Non-Thyroidal Illness Syndrome and Thyroid Autoimmunity in Hospitalized COVID-19 Patients: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Anti-Tg | Anti-thyroglobulin antibody |
| Anti-TPO | Anti-thyroid peroxidase antibody |
| BiPAP | Bilevel positive airway pressure |
| COVID-19 | Coronavirus Disease 2019 |
| CPAP | Continuous positive airway pressure |
| fT3 | Free triiodothyronine |
| fT4 | Free thyroxine |
| HPT axis | Hypothalamic–pituitary–thyroid axis |
| ICU | Intensive Care Unit |
| IQR | Interquartile range |
| NTIS | Non-thyroidal Illness Syndrome |
| rT3 | Reverse triiodothyronine |
| TFTs | Thyroid function tests |
| TRAb | Thyrotropin receptor antibody |
| TSH | Thyroid-stimulating hormone |
References
- Panesar, A.; Gharanei, P.; Khovanova, N.; Young, L.; Grammatopoulos, D. Thyroid function during COVID-19 and post-COVID complications in adults: A systematic review. Front. Endocrinol. 2024, 15, 1477389. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, N.; Rezaei, N. Autoimmune complications of COVID-19. J. Med. Virol. 2022, 94, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, M.; Nazer, M.R.; Shahali, H.; Nouri, M. Association of thyroid dysfunction and COVID-19: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 947594. [Google Scholar] [CrossRef]
- Fliers, E.; Boelen, A. An update on non-thyroidal illness syndrome. J. Endocrinol. Investig. 2021, 44, 1597–1607. [Google Scholar] [CrossRef]
- Ashrafi, S.; Hatami, H.; Bidhendi-Yarandi, R.; Panahi, M.H. The prevalence of thyroid disorders in COVID-19 patients: A systematic review and meta-analysis. BMC Endocr Disord. 2024, 24, 5. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Deandreis, D.; Siracusa, M.; Tozzoli, R.; Petranović Ovčariček, P.; Giovanella, L. SARS-CoV-2-related immune-inflammatory thyroid disorders: Facts and perspectives. Expert. Rev. Clin. Immunol. 2021, 17, 737–759. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef]
- Lania, A.; Sandri, M.T.; Cellini, M.; Mirani, M.; Lavezzi, E.; Mazziotti, G. Thyrotoxicosis in patients with COVID-19 (THYRCOV study). Endocrine 2020, 68, 473–478. [Google Scholar] [CrossRef]
- Kurtulmus, N.; Kayikci, K. Subacute thyroiditis following SARS-CoV-2 vaccines: Six cases report and review of the literature. Horm. Metab. Res. 2022, 54, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Freund, O.; Eviatar, T.; Bornstein, G. Concurrent myopathy and inflammatory cardiac disease in COVID-19 patients: A case series and literature review. Rheumatol. Int. 2022, 42, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Mariette, X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat. Rev. Rheumatol. 2021, 17, 315–332. [Google Scholar] [CrossRef]
- Anaya, J.M.; Herran, M.; Beltrán, S.; Rojas, M. Is post-COVID syndrome an autoimmune disease? Expert. Rev. Clin. Immunol. 2022, 18, 653–666. [Google Scholar] [CrossRef]
- Llamas, M.; Garo, M.L.; Giovanella, L. Low free-T3 serum levels and prognosis of COVID-19: Systematic review and meta-analysis. Clin. Chem. Lab. Med. 2021, 59, 1906–1913. [Google Scholar] [CrossRef]
- Patel, D.; Naik, D.; Kamalanathan, S.; Tamilarasu, K.; Sahoo, J.; Roy, A.; Merugu, C.; Suryadevara, V. Thyroid Function Abnormalities and Outcomes in Hospitalized Patients with COVID-19 Infection: A Cross-Sectional Study. Horm. Metab. Res. 2023, 55, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.T.W.; Lee, C.H.; Woo, Y.C.; Hung, I.F.N.; Lam, K.S.L. Thyroid dysfunction in COVID-19. Nat. Rev. Endocrinol. 2024, 20, 336–348. [Google Scholar] [CrossRef]
- Zhong, M.; Gao, Y.; Hu, H.; Zhu, X.; Gan, L.; Li, L.; Xiang, C.; Yan, Y.; Dai, Z. Transient low T3 syndrome in patients with COVID-19: A new window for prediction of disease severity. Front. Endocrinol. 2023, 14, 1154007. [Google Scholar] [CrossRef]
- Fallahi, P.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Balestri, E.; Mazzi, V.; Benvenga, S.; Varricchi, G.; Gragnani, L.; et al. Thyroid autoimmunity and SARS-CoV-2 infection. J. Clin. Med. 2023, 12, 6365. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Capalbo, C.; Napoli, C.; Anibaldi, P.; Salvati, V.; De Vitis, C.; Mancini, R.; Coluzzi, F.; Rocco, M. Nonthyroidal Illness Syndrome: To Treat or Not to Treat? Have We Answered the Question? A Review of Metanalyses. Front. Endocrinol. 2022, 13, 850328. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hong, Y.; Wang, Z.; Li, Y. Prognostic Value of Thyroid Hormone FT3 in General Patients Admitted to the Intensive Care Unit. Biomed. Res Int. 2020, 2020, 6329548. [Google Scholar] [CrossRef]
- Sror-Turkel, O.; El-Khatib, N.; Sharabi-Nov, A.; Avraham, Y.; Merchavy, S. Low TSH and low T3 hormone levels as a prognostic for mortality in COVID-19 intensive care patients. Front. Endocrinol. 2024, 15, 1322487. [Google Scholar] [CrossRef]
- Beltrão, F.E.D.L.; Beltrão, D.C.D.A.; Carvalhal, G.; Beltrão, F.E.D.L.; Brito, A.D.S.; Capistrano, K.H.R.D.; Bastos, I.H.D.A.; Hecht, F.; Daltro, C.H.D.C.; Bianco, A.C.; et al. Thyroid hormone levels during hospital admission inform disease severity and mortality in COVID-19. Thyroid 2021, 31, 902–910. [Google Scholar] [CrossRef]
- Baldelli, R.; Nicastri, E.; Petrosillo, N.; Marchioni, L.; Gubbiotti, A.; Sperduti, I.; Di Giacinto, P.; Rizza, L.; Rota, F.; Franco, M.; et al. Thyroid dysfunction in COVID-19 patients. J. Endocrinol. Investig. 2021, 44, 2735–2739. [Google Scholar] [CrossRef]
- Özportakal, H.; Menekşe, Ş.; Altınay, E.; Oğuş, H.; Kırali, K. Thyroid Dysfunction among the Patients with Critical COVID-19. Infect. Dis. Clin. Microbiol. 2022, 4, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; De Tullio, A.; Jirillo, E.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Triggiani, V. Thyroid and COVID-19: A review on pathophysiological, clinical and organizational aspects. J. Endocrinol. Investig. 2021, 44, 1801–1814. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the thyroid gland: An update. Rev. Endocr. Metab. Disord. 2021, 22, 803–815. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Jernbom, A.F.; Skoglund, L.; Pin, E.; Sjöberg, R.; Tegel, H.; Hober, S.; Rostami, E.; Rasmusson, A.; Cunningham, J.L.; Havervall, S.; et al. Prevalent and persistent new-onset autoantibodies in mild to severe COVID-19. Nat Commun. 2024, 15, 8941. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Coperchini, F.; Ricci, G.; Denegri, M.; Croce, L.; Ngnitejeu, S.T.; Villani, L.; Magri, F.; Latrofa, F.; Chiovato, L. Detection of SARS-CoV-2 receptor ACE-2 mRNA in thyroid cells: A clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Investig. 2021, 44, 1085–1090. [Google Scholar] [CrossRef]
- Rossini, A.; Cassibba, S.; Perticone, F.; Benatti, S.V.; Venturelli, S.; Carioli, G.; Ghirardi, A.; Rizzi, M.; Barbui, T.; Trevisan, R.; et al. Increased prevalence of autoimmune thyroid disease after COVID-19: A single-center, prospective study. Front. Endocrinol. 2023, 14, 1126683. [Google Scholar] [CrossRef]
- Yan, Y.R.; Gao, X.L.; Zeng, J.; Liu, Y.; Lv, Q.G.; Jiang, J.; Huang, H.; Tong, N.W. The association between thyroid autoantibodies in serum and abnormal function and structure of the thyroid. J. Int. Med. Res. 2015, 43, 412–423. [Google Scholar] [CrossRef]
- Duntas, L.H.; Jonklaas, J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J Endocr Soc. 2021, 5, bvab076. [Google Scholar] [CrossRef]
- Muller, I.; Cannavaro, D.; Dazzi, D.; Covelli, D.; Mantovani, G.; Muscatello, A.; Ferrante, E.; Orsi, E.; Resi, V.; Longari, V.; et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020, 8, 739–741. [Google Scholar] [CrossRef]
- Khoo, B.; Tan, T.; Clarke, S.A.; Mills, E.G.; Patel, B.; Modi, M.; Phylactou, M.; Eng, P.C.; Thurston, L.; Alexander, E.C. Thyroid function before, during, and after COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e803–e811. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, J.; Wang, T.; Wang, H.; Yao, J.; Wang, S.; Mou, Y. Thyroid diseases are associated with coronavirus disease 2019 infection: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 952049. [Google Scholar] [CrossRef] [PubMed]
- Naguib, R. Potential relationships between COVID-19 and the thyroid gland: An update. J. Int. Med. Res. 2022, 50, 03000605221082898. [Google Scholar] [CrossRef] [PubMed]
| Variable | COVID ICU n = 138 | COVID Non-ICU n = 138 | Control Group n = 110 | p-Value (ICU vs. Non-ICU) | p-Value (ICU vs. Control) | p-Value (Non-ICU vs. Control) |
|---|---|---|---|---|---|---|
| Age (years), median [Q1–Q3] | 70 [58–80] | 67 [53–78] | 50 [36–69] | 0.197 | <0.001 | <0.001 |
| Male sex, n (%) | 56 (40.6%) | 54 (39.1%) | 30 (27.3%) | 0.902 | 0.032 | 0.059 |
| Female sex, n (%) | 82 (59.4%) | 84 (60.9%) | 80 (72.7%) | |||
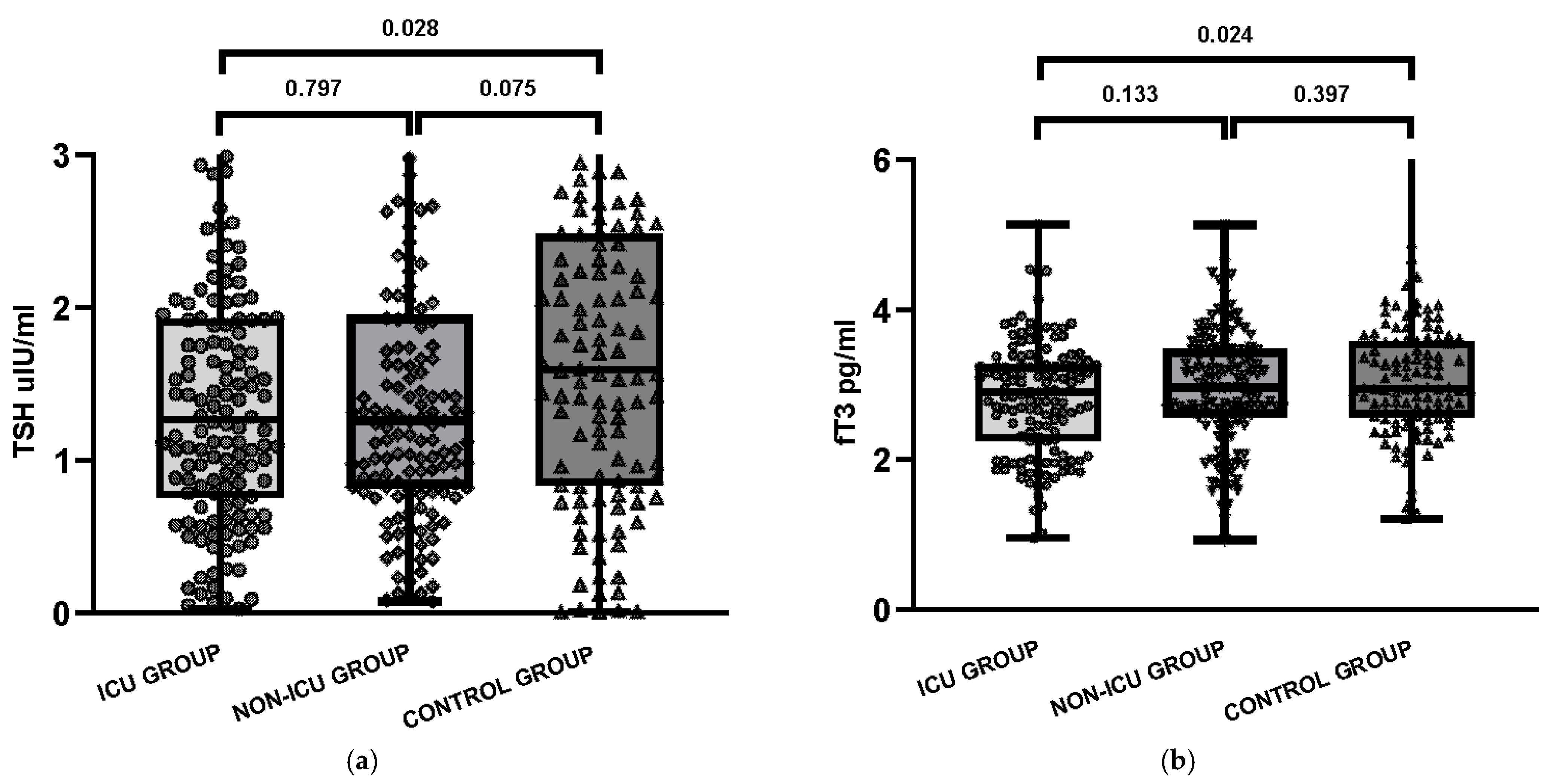
| TSH, median [Q1–Q3] | 1.27 [0.77–1.93] | 1.26 [0.82–1.94] | 1.59 [0.84–2.46] | 0.797 | 0.028 | 0.075 |
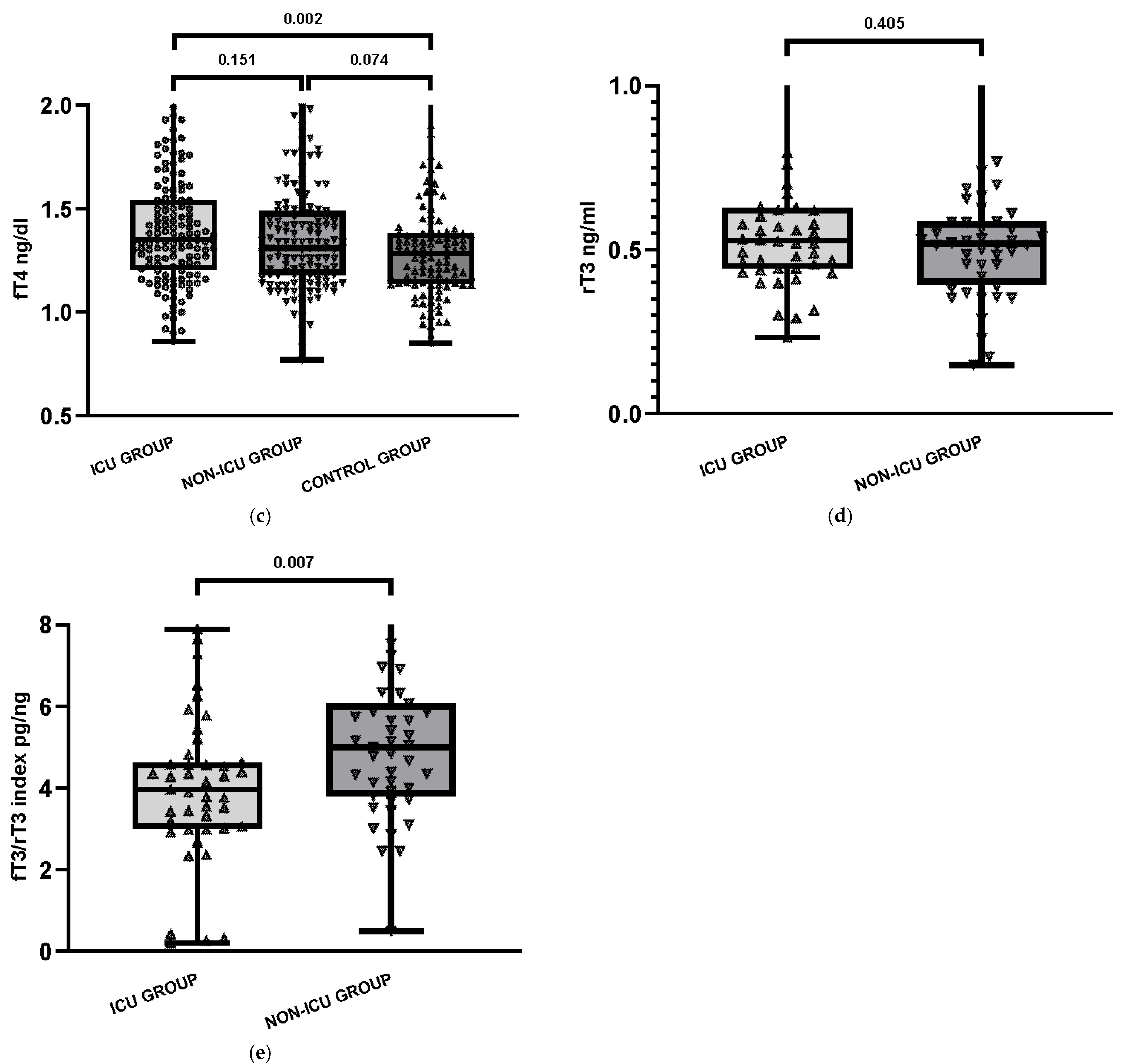
| fT4, median [Q1–Q3] | 1.35 [1.21–1.54] | 1.31 [1.18–1.49] | 1.29 [1.14–1.38] | 0.151 | 0.002 | 0.074 |
| fT3, median [Q1–Q3] | 2.90 [2.26–3.26] | 2.96 [2.57–3.47] | 2.95 [2.58–3.57] | 0.133 | 0.024 | 0.397 |
| rT3, median [Q1-Q3] | 0.53 [0.44–0.62] | 0.52 [0.40–0.58] | - | 0.405 | - | - |
| fT3/rT3 index (pg/ng) | 3.96 | 5.00 | - | 0.007 | - | - |
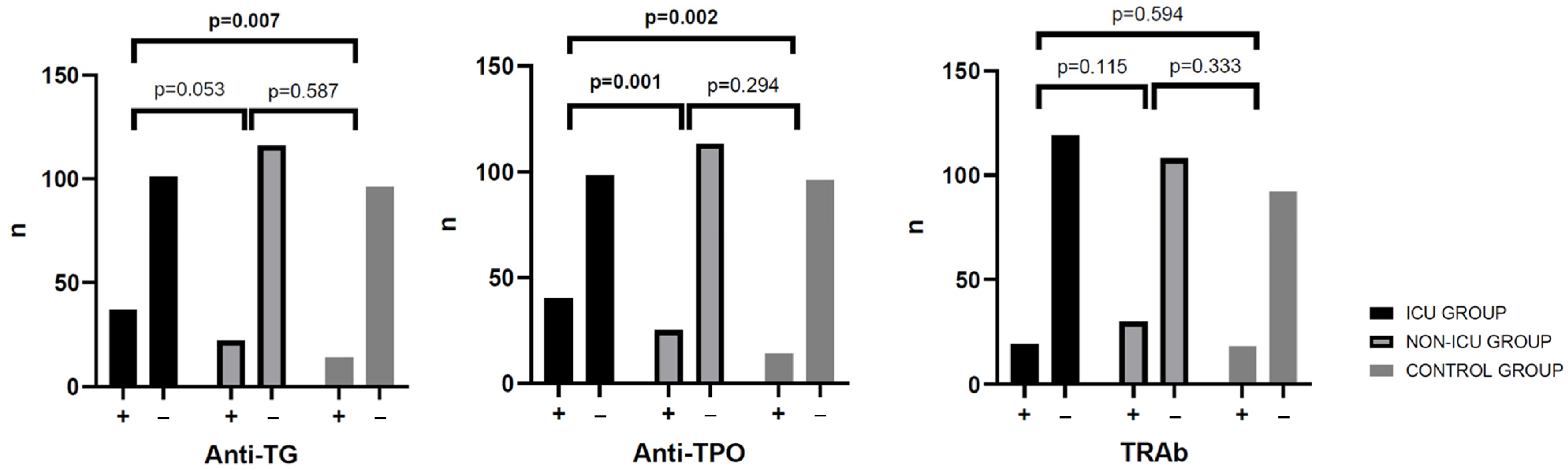
| % anti-TPO (+) | 28.9% | 25.0% | 12.7% | 0.001 | 0.002 | 0.294 |
| % anti-Tg (+) | 24.6% | 27.1% | 3.6% | 0.053 | 0.007 | 0.587 |
| % TRAb (+) | 17.4% | 24.3% | 16.4% | 0.115 | 0.594 | 0.333 |
| Comorbidities | ||||||
| Hypertension, n (%) | 32 (23.2%) | 13 (9.4%) | - | 0.003 | - | - |
| Diabetes mellitus, n (%) | 19 (13.8%) | 8 (5.8%) | - | 0.041 | - | - |
| Obesity, n (%) | 24 (17.4%) | 17 (12.3%) | - | 0.310 | - | - |
| Heart failure, n (%) | 7 (5.1%) | 3 (2.2%) | - | 0.335 | - | - |
| Coronary artery disease, n (%) | 24 (17.4%) | 10 (7.2%) | - | 0.016 | - | - |
| Chronic kidney disease, n (%) | 9 (6.5%) | 2 (1.4%) | - | 0.060 | - | - |
| Clinical condition | ||||||
| Oxygen therapy (nasal cannula/mask), n (%) | 15 (10.9%) | 16 (11.6%) | - | 1.000 | - | - |
| Non-invasive ventilation (CPAP/BiPAP), n (%) | 25 (18.1%) | 23 (16.7%) | - | 0.874 | - | - |
| Intubation, n (%) | 42 (30.4%) | 2 (1.4%) | - | <0.001 | - | - |
| Steroid treatment, n (%) | 59 (42.8%) | 24 (17.4%) | - | <0.001 | - | - |
| Heparin treatment, n (%) | 15 (10.9%) | 5 (3.6%) | - | 0.037 | - | - |
| Thyroid Autoantibodies | COVID ICU Group n = 138 | COVID Non-ICU Group n = 138 | Control Group n = 110 | p (χ2 Test ) |
|---|---|---|---|---|
| Anti-Tg +, anti-TPO +, TRAb + | 13 (9.4%) | 24 (17.4%) | 6 (5.5%) | 0.009 |
| Anti-Tg +, anti-TPO + | 21 (15.2%) | 24 (17.4%) | 7 (6.4%) | 0.031 |
| Anti-Tg + | 4 (2.9%) | 8 (5.8%) | 1 (0.9%) | 0.098 |
| Anti-TPO + | 10 (7.2%) | 2 (1.4%) | 2 (1.8%) | 0.018 |
| TRAb + | 6 (4.3%) | 4 (2.9%) | 2 (1.8%) | 0.514 |
| COVID ICU Group (n = 138) | COVID Non ICU Group (n = 138) | p (χ2 Test) | |
|---|---|---|---|
| Normal thyroid function test | 95 (68.8%) | 99 (71.7%) | 0.423 |
| Low fT3 | 18 (13.0%) | 13 (9.4%) | 0.343 |
| Isolated elevated fT4 | 12 (8.7%) | 7 (5.1%) | 0.241 |
| Subclinical thyrotoxicosis | 2 (1.4%) | 3 (2.2%) | 0.652 |
| Overt thyrotoxicosis | 6 (4.3%) | 9 (6.5%) | 0.430 |
| Subclinical hypothyroidism | 1 (0.7%) | 2 (1.4%) | 0.561 |
| Overt hypothyroidism | 3 (2.2%) | 4 (2.9%) | 0.704 |
| Isolated low fT4 | 1 (0.7%) | 1 (0.7%) | 1.000 |
| Predictor | OR | 95% CI | p-Value |
|---|---|---|---|
| Age (per 10 years) | 1.12 | 0.85–1.49 | 0.415 |
| Male sex (vs. female) | 0.94 | 0.38–2.31 | 0.889 |
| ICU (vs. non-ICU) | 0.83 | 0.33–2.07 | 0.689 |
| fT3/rT3 index (per 1) | 1.02 | 0.86–1.21 | 0.823 |
| ≥1 comorbidity (vs. none) * | 0.58 | 0.26–1.29 | 0.190 |
| Group | Predictor | Deaths with Predictor n (%) | Survivors with Predictor n (%) | Deaths Without Predictor n (%) | Survivors Without Predictor n (%) | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| ICU | NTIS | 11 (36.7) | 19 (63.3) | 29 (26.9) | 79 (73.1) | 1.58 | 0.67–3.71 |
| Any Ab + | 21 (38.2) | 34 (61.8) | 19 (22.9) | 64 (77.1) | 2.08 | 0.99–4.39 | |
| non-ICU | NTIS | 2 (10.0) | 18 (90.0) | 13 (11.1) | 104 (88.9) | 0.89 | 0.19–4.28 |
| Any Ab + | 10 (21.7) | 36 (78.3) | 5 (5.5) | 86 (94.5) | 4.78 | 1.53–14.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, E.; Małecka-Giełdowska, M.; Ciepiela, O. Non-Thyroidal Illness Syndrome and Thyroid Autoimmunity in Hospitalized COVID-19 Patients: A Retrospective Study. J. Clin. Med. 2025, 14, 6784. https://doi.org/10.3390/jcm14196784
Kozłowska E, Małecka-Giełdowska M, Ciepiela O. Non-Thyroidal Illness Syndrome and Thyroid Autoimmunity in Hospitalized COVID-19 Patients: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(19):6784. https://doi.org/10.3390/jcm14196784
Chicago/Turabian StyleKozłowska, Ewa, Milena Małecka-Giełdowska, and Olga Ciepiela. 2025. "Non-Thyroidal Illness Syndrome and Thyroid Autoimmunity in Hospitalized COVID-19 Patients: A Retrospective Study" Journal of Clinical Medicine 14, no. 19: 6784. https://doi.org/10.3390/jcm14196784
APA StyleKozłowska, E., Małecka-Giełdowska, M., & Ciepiela, O. (2025). Non-Thyroidal Illness Syndrome and Thyroid Autoimmunity in Hospitalized COVID-19 Patients: A Retrospective Study. Journal of Clinical Medicine, 14(19), 6784. https://doi.org/10.3390/jcm14196784







