Trajectory of Iron and Red Cell Parameters in Moderately Anemic Iron-Deficient Pregnant Women Receiving Daily Iron–Folic Acid Supplementation: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Size
2.3. Study Procedure
2.4. Treatment Dosage
2.5. Analysis of Samples
2.6. Statistical Analysis
3. Results
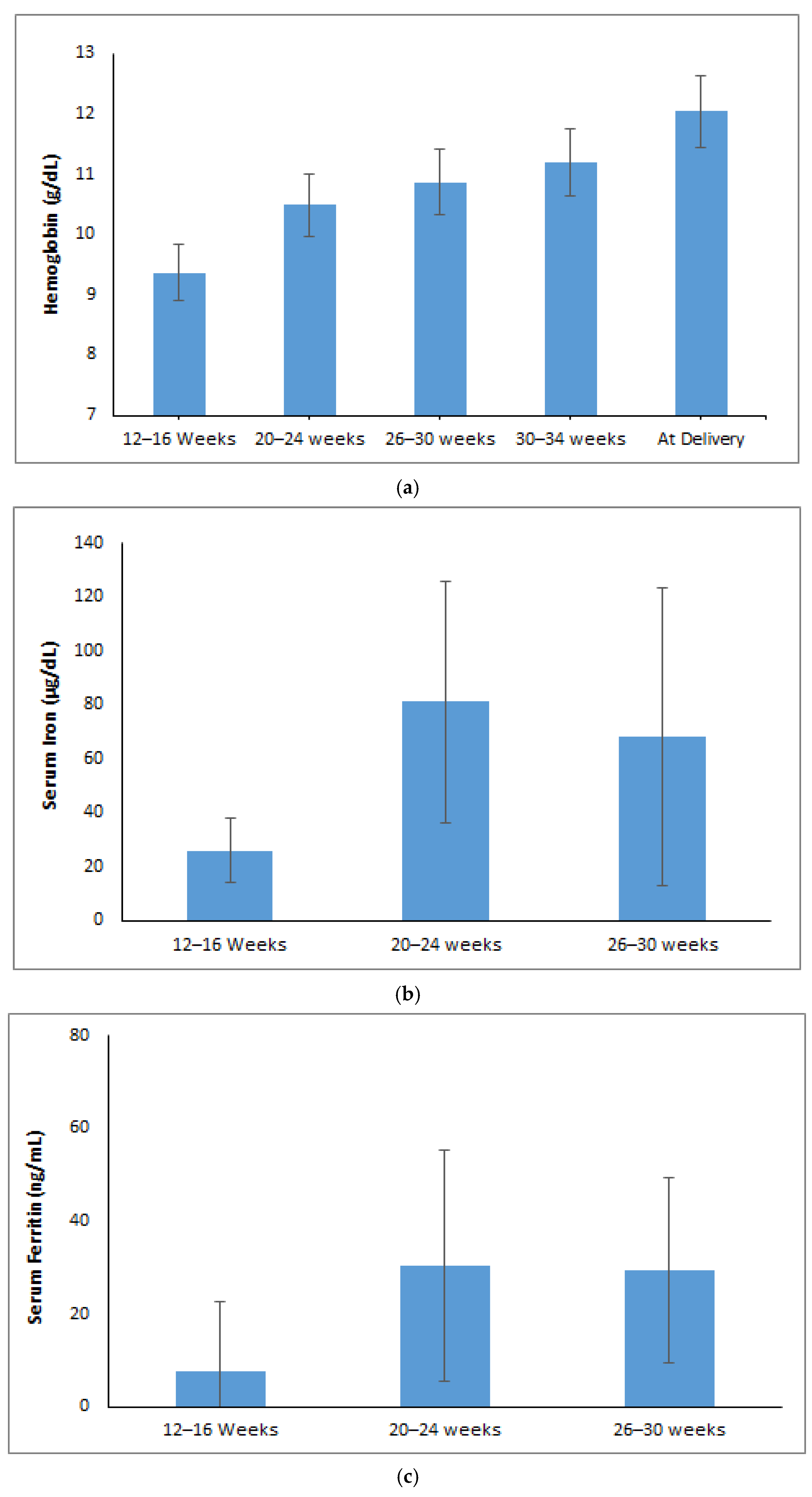
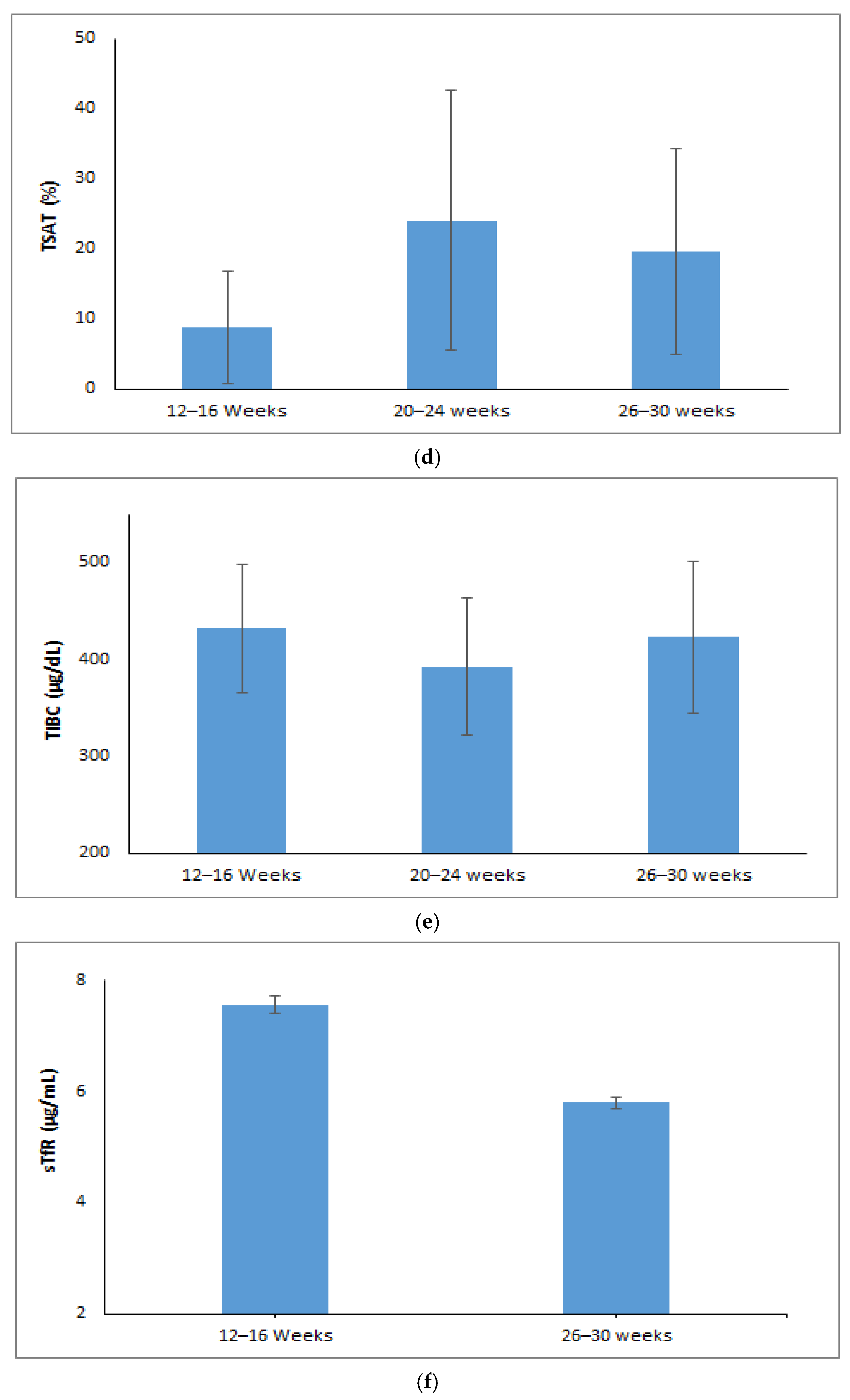
3.1. Changes in Maternal Red Cell Indices During Pregnancy
3.2. Trajectory or Trends of Maternal Iron Parameters During Pregnancy
4. Discussion
4.1. The Main Findings
4.2. Trimester Specific Strategy and Counselling Support
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Iron Deficiency Anemia: Assessment, Prevention, and Control; A Guide for Programme Managers; WHO: Geneva, Switzerland, 2001; pp. 47–62.
- Ministry of Health & Family Welfare Government of India. National Family Health Survey 5. 2021. Available online: https://dhsprogram.com/publications/publication-OF43-Other-Fact-Sheets.cfm (accessed on 28 March 2025).
- Fisher, A.L.; Nemeth, E. Iron Homeostasis during Pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef]
- Fa, O.; Naiman, J.L. Hematologic Problems in the Newborn. Major Probl. Clin. Pediatr. 1982, 4, 1–360. [Google Scholar] [PubMed]
- McArdle, H.J.; Gambling, L.; Kennedy, C. Iron Deficiency during Pregnancy: The Consequences for Placental Function and Fetal Outcome. Proc. Nutr. Soc. 2013, 73, 9–15. [Google Scholar] [CrossRef]
- Brittenham, G. Disorders of iron metabolism: Iron deficiency and overload. In Hematology: Basic Principles and Practice, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 478–490. [Google Scholar]
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral Iron Supplementation in Iron-Deficient Women: How Much and How Often? Mol. Asp. Med. 2020, 75, 100865. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M. Optimizing Diagnosis and Treatment of Iron Deficiency and Iron Deficiency Anemia in Women and Girls of Reproductive Age: Clinical Opinion. Int. J. Gynaecol. Obstet. 2023, 162, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily Oral Iron Supplementation during Pregnancy. Cochrane Database Syst. Rev. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Derman, R.J.; Goudar, S.S.; Thind, S.; Bhandari, S.; Aghai, Z.; Auerbach, M. RAPIDIRON: Reducing Anaemia in Pregnancy in India—A 3-Arm, Randomized-Controlled Trial Comparing the Effectiveness of Oral Iron with Single-Dose Intravenous Iron in the Treatment of Iron Deficiency Anaemia in Pregnant Women and Reducing Low Birth Weight Deliveries. Trials 2021, 22, 649. [Google Scholar] [CrossRef]
- Anemia Mukt Bharat. Available online: https://anemiamuktbharat.info/programme/interventions#link-1 (accessed on 29 March 2025).
- Beressa, G.; Lencha, B.; Bosha, T.; Egata, G. Utilization and Compliance with Iron Supplementation and Predictors among Pregnant Women in Southeast Ethiopia. Sci. Rep. 2022, 12, 16253. [Google Scholar] [CrossRef]
- Molla, T.; Guadu, T.; Muhammad, E.A.; Hunegnaw, M.T. Factors Associated with Adherence to Iron Folate Supplementation among Pregnant Women in West Dembia District, Northwest Ethiopia: A Cross Sectional Study. BMC Res. Notes 2019, 12, 6. [Google Scholar] [CrossRef]
- Haruna, S.; Patience Kanyiri, G.; Mogre, V. Adherence to Iron and Folic Acid Supplementation among Pregnant Women from Northern Ghana. Nutr. Metab. Insights 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmy, R.; Lavanya, P.; Rajaa, S.; Mahalakshmy, T. Adherence to Iron and Folic Acid Supplementation among Antenatal Mothers Attending a Tertiary Care Center, Puducherry: A Mixed-Methods Study. J. Fam. Med. Prim. Care 2020, 9, 5205–5211. [Google Scholar] [CrossRef]
- Agegnehu, G.; Atenafu, A.; Dagne, H.; Dagnew, B. Adherence to Iron and Folic Acid Supplement and Its Associated Factors among Antenatal Care Attendant Mothers in Lay Armachiho Health Centers, Northwest, Ethiopia, 2017. Int. J. Reprod. Med. 2019, 1, 5863737. [Google Scholar] [CrossRef]
- Ugwu, E.; Olibe, A.; Obi, S.; Ugwu, A. Determinants of Compliance to Iron Supplementation among Pregnant Women in Enugu, Southeastern Nigeria. Niger. J. Clin. Pract. 2014, 17, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Habib Zein Alabdin, E.; Alenazy, M.; Nooh, R. Compliance to Iron Supplementation during Pregnancy. J. Obstet. Gynaecol. 2009, 29, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral Iron Supplements Increase Hepcidin and Decrease Iron Absorption from Daily or Twice-Daily Doses in Iron-Depleted Young Women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Oshodi, Y.A.; Fabamwo, A.O.; Akinola, O.I. Haematological Indices in Pregnancy and Puerperium. Int. J. Curr. Res. 2017, 9, 54712–54721. [Google Scholar]
- Davidson, R.J.; Hamilton, P.J. High Mean Red Cell Volume: Its Incidence and Significance in Routine Haematology. J. Clin. Pathol. 1978, 31, 493–498. [Google Scholar] [CrossRef]
- Chandra, S.; Tripathi, A.K.; Mishra, S.; Amzarul, M.; Vaish, A.K. Physiological Changes in Hematological Parameters during Pregnancy. Indian J. Hematol. Blood Transfus. 2012, 28, 144–146. [Google Scholar] [CrossRef]
- Abdelrahman, E.G.; Gasim, G.I.; Musa, I.R.; Elbashir, L.M.; Adam, I. Red Blood Cell Distribution Width and Iron Deficiency Anemia among Pregnant Sudanese Women. Diagn. Pathol. 2012, 7, 168. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Gomez Malave, H.; Flores-Urrutia, M.C.; Dowswell, T. Intermittent Oral Iron Supplementation during Pregnancy. Cochrane Database Syst. Rev. 2015, 10. [Google Scholar] [CrossRef]
- Davis, B.H.; Ornvold, K.; Bigelow, N.C. Flow Cytometric Reticulocyte Maturity Index: A Useful Laboratory Parameter of Erythropoietic Activity in Anemia. Cytometry 1995, 22, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Goyal, L.; Kaushik, D.; Gulati, S.; Sharma, N.; Harshvardhan, L.; Gupta, N. Reticulocyte Hemoglobin vis-à-vis Immature Reticulocyte Fraction, as the Earliest Indicator of Response to Therapy in Iron Deficiency Anemia. J. Assoc. Physicians India 2017, 65, 14–17. [Google Scholar] [PubMed]
- Mast, A.E.; Blinder, M.A.; Lu, Q.; Flax, S.; Dietzen, D.J. Clinical Utility of the Reticulocyte Hemoglobin Content in the Diagnosis of Iron Deficiency. Blood 2002, 99, 1489–1491. [Google Scholar] [CrossRef]
- Parodi, E.; Giraudo, M.T.; Ricceri, F.; Aurucci, M.L.; Mazzone, R.; Ramenghi, U. Absolute Reticulocyte Count and Reticulocyte Hemoglobin Content as Predictors of Early Response to Exclusive Oral Iron in Children with Iron Deficiency Anemia. Anemia 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Noshiro, K.; Umazume, T.; Hattori, R.; Kataoka, S.; Yamada, T.; Watari, H. Hemoglobin Concentration during Early Pregnancy as an Accurate Predictor of Anemia during Late Pregnancy. Nutrients 2022, 14, 839. [Google Scholar] [CrossRef]
- Lee, J.I.; Kang, S.A.; Kim, S.K.; Lim, H.S. A Cross Sectional Study of Maternal Iron Status of Korean Women during Pregnancy. Nutr. Res. 2002, 22, 1377–1388. [Google Scholar] [CrossRef]
- Kirthan, J.A.; Somannavar, M.S. Pathophysiology and Management of Iron Deficiency Anaemia in Pregnancy: A Review. Ann. Hematol. 2023, 103, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Carriaga, M.T.; Skikne, B.S.; Finley, B.; Cutler, B.; Cook, J.D. Serum Transferrin Receptor for the Detection of Iron Deficiency in Pregnancy. Am. J. Clin. Nutr. 1991, 54, 1077–1081. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Ganz, T.; Nemeth, E. Iron Homeostasis during Pregnancy: Maternal, Placental, and Fetal Regulatory Mechanisms. Annu. Rev. Nutr. 2023, 43, 279–300. [Google Scholar] [CrossRef]
| Variables | n (%)/Median (Q1, Q3) |
|---|---|
| Maternal Age (years) | |
| 18–25 | 231 (73.3) |
| 26–30 | 60 (19.0) |
| ≥31 | 24 (7.6) |
| Maternal Hb (at recruitment) | 9.36 (8.55, 9.74) |
| BMI | |
| Underweight | 106 (33.7) |
| Normal weight | 183 (58.1) |
| Overweight | 22 (7.0) |
| Obesity | 4 (1.3) |
| Blood Pressure | |
| Systolic Blood Pressure (mmHg) | 101 (94,109) |
| Diastolic Blood Pressure (mmHg) | 64 (60,70) |
| Parity | |
| Nulliparous | 134 (42.5) |
| Primiparous | 90 (28.6) |
| Multiparous | 91 (28.9) |
| Diet | |
| Vegetarian | 23 (7.3) |
| Lacto-vegetarian | 47 (14.9) |
| Ovo-vegetarian | 54 (17.1) |
| Mixed diet | 191 (60.6) |
| Reason | Iron Tablets n = 17 (%) | Folic Acid Tablets n = 11 (%) |
|---|---|---|
| Forgot to take tablets | 2 (0.63) | 2 (0.63) |
| Tablets made me feel sick | 14 (4.44) | 8 (2.54) |
| Tablets got lost | 0 (0) | 0 (0) |
| Did not understand the instructions for taking tablet | 1 (0.32) | 1 (0.32) |
| Parameters | Time Points | t-Test/Z Statistic | p-Value | |
|---|---|---|---|---|
| 12–16 Weeks GA | 26–30 Weeks GA | |||
| RBC (106 cells/µL) # | 4.19 ± 0.42 | 4.05 ± 0.44 | 7.39 | <0.001 * |
| MCV (fL) # | 72.16 ± 7.90 | 83.47 ± 7.65 | 26.95 | <0.001 * |
| MCH (pg) # | 22.44 ± 3.01 | 26.77 ± 3.08 | 24.41 | <0.001 * |
| MCHC (g/dL) # | 31.03 ± 1.14 | 32.01 ± 1.21 | 12.26 | <0.001 * |
| RDW (%) ¥ | 17 (15.5, 18.10) | 16.9 (15.1, 19.7) | 3.04 | <0.001 * |
| HCT (%) # | 29.93 ± 2.87 | 33.71 ± 3.69 | 15.02 | <0.001 * |
| Reticulocyte Hb (pg) # | 23.30 ± 3.03 | 27.84 ± 3.83 | 18.40 | <0.001 * |
| Immature Reticulocyte Fraction (%) ¥ | 6.90 (4.30, 9.50) | 7.30 (4.3, 11.0) | 3.59 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akshaykirthan, J.P.; Somannavar, M.S.; Yogeshkumar, S.; Deepthy, M.S.; Charantimath, U.; Patil, A.; Bellad, M.B.; Derman, R.; Goudar, S.S. Trajectory of Iron and Red Cell Parameters in Moderately Anemic Iron-Deficient Pregnant Women Receiving Daily Iron–Folic Acid Supplementation: A Prospective Cohort Study. J. Clin. Med. 2025, 14, 6787. https://doi.org/10.3390/jcm14196787
Akshaykirthan JP, Somannavar MS, Yogeshkumar S, Deepthy MS, Charantimath U, Patil A, Bellad MB, Derman R, Goudar SS. Trajectory of Iron and Red Cell Parameters in Moderately Anemic Iron-Deficient Pregnant Women Receiving Daily Iron–Folic Acid Supplementation: A Prospective Cohort Study. Journal of Clinical Medicine. 2025; 14(19):6787. https://doi.org/10.3390/jcm14196787
Chicago/Turabian StyleAkshaykirthan, J. P., Manjunath S. Somannavar, S. Yogeshkumar, M. S. Deepthy, Umesh Charantimath, Amaresh Patil, Mrutyunjaya B. Bellad, Richard Derman, and Shivaprasad S. Goudar. 2025. "Trajectory of Iron and Red Cell Parameters in Moderately Anemic Iron-Deficient Pregnant Women Receiving Daily Iron–Folic Acid Supplementation: A Prospective Cohort Study" Journal of Clinical Medicine 14, no. 19: 6787. https://doi.org/10.3390/jcm14196787
APA StyleAkshaykirthan, J. P., Somannavar, M. S., Yogeshkumar, S., Deepthy, M. S., Charantimath, U., Patil, A., Bellad, M. B., Derman, R., & Goudar, S. S. (2025). Trajectory of Iron and Red Cell Parameters in Moderately Anemic Iron-Deficient Pregnant Women Receiving Daily Iron–Folic Acid Supplementation: A Prospective Cohort Study. Journal of Clinical Medicine, 14(19), 6787. https://doi.org/10.3390/jcm14196787





