholar] [PubMed]
- Granata, R.; Miceli, R.; Orlandi, E.; Perrone, F.; Cortelazzi, B.; Franceschini, M.; Locati, L.D.; Bossi, P.; Bergamini, C.; Mirabile, A.; et al. Tumor stage, human papillomavirus and smoking status affect the survival of patients with oropharyngeal cancer: An Italian validation study. Ann. Oncol. 2012, 23, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- St Guily, J.L.; Rousseau, A.; Baujat, B.; Périé, S.; Schultz, P.; Barry, B.; Dufour, X.; Malard, O.; Pretet, J.L.; Clavel, C.; et al. Oropharyngeal cancer prognosis by tumour HPV status in France: The multicentric papillophar study. Oral Oncol. 2017, 67, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Lacroix, L.; Rossoni, C.; Sun, R.; Aupérin, A.; Casiraghi, O.; Villepelet, A.; Lacave, R.; Faucher, G.; Marty, V.; et al. Does smoking alter the mutation profile of human papillomavirus-driven head and neck cancers? Eur. J. Cancer 2018, 94, 61–69. [Google Scholar] [CrossRef]
- Nguyen-Tan, P.F.; Zhang, Q.; Ang, K.K.; Weber, R.S.; Rosenthal, D.I.; Soulieres, D.; Kim, H.; Silverman, C.; Raben, A.; Galloway, T.J.; et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long-term report of efficacy and toxicity. J. Clin. Oncol. 2014, 32, 3858–3866. [Google Scholar] [CrossRef]
- Gabani, P.; Lin, A.J.; Barnes, J.; Oppelt, P.; Adkins, D.R.; Rich, J.T.; Zevallos, J.P.; Daly, M.D.; Gay, H.A.; Thorstad, W.L. Radiation therapy dose de-escalation compared to standard dose radiation therapy in definitive treatment of HPV-positive oropharyngeal squamous cell carcinoma. Radiother. Oncol. 2019, 134, 81–88. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Huang, S.H.; Siu, L.L.; Waldron, J.; Zhao, H.; Perez-Ordonez, B.; Weinreb, I.; Kim, J.; Ringash, J.; Bayley, A.; et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 2013, 31, 543–550. [Google Scholar] [CrossRef]
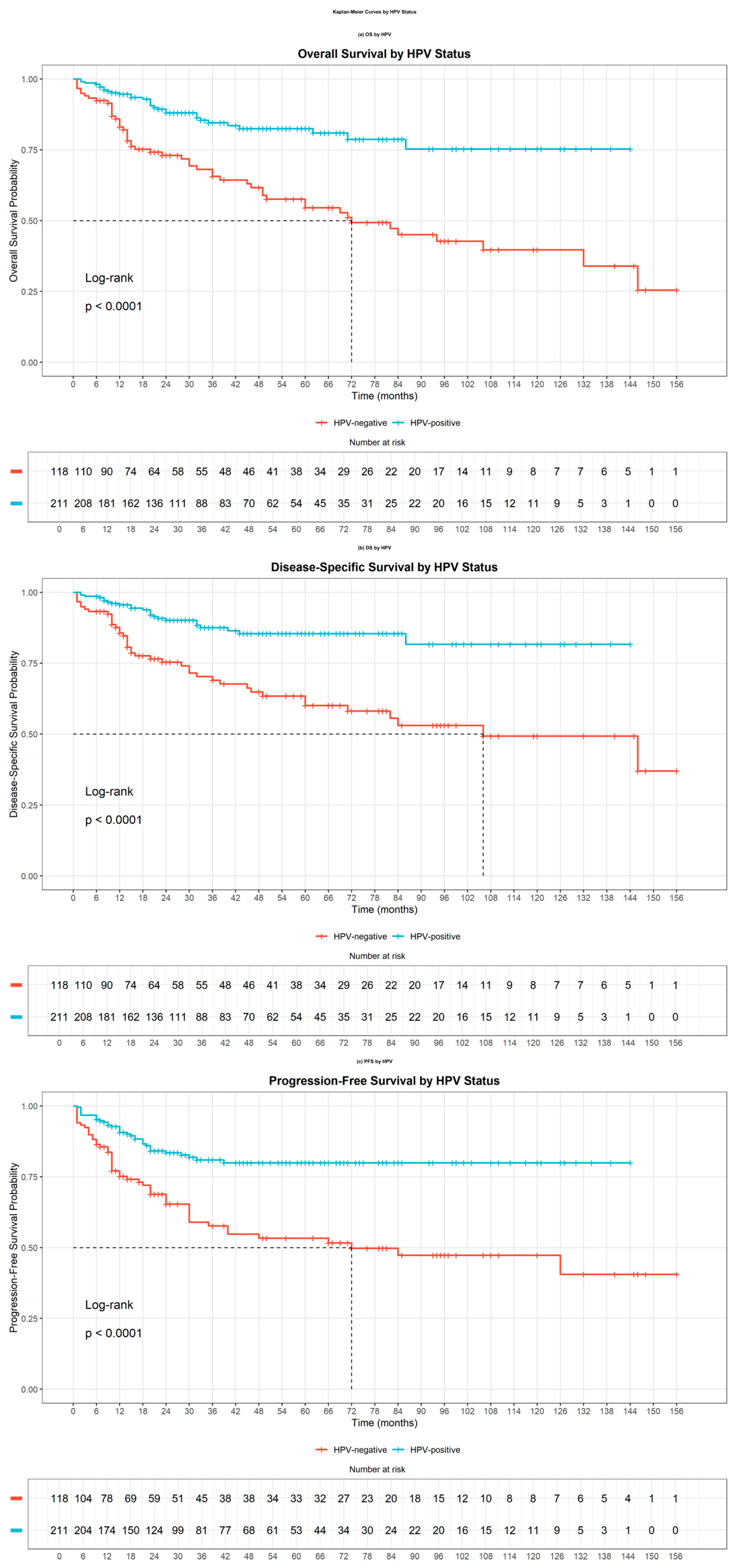
| Characteristics | HPV a Negative (n = 118) | HPV a Positive (n = 211) | p b Value | |||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |||
| Gender | 0.007 | |||||
| Male | 101 | 85.6 | 153 | 72.5 | ||
| Female | 17 | 14.4 | 58 | 27.5 | ||
| Age (years) | 0.040 | |||||
| Mean ± SD | 59.75 ± 9.95 | 57.35 ± 10.28 | ||||
| Tobacco use | <0.001 | |||||
| Smoker | 87 | 73.7 | 94 | 44.5 | ||
| Non-smoker | 31 | 26.3 | 117 | 55.5 | ||
| Alcohol consumption | <0.001 | |||||
| Drinker | 67 | 56.8 | 59 | 28.0 | ||
| Non-drinker | 51 | 43.2 | 152 | 72.0 | ||
| Adult comorbidity score | 0.491 | |||||
| None | 62 | 52.5 | 126 | 59.7 | ||
| Mild | 25 | 21.2 | 44 | 20.8 | ||
| Moderate | 29 | 24.6 | 40 | 19.0 | ||
| Severe | 2 | 1.7 | 1 | 0.5 | ||
| Tumor site | <0.001 | |||||
| Tonsil | 36 | 30.5 | 153 | 72.5 | ||
| Base of tongue | 52 | 44.1 | 48 | 22.7 | ||
| Soft palate, lateral/posterior pharyngeal wall, or NOS c | 30 | 25.4 | 10 | 4.7 | ||
| AJCC d clinical stage 8th | <0.001 | |||||
| I | 3 | 2.5 | 111 | 52.6 | ||
| II | 23 | 19.5 | 71 | 33.7 | ||
| III | 23 | 19.5 | 26 | 12.3 | ||
| IV | 69 | 58.5 | 3 | 1.4 | ||
| Primary treatment modality | 0.005 | |||||
| Surgical e | 57 | 48.3 | 68 | 32.2 | ||
| Non-surgical f | 61 | 51.7 | 143 | 67.8 | ||
| A. Without Interaction Terms | |||||||
| Variable | β b | SE c | HR d | Lower 95% CL e | Upper 95% CL | P1 | p * |
| HPV a-positive | −0.633 | 0.2416 | 0.531 | 0.3307 | 0.8526 | 0.00879 | 0.0352 |
| Smoking | 1.0556 | 0.3095 | 2.874 | 1.5669 | 5.2705 | 0.000647 | 0.00259 |
| T_new f | 0.3273 | 0.133 | 1.387 | 1.069 | 1.8002 | 0.01383 | 0.0553 |
| N_new g | 0.319 | 0.1327 | 1.376 | 1.0606 | 1.7843 | 0.01623 | 0.0649 |
| B. With Interaction Terms | |||||||
| HPV-positive | −1.9118 | 0.56 | 0.1478 | 0.04932 | 0.443 | 0.00064 | 0.003202 |
| Smoking | 0.2894 | 0.3763 | 1.3357 | 0.63884 | 2.793 | 0.44181 | 0.4418 |
| T_new f | 0.3202 | 0.131 | 1.3775 | 1.06564 | 1.781 | 0.01447 | 0.01809 |
| N_new g | 0.3554 | 0.1335 | 1.4268 | 1.09839 | 1.853 | 0.00774 | 0.01788 |
| HPV-positive: Smoking | 1.5695 | 0.6151 | 4.8043 | 1.43886 | 16.041 | 0.01073 | 0.01788 |
| A. Without Interaction Terms | |||||||
| Variable | β b | SE c | HR d | Lower 95% CL e | Upper 95% CL | P1 | p * |
| HPV a-positive | −0.6278 | 0.2689 | 0.5338 | 0.3151 | 0.9041 | 0.01955 | 0.02607 |
| Smoking | 1.404 | 0.392 | 4.0715 | 1.8883 | 8.7787 | 0.000342 | 0.001366 |
| T_new f | 0.4373 | 0.1483 | 1.5486 | 1.158 | 2.0708 | 0.003183 | 0.006366 |
| N_new g | 0.3019 | 0.1491 | 1.3524 | 1.0098 | 1.8113 | 0.04285 | 0.04285 |
| B. With Interaction Terms | |||||||
| HPV-positive | −3.25422 | 1.07021 | 0.03868 | 0.004748 | 0.3151 | 0.00237 | 0.008615 |
| Smoking | 0.317 | 0.42332 | 1.37301 | 0.59886 | 3.1478 | 0.45395 | 0.4539 |
| T_new f | 0.42602 | 0.14565 | 1.53115 | 1.15089 | 2.037 | 0.00345 | 0.008615 |
| N_new g | 0.34551 | 0.14986 | 1.4127 | 1.05314 | 1.895 | 0.02114 | 0.02642 |
| HPV-positive: Smoking | 2.96298 | 1.10498 | 19.35553 | 2.21943 | 168.7984 | 0.00733 | 0.01222 |
| A. Without Interaction Terms | |||||||
| Variable | β b | SE c | HR d | Lower 95% CL e | Upper 95% CL | P1 | p * |
| HPV a-positive | −0.6001 | 0.2329 | 0.5488 | 0.3477 | 0.8662 | 0.009973 | 0.009973 |
| Smoking | 1.0008 | 0.2918 | 2.7203 | 1.5354 | 4.8198 | 0.000605 | 0.002421 |
| T_new f | 0.3375 | 0.13 | 1.4015 | 1.0863 | 1.808 | 0.009397 | 0.009973 |
| N_new g | 0.3827 | 0.1329 | 1.4662 | 1.13 | 1.9024 | 0.003978 | 0.007955 |
| B. With Interaction Terms | |||||||
| HPV-positive | −1.6537 | 0.5049 | 0.1913 | 0.07113 | 0.5147 | 0.00106 | 0.005279 |
| Smoking | 0.3016 | 0.3735 | 1.352 | 0.65026 | 2.811 | 0.41936 | 0.4194 |
| T_new f | 0.3338 | 0.1283 | 1.3963 | 1.0858 | 1.7956 | 0.00929 | 0.01549 |
| N_new g | 0.4063 | 0.1333 | 1.5012 | 1.15595 | 1.9496 | 0.00231 | 0.005783 |
| HPV-positive: Smoking | 1.3121 | 0.5629 | 3.7138 | 1.23214 | 11.1939 | 0.01976 | 0.02471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Diao, W.; Zhu, X.; Yu, S.; Xia, X.; Han, W.; Chen, X. Impact of Smoking on Outcomes in HPV-Positive Oropharyngeal Squamous Cell Carcinoma in a Chinese Cohort Under AJCC 8th Edition Staging. J. Clin. Med. 2025, 14, 6802. https://doi.org/10.3390/jcm14196802
Zhu Y, Diao W, Zhu X, Yu S, Xia X, Han W, Chen X. Impact of Smoking on Outcomes in HPV-Positive Oropharyngeal Squamous Cell Carcinoma in a Chinese Cohort Under AJCC 8th Edition Staging. Journal of Clinical Medicine. 2025; 14(19):6802. https://doi.org/10.3390/jcm14196802
Chicago/Turabian StyleZhu, Yingying, Wenwen Diao, Xiaoli Zhu, Shuting Yu, Xin Xia, Wei Han, and Xingming Chen. 2025. "Impact of Smoking on Outcomes in HPV-Positive Oropharyngeal Squamous Cell Carcinoma in a Chinese Cohort Under AJCC 8th Edition Staging" Journal of Clinical Medicine 14, no. 19: 6802. https://doi.org/10.3390/jcm14196802
APA StyleZhu, Y., Diao, W., Zhu, X., Yu, S., Xia, X., Han, W., & Chen, X. (2025). Impact of Smoking on Outcomes in HPV-Positive Oropharyngeal Squamous Cell Carcinoma in a Chinese Cohort Under AJCC 8th Edition Staging. Journal of Clinical Medicine, 14(19), 6802. https://doi.org/10.3390/jcm14196802







