Abstract
Background/Objectives: Untreated dental caries, the single most common health condition globally, is strongly associated with behavioural factors. This study examined dental status and oral health habits in child–parent and sibling pairs. Methods: We retrospectively analysed records from a dental practice in northeastern Poland, including 90 child–parent pairs and 27 sibling pairs. Dental status was assessed using the Decayed-Missing-Filled Teeth (DMFT) index, and treatment completion was measured with the Dental Treatment Index (DTI). Oral health behaviours were also evaluated. Results: Significant differences between children and parents were observed only in the mild-to-moderate caries groups (DMFT I: 27 children vs. 12 parents; DMFT II: 15 children vs. 32 parents). No differences were found in the severe caries or caries-free groups. Children had lower treatment completion than parents in the poorest care group (DTI 1: 20 children vs. 7 parents), but similar outcomes in higher DTI categories. Among siblings, differences appeared only in the DMFT I group, with no differences in treatment completion or behaviours. Conclusions: Strong similarities in extreme dental characteristics between children and parents, comparable DTI values in most groups, and consistent sibling outcomes suggest that family environment strongly influences oral health.
1. Introduction
Oral diseases are a major global health concern, with dental caries being one of the most common non-communicable diseases alongside diabetes and cardiovascular disease [1,2,3]. Socio-behavioural and environmental factors play crucial roles in disease development, accounting for approximately 40% of deaths annually in the US [2,3,4]. In response, the World Health Organization (WHO) has emphasised “Healthy Living” policies, highlighting the influence of social and community contexts on health outcomes [5,6].
Understanding how these health patterns develop requires examining their origins in family environments. Health behaviours are shaped primarily within the family. They are acquired through observation, imitation, and shared daily routines, a process often referred to as “community learning” [7,8,9]. Families provide the strongest environment for this learning because of emotional bonds, shared time, unlimited behavioural observation, and repetitive exposure [10]. These patterns are effectively “transmitted” across generations, much like genetic traits [11,12,13,14,15].
This family influence is especially visible in oral health, where caries reflects both biological and social determinants. Key factors influencing children’s oral health outcomes include socioeconomic status, parental education, family affluence, and access to preventive services [16,17,18]. Early social conditions establish oral health trajectories that persist over time, reinforcing inequalities in diet and access to treatment through structural barriers such as healthcare financing and community resources [19,20]. These persistent inequalities are driven by upstream social gradients and have been further exacerbated by global challenges such as the COVID-19 syndemic [5,6,7,21].
Beyond these structural determinants, psychological aspects of oral health also show intrafamilial transmission. Studies have found associations between parental dental anxiety and children’s dental fear, phobia symptoms, and oral health outcomes [22,23]. Poor parental knowledge has similarly been linked to higher dental anxiety in children after dental trauma [24]. Together, these findings indicate that both cognitive and emotional dimensions of oral health can be transmitted within families.
The universality of family influence on oral health, however, manifests differently across healthcare systems and cultural contexts. International research across diverse healthcare systems reveals significant cross-cultural variations in intrafamilial oral health patterns and anxiety transmission. A comprehensive six-country European study of 731 families from the Balkans and Central Europe found regional differences in the prevalence of dental anxiety among children, with children’s high anxiety levels ranging from 10.94% in Bosnia and Herzegovina to 26.67% in North Macedonia, though the correlation between parents and children remained consistent (r = 0.4, p < 0.01) [25]. Such variation suggests that healthcare system design and cultural family dynamics modify the way biological and behavioural transmission mechanisms are expressed. For example, Nordic countries provide comprehensive public dental care for children, reducing family-level disparities compared with mixed public–private systems more common in Central and Southern Europe [26,27,28]. These international comparisons underline that while family environment is a universal determinant of oral health, its impact is shaped by system- and culture-specific factors [29].
Building on this background, the aim of this study was to examine associations between family environment and oral health outcomes in children. Specifically, we compared the following variables: (1) dental status, measured by the Decayed-Missing-Filled Teeth (DMFT) index, and treatment completion, measured by the Dental Treatment Index (DTI), between children and their parents and between siblings; and (2) oral health behaviour patterns between siblings.
We tested the null hypothesis that there are no statistically significant differences in dental status and behavioural habits between children and their parents, or between siblings.
2. Materials and Methods
2.1. Study Design and Setting
We conducted a retrospective cross-sectional study using dental records collected between 2017 and 2019 from a single dental practice in northeastern Poland. The clinic serves both urban (67%) and rural (33%) populations. The practice follows standard Polish dental care protocols and serves families across diverse socioeconomic backgrounds typical of the regional population. The study was approved by the Biomedical Committee of the Medical University of Bialystok (resolution nr R-I-002/36/2019).
2.2. Study Population
From 203 paediatric patient records, we identified 90 children whose parents also had complete treatment documentation at the same practice. When both parents met the inclusion criteria, the parent with the greater number of documented visits was selected. This process yielded 90 child–parent pairs for analysis.
We also identified 27 sibling pairs who both received treatment at the clinic and had complete dental documentation. Subjects with incomplete records were excluded. Detailed inclusion and exclusion criteria, along with sample size calculation, are presented in Appendix A.
2.3. Dental Status Assessment
Dental status was evaluated using the Decayed-Missing-Filled Teeth (DMFT) index. To allow comparisons across age groups with different numbers of teeth, DMFT values were converted to percentages (see Appendix A for details). Clinical examinations were performed by a calibrated dentist following WHO criteria (see Appendix A for calibration details), under artificial lighting, using dental mirrors and probes.
Treatment completion was assessed with the Dental Treatment Index (DTI), calculated for individuals with DMFT > 0 as the ratio of filled teeth to the sum of decayed and filled teeth. A higher DTI indicates more effective treatment (see Appendix A).
2.4. Group Classification
Participants were categorised into subgroups based on caries severity (DMFT 0–IV) and treatment completion (DTI 1–4), as shown in Table 1. For sibling pairs, we also compared oral health behaviours, including toothbrush type, brushing frequency, dental visit attendance, and sweet consumption. Behavioural data were obtained from routine questionnaires completed at the first visit and updated every six months.

Table 1.
Criteria and characteristics for Decayed-Missing-Filled Teeth (DMFT) index and Dental Treatment Index (DTI) subgroups.
2.5. Statistical Analysis
Associations between categorical variables were tested using the chi-square independence test. For paired variables, McNemar’s test was applied. Analyses were performed with GraphPad Prism 9.4 software (Boston, MA, USA), and statistical significance was set at p < 0.05.
3. Results
3.1. Demographics
The subjects of the study included 90 children (53 boys, 37 girls) and their parents, most of whom were mothers (Figure 1A). We also identified 27 sibling pairs. Figure 1B shows the urban-rural distribution of participants, while Figure 1C presents the age distributions for children and parents (children: 9.3 ± 0.4 years; parents: 36.6 ± 5.7 years, mean ± SEM, where SEM = standard error of the mean). No significant age differences were observed between siblings (Figure 1D). For subgroup analysis, children were divided according to whether they were younger or older than 10 years of age (χ2 = 0.071, p = ns, OR = 0.75, 95% CI: 0.214–2.465).
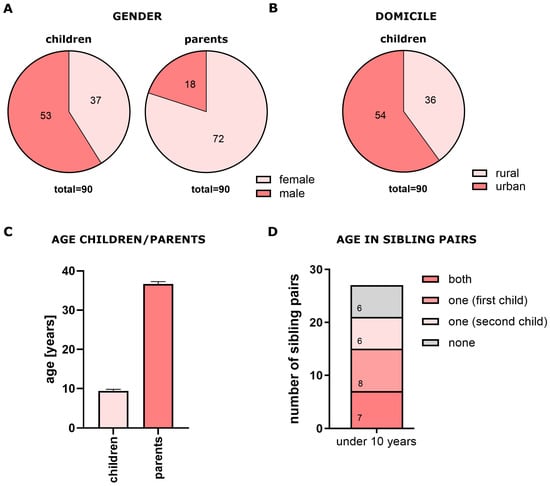
Figure 1.
Demographic characteristics of study population. (A) Sex distribution of children and parents; (B) urban–rural distribution; (C) age distribution of children and parents (mean ± SEM); (D) age distribution of siblings.
3.2. Parent–Child Comparisons
We compared dental status and treatment completion between children and their parents using paired analysis. Participants were classified into five DMFT subgroups and four DTI subgroups (Table 1).
Of the 90 child–parent pairs, 81 had DMFT > 0. No pair had both parent and child with DMFT = 0, though this difference was not statistically significant.
DMFT Analysis: Significant differences between parents and children were observed only in the intermediate categories:

Table 2.
Distribution and statistical analysis of Decayed-Missing-Filled Teeth (DMFT) and Decayed Teeth Index (DTI) groups in parent–child pairs. ns = not significant. p < 0.05 (chi-square test). * denotes statistically significant difference.
Table 2.
Distribution and statistical analysis of Decayed-Missing-Filled Teeth (DMFT) and Decayed Teeth Index (DTI) groups in parent–child pairs. ns = not significant. p < 0.05 (chi-square test). * denotes statistically significant difference.
| Chi-Square | 95% Confidence | ||||
|---|---|---|---|---|---|
| Decayed-Missing-Filled Teeth | Value | p-Value | Odds Ratio | Lower | Upper |
| DMFT 0 | 1.78 | p = ns | 0.29 | 0.03 | 1.50 |
| DMFT I | 5.03 | * p < 0.05 | 0.44 | 0.21 | 0.91 |
| DMFT II | 5.45 | * p < 0.05 | 2.13 | 1.12 | 4.24 |
| DMFT III | 1.44 | p = ns | 1.62 | 0.77 | 3.51 |
| DMFT IV | 0.75 | p = ns | 0.50 | 0.11 | 1.87 |
| Dental Treatment Index | |||||
| DTI 1 | 5.33 | * p < 0.05 | 0.35 | 0.13 | 0.86 |
| DTI 2 | 0.00 | p = ns | 0.94 | 0.43 | 2.03 |
| DTI 3 | 1.63 | p = ns | 0.58 | 0.25 | 1.28 |
| DTI 4 | 1.24 | p = ns | 0.61 | 0.26 | 1.37 |
No differences were observed in the extreme categories (caries-free, severe, or very severe caries groups), suggesting strong parent–child concordance in these groups (Figure 2).
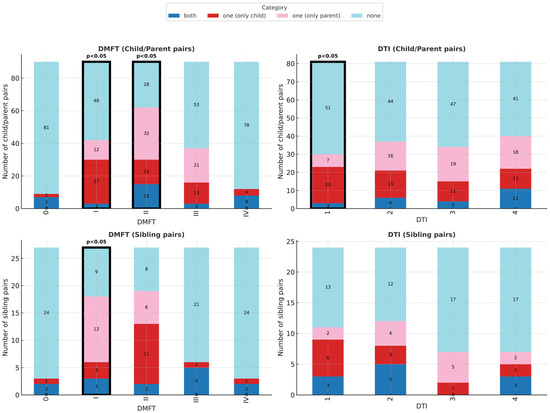
Figure 2.
Dental status and treatment outcomes in child–parent and sibling pairs. Distribution across DMFT (caries severity) and DTI (treatment completion) subgroups. Significant differences were found in DMFT I and II (child–parent pairs), DTI 1 (child–parent pairs), and DMFT I (sibling pairs).
DTI Analysis: DTI was calculated for 81 pairs (excluding seven children and two parents with DMFT = 0). Children had significantly lower treatment completion than parents only in the poorest care category (DTI 1: OR = 0.35, 95% CI: 0.13–0.86, p < 0.05; Table 3). In higher categories (DTI 2–4), outcomes were comparable (Figure 2).

Table 3.
Distribution and statistical analysis of Decayed-Missing-Filled Teeth (DMFT), Decayed Teeth Index (DTI) and oral health behaviours in sibling pairs. ns = not significant. p < 0.05 (chi-square test). * denotes statistically significant difference.
3.3. Sibling Comparisons
Analysis of sibling pairs (n = 27, limited statistical power) revealed significant differences only in the mild caries group (DMFT I: OR = 4.0, 95% CI: 1.08–22.09, p < 0.05; Table 3). No differences were found in treatment completion (DTI) or in oral health behaviours such as toothbrush type, brushing frequency, dental visits, or sweet consumption (Figure 3).
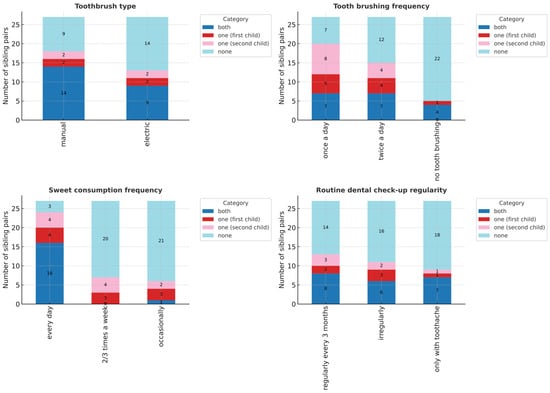
Figure 3.
Oral health behaviours in sibling pairs. Comparison of toothbrush type, brushing frequency, dental attendance, and sweet consumption. No statistically significant differences were observed.
Overall, siblings demonstrated high behavioural consistency, with individual variation appearing only in early caries development.
4. Discussion
The public health principle “health begins at home” highlights the central role of families in shaping health behaviours. Lifestyle—defined as the sum of health-promoting and health-damaging behaviours learned in childhood and carried into adulthood—is among the strongest determinants of health and wellbeing [18,30,31,32,33]. Dental caries, like other chronic diseases such as diabetes or cardiovascular disease, is largely driven by non-medical factors, with around 80% of its risk attributable to behavioural and environmental influences [3,19,21,32].
4.1. Parent–Child Relationships in Oral Health
Our findings show clear parent–child similarities in dental health. Mothers predominantly accompanied children to dental visits (Figure 1), reflecting their dominant role in modelling health behaviours [33,34]. Mothers may be best positioned to monitor disease progression and treatment management, as well as treatment guidelines and recommendations for their children. This highlights mothers as key targets for prevention and education programs.
Strong concordance appeared in extreme categories: families were either consistently caries-free or severely affected (DMFT III–IV). In contrast, mild-to-moderate categories (DMFT I–II) showed greater variation, suggesting that families are most similar when disease burden is very low or very high, but more heterogeneous in intermediate states. Differences in these groups may reflect biological susceptibility or parental underestimation of early disease in children. Strong concordance in extreme categories (caries-free and severe disease) reflects consistent family behavioural patterns—families with excellent hygiene maintain health across members, while families with poor habits show universal disease burden.
4.2. Quality Disparities
The DTI analysis revealed concerning disparities in treatment quality. Group DTI 1 (poorest treatment completion) showed the most significant parent–child differences, with children receiving inferior care in 20 pairs compared to their parents (Figure 2). This is particularly troubling given that caries progresses most rapidly during childhood. Clinicians notice that most dental interventions concern teeth initially treated during childhood and adolescence (secondary or atypical caries). The superior parental dental status in this group suggests these parents benefited from better childhood dental care but failed to provide equivalent care for their children.
One possible explanation is structural. Until recently, school-based dental programs provided universal care for Polish children, but these services were discontinued. Parents may have benefited from school-based care in their youth, while today’s children must rely on less accessible clinic-based services. It could be inferred that this change in public health strategy has acted to the disadvantage of children, contributing to inferior dental status compared to their parents.
Other factors, such as limited provider comfort with young patients or scheduling difficulties, may also contribute, even though financial coverage for paediatric care is provided by the National Health Fund.
4.3. Sibling Comparisons and Family Environment
Sibling analysis, though limited by sample size, demonstrated striking behavioural concordance. Brushing habits, dental attendance, and sweet consumption were nearly identical within families (Figure 3). This supports the strong role of family environment in shaping oral health behaviours. Children observe and replicate behaviours within their family unit, particularly from parents and older siblings [35].
Despite behavioural similarities, some variation emerged in early disease expression (DMFT I). This likely reflects individual biological differences in susceptibility or parental attention. Importantly, the absence of differences in treatment completion (DTI) suggests that once caries is diagnosed, families provide similar treatment access to all children.
4.4. International Context
Our findings need to be considered in light of healthcare system differences. The Polish healthcare system presents a particularly instructive case study, representing a mixed public–private model characteristic of post-transition economies in Central Europe. In Poland, children are entitled to free dental care until age 18, but oral health accounts for only 2.7% of public healthcare spending, with state-financed oral services utilised by approximately 30% of children and adolescents and just 15% of adults [36]. This limited public coverage, combined with basic dental materials being the only state-funded options, creates substantial reliance on private care for comprehensive treatment. This leads to significant socioeconomic disparities: 92% of Polish teenagers experience caries, and rural children often lack access to public services [37].
Such healthcare infrastructure characteristics may amplify intrafamilial oral health clustering in several ways. In mixed public–private systems such as Poland’s, treatment choices may be shaped by financial considerations, intensifying parental influence on children’s oral health outcomes. This contrasts with universal public models, such as those in Nordic countries, where family-level socioeconomic differences are less likely to determine treatment access [26,27,28]. Such comparisons highlight the importance of tailoring prevention strategies to healthcare financing structures and cultural contexts.
4.5. Clinical and Public Health Implications
These findings challenge models that attribute caries solely to infectious transmission, instead emphasizing the importance of lifestyle, behaviour, and education [38,39]. Families emerge as critical units for interventions, as summarised in Table 4.

Table 4.
Summary of study findings, results, conclusions, and clinical significance.
Routine family-based screening should be considered: when one family member has extreme oral health status, other members should be assessed and appropriate preventive or therapeutic measures provided. Prevention efforts should target high-risk families where parents have poor dental status or low treatment completion, since children in these households are most vulnerable.
Public health campaigns should also raise awareness of the importance of early dental visits. Regular dental attendance from early childhood significantly impacts lifelong oral health [40]. However, while parents often attend medical appointments for young children, they may neglect dental care in early childhood [41,42]. Strengthening awareness of dentists’ role in paediatric health could improve long-term outcomes.
4.6. Study Limitations and Strengths
Limitations. Our study has several important limitations. The retrospective cross-sectional design prevents establishing causal relationships between parental dental status and children’s outcomes or determining whether shared behaviours cause similar outcomes. Significant unmeasured confounders include genetics, environmental factors (water quality, pollution), and, most importantly, socioeconomic status. Socioeconomic status is particularly important, as it influences oral health through multiple pathways: direct effects via access to preventive care, healthy foods, and quality oral hygiene products; indirect effects through health literacy, parental supervision time, and stress levels affecting health prioritisation; and environmental effects, including neighbourhood water fluoridation, local food environments, and school-based prevention programs. Our observed family concordance may largely reflect shared socioeconomic circumstances rather than direct behavioural transmission. In future research, we could stratify families by their household income to remove its confounding effect.
The single-practice design and modest sample size (90 child–parent pairs, 27 sibling pairs) limit generalisability and statistical power, especially for subgroup analyses. It also introduces selection bias, as families may share similar socioeconomic characteristics and healthcare-seeking behaviours. Families with poor oral health or irregular attendance may also be underrepresented (survival bias), potentially leading our sample to over-represent families with better oral health outcomes and more consistent healthcare utilisation.
Strengths. Despite these limitations, our study provides valuable evidence for the underestimated role of family environment in oral health outcomes through an innovative intrafamilial design that addresses a significant gap in the literature.
Strengths include the intrafamilial design, which allowed direct comparisons between related individuals, the use of objective clinical indices (DMFT, DTI), and the inclusion of both parent–child and sibling analyses. Using medical records rather than self-reports adds reliability, and the dual-relationship design offers insights into both intergenerational and household-level influences.
To our knowledge, this represents one of the first studies to conduct systematic comparative analysis of dental status among multiple family members across different age groups using standardised clinical measures. Existing literature primarily examines associations between children’s oral health and parental sociodemographic characteristics (education, residence, socioeconomic status) rather than direct intrafamilial clinical concordance. Our direct comparison approach between related individuals provides new insights into family-level oral health patterns and offers a foundation for developing family-centred prevention strategies.
4.7. Conclusions and Future Directions
Our study highlights the strong influence of family environment on oral health. Key findings include
- Selective concordance between parents and children in mild-to-moderate caries, with strong similarities in extreme categories (caries-free and severe disease).
- Treatment disparities, with children receiving less complete care than parents in families with the poorest oral health.
- Behavioural consistency among siblings, indicating powerful family-level influence, despite individual differences in early disease expression.
- Support for family-centred strategies, suggesting that prevention and screening should target entire households rather than individuals.
These results challenge traditional individual-focused care models and argue for family-based screening protocols, especially in mixed public–private healthcare systems where socioeconomic status strongly affects treatment access and quality.
Future research should expand to multi-centre samples and include socioeconomic stratification to account for confounding effects. Advanced analytical approaches, such as clustering or similarity-based methods, may help identify distinct family oral health profiles. By doing so, we can design more effective prevention strategies that address both intergenerational and household-level influences.
Author Contributions
Conceptualization, M.N., Z.B. and K.S.; Methodology, M.N., J.B. and K.S.; Software, Z.B. and J.M.H.; Validation, M.N. and Z.B.; Formal Analysis, M.N. and Z.B.; Investigation, K.S. and K.W.; Resources, M.N., Z.B. and J.B.; Data Curation, Z.B., M.N. and K.S.; Writing—Original Draft Preparation, Z.B. and M.N.; Writing—Review & Editing, Z.B. and M.N.; Translation to English, Z.B.; Visualization, Z.B. and J.M.H.; Supervision, M.N.; Project Administration, M.N.; Funding Acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by grant No. B. SUB. 25.224. from the Medical University of Bialystok, Poland (Uniwersytet Medyczny w Bialymstoku).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Bialystok, Poland; no.: R-I-002/36/2019, date of approval: 31 January 2019.
Informed Consent Statement
Patient consent was waived due to retrospective nature of the study and acquisition of appropriate approval from the Ethics Committee of the Medical University of Bialystok. Approval from the Ethics Committee was conditional upon researchers’ written commitment to maintain confidentiality and not disclose any personal data or information that could enable unauthorized access to participant data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (the data are not publicly available due to privacy or ethical restrictions).
Acknowledgments
The authors gratefully acknowledge the dental practice in northeastern Poland for providing access to patient medical records that made this research possible. We thank the dental staff for their cooperation and assistance during the data collection process. We also acknowledge the patients and families whose medical records contributed to this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DMFT | Decayed Missed Filled Teeth |
| DTI | Dental Treatment Index |
| SEM | Standard Error of the Mean |
Appendix A. Materials and Methods
Appendix A.1. Sample Size Calculation
The minimum sample size was determined based on population data from the most recent Polish oral health monitoring report [43]. Using a population proportion of 90% derived from this national survey data, the minimum required sample size was calculated to be 83. This calculation ensures adequate statistical power to detect meaningful differences in oral health outcomes within the study population while maintaining appropriate precision for the estimated proportions.
Appendix A.2. DMFT Index Conversion to Percentage
To assess the dental condition, an original method was used that enables comparison of caries experience between participants of different ages. To obtain comparable values, for each child and parent, the percentage of teeth affected by caries disease was determined, expressed as DMFT + dmft/X. X represents the number of teeth determined as follows:
- -
- In the children’s group, X was taken as the sum of the number of primary and permanent teeth present in the oral cavity plus the number of teeth extracted due to caries (MT/mt) if such extractions were performed. Primary teeth lost due to physiological tooth exfoliation, unerupted primary and permanent teeth, teeth lost due to trauma, and teeth removed for orthodontic reasons were not counted as MT/mt.
- -
- In the adult group, X was taken as the sum of the number of permanent teeth present in the oral cavity, including third molars, plus the number of teeth extracted due to caries (MT), if such extractions were performed. Unerupted teeth, teeth lost due to trauma or periodontal disease, and teeth removed for orthodontic reasons were not counted as MT.
Appendix A.3. DMFT Examiner Calibration
The examination was conducted by one researcher (KS) according to WHO recommendations and based on the criteria for clinical condition classification (Oral Health Surveys. Basic Data. WHO Geneva 1997). The researcher was trained by a member of the epidemiological team of calibrated researchers (MN) in a training and calibration process for the purpose of conducting epidemiological studies titled: “Monitoring the oral health status of the Polish population in 2016–2020” in the northeastern region of Poland [44]. Differences in the two examinations concerned only the determined number of D (decayed teeth). Inter-examiner reliability was assessed using 18 patients examined by both the principal investigator (MN) and trained researcher (KS). Cohen’s kappa coefficient for the decayed teeth component was κ = 0.923. Intra-examiner reliability was evaluated by having researcher KS re-examine the same 18 patients after 10 days, yielding κ = 0.943, indicating excellent agreement for both inter- and intra-examiner reliability.
Appendix A.4. DTI Calculation
The DTI was determined for permanent dentition using the formula F/D + F, for primary dentition: f/d + f, and for mixed dentition (F + f)/(D + d) + (F + f). Values range from 0 to 1, where 1 means that all teeth with caries were treated, while 0 means that no tooth with caries was treated. The result (being a fraction) was then converted to %. It was determined for individuals with DMFT (or dmft) > 0 [45,46]—methodological references.

Table A1.
Inclusion and exclusion criteria.
Table A1.
Inclusion and exclusion criteria.
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| General Criteria (All Participants) |
|
|
| Child–Parent Pairs |
|
|
| Sibling Pairs |
|
|
References
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31 (Suppl. S1), 3–24. [Google Scholar] [CrossRef]
- Pitts, N.; Melo, P.; Martignon, S.; Ekstrand, K.; Ismail, A. Caries risk assessment, diagnosis and synthesis in the context of a European Core Curriculum in Cariology. Eur. J. Dent. Educ. 2011, 15 (Suppl. S1), 23–31. [Google Scholar] [CrossRef]
- McGinnis, J.M.; Williams-Russo, P.; Knickman, J.R. The case for more active policy attention to health promotion. Health Aff. 2002, 21, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Cockerham, W.C. Health lifestyle theory and the convergence of agency and structure. J. Health Soc. Behav. 2005, 46, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Pampel, F.C.; Krueger, P.M.; Denney, J.T. Socioeconomic disparities in health behaviors. Annu. Rev. Sociol. 2010, 36, 349–370. [Google Scholar] [CrossRef]
- Kuntsche, E.; Ravens-Sieberer, U. Monitoring adolescent health behaviours and social determinants cross-nationally over more than a decade: Introducing the Health Behaviour in School-aged Children (HBSC) study supplement on trends. Eur. J. Public Health 2015, 25 (Suppl. S2), 1–3. [Google Scholar] [CrossRef]
- Currie, C.; Zanotti, C.; Morgan, A.; Currie, D.; De Looze, M.; Roberts, C.; Samdal, O.; Smith, O.R.; Barnekow, V. (Eds.) Social determinants of health and well-being among young people. In Health Behaviour in School-Aged Children (HBSC) Study: International Report from the 2009/2010 Survey; WHO Regional Office for Europe: Copenhagen, Denmark, 2012. [Google Scholar]
- Currie, C.; Nic Gabhainn, S.; Godeau, E.; Roberts, C.; Smith, R.; Currie, D.; Pickett, W.; Richter, M.; Morgan, A.; Barnekow, V. (Eds.) Inequalities in Young People’s Health: HBSC International Report from the 2005/06 Survey; WHO Regional Office for Europe: Copenhagen, Denmark, 2008. [Google Scholar]
- Brooks, F.; Zaborskis, A.; Tabak, I.; Alcón, M.d.C.G.; Zemaitiene, N.; de Roos, S.; Klemera, E. Trends in adolescents’ perceived parental communication across 32 countries in Europe and North America from 2002 to 2010. Eur. J. Public Health 2015, 25 (Suppl. S2), 46–50. [Google Scholar] [CrossRef]
- Turagabeci, A.R.; Nakamura, K.; Kizuki, M.; Takano, T. Family structure and health, how companionship acts as a buffer against ill health. Health Qual. Life Outcomes 2007, 5, 61. [Google Scholar] [CrossRef]
- Chen, M.Y.; Shio, Y.C.; Gau, Y.M. Comparison of adolescent health-related behavior in different family structures. J. Nurs. Res. 2007, 15, 1–10. [Google Scholar] [CrossRef]
- Kitzman-Ulrich, H.; Wilson, D.K.; George, S.M.S.; Lawman, H.; Segal, M.; Fairchild, A. The integration of a family system approach for understanding youth obesity, physical activity, and dietary programs. Clin. Child Fam. Psychol. Rev. 2010, 13, 231–253. [Google Scholar] [CrossRef]
- Farrell, M.P.; Barnes, G.M.; Banerjee, S. Family cohesion as a buffer against the effects of problem-drinking fathers on psychological distress, deviant behavior and heavy drinking in adolescents. J. Health Soc. Behav. 1995, 36, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Coonrod, D.V.; Balcazar, H.; Brady, J.; Garcia, S.; Van Tine, M. Smoking, acculturation and family cohesion in Mexican-American women. Ethn. Dis. 1999, 9, 434–440. [Google Scholar] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Pförtner, T.; Moor, I.; Rathmann, K.; Hublet, A.; Molcho, M.; Kunst, A.E.; Richter, M. The association between family affluence and smoking among 15-year-old adolescents in 33 European countries, Israel and Canada: The role of national wealth. Addiction 2015, 110, 162–173. [Google Scholar] [CrossRef]
- Franko, D.L.; Thompson, D.; Affenito, S.G.; Barton, B.A.; Striegel-Moore, R.H. What mediates the relationship between family meals and adolescents health issues. Health Psychol. 2008, 27, 109–117. [Google Scholar] [CrossRef]
- Kliewer, W.; Murrelle, L.; Prom, E.; Ramirez, M.; Obando, P.; Sandi, L.; Karenkeris, M.D.C. Violence exposure and drug use in Central American youth: Family cohesion and parental monitoring as protective factors. J. Res. Adolesc. 2006, 16, 455–478. [Google Scholar] [CrossRef]
- Guido, J.A.; Martinez Mier, E.A.; Soto, A.; Eggertsson, H.; Sanders, B.J.; Jones, J.E.; Weddell, J.A.; Villanueva Cruz, I.; Anton de la Concha, J.L. Caries prevalence and its association with brushing habits, water availability, and the intake of sugared beverages. Int. J. Pediatr. Dent. 2011, 21, 432–440. [Google Scholar] [CrossRef]
- Kalavana, T.; Lazarou, C.; Christodoulou, C. Family environment in relations to eating and health risk behaviors in adolescents. Med. Health Sci. J. 2011, 7, 15–25. [Google Scholar]
- Tsakos, G.; Watt, R.G.; Guarnizo-Herreño, C.C. Reflections on oral health inequalities: Theories, pathways and next steps for research priorities. Community Dent. Oral Epidemiol. 2023, 51, 17–27. [Google Scholar] [CrossRef]
- Petrović, D.; Cicvarić, O.; Šimunović-Erpušina, M.; Ivančić Jokić, N.; Bakarčić, D.; Bučević Sojčić, P.; Jurić, H. The Role of Family Factors in the Development of Dental Anxiety in Children. Medicina 2024, 60, 180. [Google Scholar] [CrossRef]
- Samal, A.; Menon, I.; Jha, K.; Kumar, G.; Singh, A.; Dash, K.S.; Saxsena, A. Influence of Parental Dental Anxiety on Children’s Oral Health Status—A Cross Sectional Survey. J. Health Sci. Med. Res. 2024, 43, 20241134. [Google Scholar] [CrossRef]
- Kvesić, A.J.; Hrelja, M.; Lovrić, Ž.; Šimunović, L.; Špiljak, B.; Supina, N.; Vranić, L.; Vranić, D.N. Possible Risk Factors for Dental Fear and Anxiety in Children Who Suffered Traumatic Dental Injury. Dent. J. 2023, 11, 190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šimunović, L.; Špiljak, B.; Radulović, M.; Vlahovljak, A.; Ostojić, M.; Krlev, J.; Ibrahimpašić, A.; Vranić, L.; Negovetić Vranić, D. Relationship between Children’s and Parents’ Dental Anxiety: A Cross-Sectional Study on the Six European Countries. Dent. J. 2022, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Hugoson, A.; Koch, G.; Helkimo, A.N.; Lundin, S.A. Caries prevalence and distribution in individuals aged 3–20 years in Jönköping, Sweden, over a 30-year period (1973–2003). Int. J. Paediatr. Dent. 2008, 18, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Holst, D.; Schuller, A.A. Oral health in a life-course: Birth-cohorts from 1929 to 2006 in Denmark and the Netherlands. Community Dent. Oral Epidemiol. 2012, 40, 352–358. [Google Scholar]
- Grytten, J.; Holst, D.; Laake, P. Accessibility of dental services according to family income in a non-insured population. Soc. Sci. Med. 1990, 31, 327–330. [Google Scholar] [CrossRef]
- Pine, C.M.; Adair, P.M.; Nicoll, A.D.; Burnside, G.; Petersen, P.E.; Beighton, D.; Gillett, A.; Anderson, R.; Anwar, S.; Brailsford, S.; et al. International comparisons of health inequalities in childhood dental caries. Community Dent. Health 2004, 21 (Suppl. S1), 121–130. [Google Scholar]
- Scherer, M.; Worthington, E.L.; Hook, J.N.; Campana, K.L.; West, S.L.; Gartner, A.L. Forgiveness and cohesion in familiar perceptions of alcohol misuse. J. Couns. Dev. 2012, 90, 160–168. [Google Scholar] [CrossRef]
- Maltz, M.; Koppe, B.; Jardim, J.J.; Alves, L.S.; de Paula, L.M.; Yamaguti, P.M.; Almeida, J.C.F.; Moura, M.S.; Mestrinho, H.D. Partial caries removal in deep caries lesions: A 5-year multicenter randomized controlled trial. Clin. Oral Investig. 2018, 22, 1337–1343. [Google Scholar] [CrossRef]
- Petersen, P.E. Socio-behavioural risk factors in dental caries—An international perspective. Community Dent. Oral Epidemiol. 2005, 52, 1–9. [Google Scholar]
- Vandeleur, C.L.; Jeanpretre, N.; Perrez, M.; Schoebi, D. Cohesion, satisfaction with family bonds and emotional well-being in families with adolescents. J. Marriage Fam. 2009, 71, 1205–1219. [Google Scholar] [CrossRef]
- Łaguna, W.; Bagińska, J.; Oniśko, A. Bayesian network modelling in discovering risk factors of dental caries in tree-year-old children. Prog. Health Sci. 2019, 9, 118–125. [Google Scholar] [CrossRef]
- Waligóra, J.; Michalak, E.; Pytko-Polończyk, J. Reasons for dental visits of children and their parents at the dental clinics in Cracow. Prz. Epidemiol. 2021, 75, 413–423. [Google Scholar] [CrossRef]
- Malkiewicz, K.; Malkiewicz, E.; Eaton, K.A.; Widström, E. The healthcare system and the provision of oral healthcare in European Union Member States. Part 6: Poland. Br. Dent. J. 2016, 221, 501–507. [Google Scholar] [CrossRef]
- Najwyższa Izba Kontroli [Supreme Audit Office] (nik.gov.pl). NIK on Dental Care in Poland—Supreme Audit Office. 2013. Available online: https://www.nik.gov.pl/en/news/nik-on-dental-care-in-poland.html (accessed on 4 September 2025).
- World Health Organization. WHO Expert Consultation on Public Health Intervention Against Early Childhood Caries: Report of a Meeting, Bangkok, Thailand, 26–28 January 2016; WHO/NMH/PND/17.1. 2017. Available online: https://www.who.int/publications/i/item/who-expert-consultation-on-public-health-intervention-against-early-childhood-caries (accessed on 4 September 2025).
- Phantumvanit, P.; Makino, Y.; Ogawa, H.; Rugg-Gunn, A.; Moynihan, P.; Petersen, P.E.; Evans, W.; Feldens, C.A.; Lo, E.; Khoshnevisan, M.H.; et al. WHO Global Consultation on Public Health Intervention against Early Childhood Caries. Community Dent. Oral Epidemiol. 2018, 46, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Droogers, M.; Jansen, D.; Lindert, J.; Saboga-Nunes, L.; Rudén, M.; Guichardon, M.; Zeegers, D. Pagetc. Health-related Sustainable Development Goals: Countdown on alcohol use, smoking prevalence, child overweight and suicide mortality. Eur. J. Public Health 2020, 30 (Suppl. S1), i10–i13. [Google Scholar] [CrossRef] [PubMed]
- William, N.J.; Whittle, J.G.; Gatrell, A.C. The relationship between socio-demographic characteristics and dental knowledge and attitudes of parents with young children. Br. Dent. J. 2002, 193, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Vallejos-Sanchez, A.A.; Medina-Solis, C.E.; Maupome, G.; Casanova-Rosado, J.F.; Pontigo-Loyola, A.P. Socio-behavioral factors influencing tooth brushing frequency among schoolchildren. J. Am. Dent. Assoc. 2008, 139, 743–749. [Google Scholar] [CrossRef]
- Olczak-Kowalczyk, D. Choroba Próchnicowa i Stan Tkanek Przyzębia Populacji Polskiej: Podsumowanie Wyników Badań z Lat 2016–2019 (Poprawione v15012021) [Caries Disease and Periodontal Tissue Status of the Polish Population: Summary of Study Results from 2016–2019 (Revised v15012021)]. Ministry of Health: Singapore, 2021. Available online: https://www.gov.pl/web/zdrowie/zdrowie-jamy-ustnej (accessed on 4 September 2025).
- Ministerstwo Zdrowia [Ministry of Health]. Monitorowanie Stanu Zdrowia Jamy Ustnej Populacji Polskiej na Lata 2016–2020. Monitorowanie stanu Zdrowia Jamy Ustnej Populacji Polskiej na Lata 2016–2020—Ministerstwo Zdrowia—Portal Gov.pl. 2018. Available online: https://www.gov.pl/web/zdrowie/monitorowanie-stanu-zdrowia-jamy-ustnej-populacji-polskiej-w-latach-2016-2020 (accessed on 4 September 2025).
- Hajto-Bryk, J.; Dobroś, K.; Zarzecka, J. Dental anxiety level and dental status in 18-year-old patients in Poland. J. Stomatol. 2015, 68, 669–680. [Google Scholar]
- Broniarek-Machnik, M.; Colonna-Walewska, M.; Płuciennik-Stronias, M.; Bołtacz-Rzepkowska, E. Evaluation of dental status and effectiveness of treatment among children and adolescents in the reference groups aged 6, 12, and 18 years from Skierniewice and its region in 2017–2020. Prz. Epidemiol. 2021, 75, 119–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).