Intra- and Inter-Rater Reproducibility of Measures of Physical Performance in Patients with COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
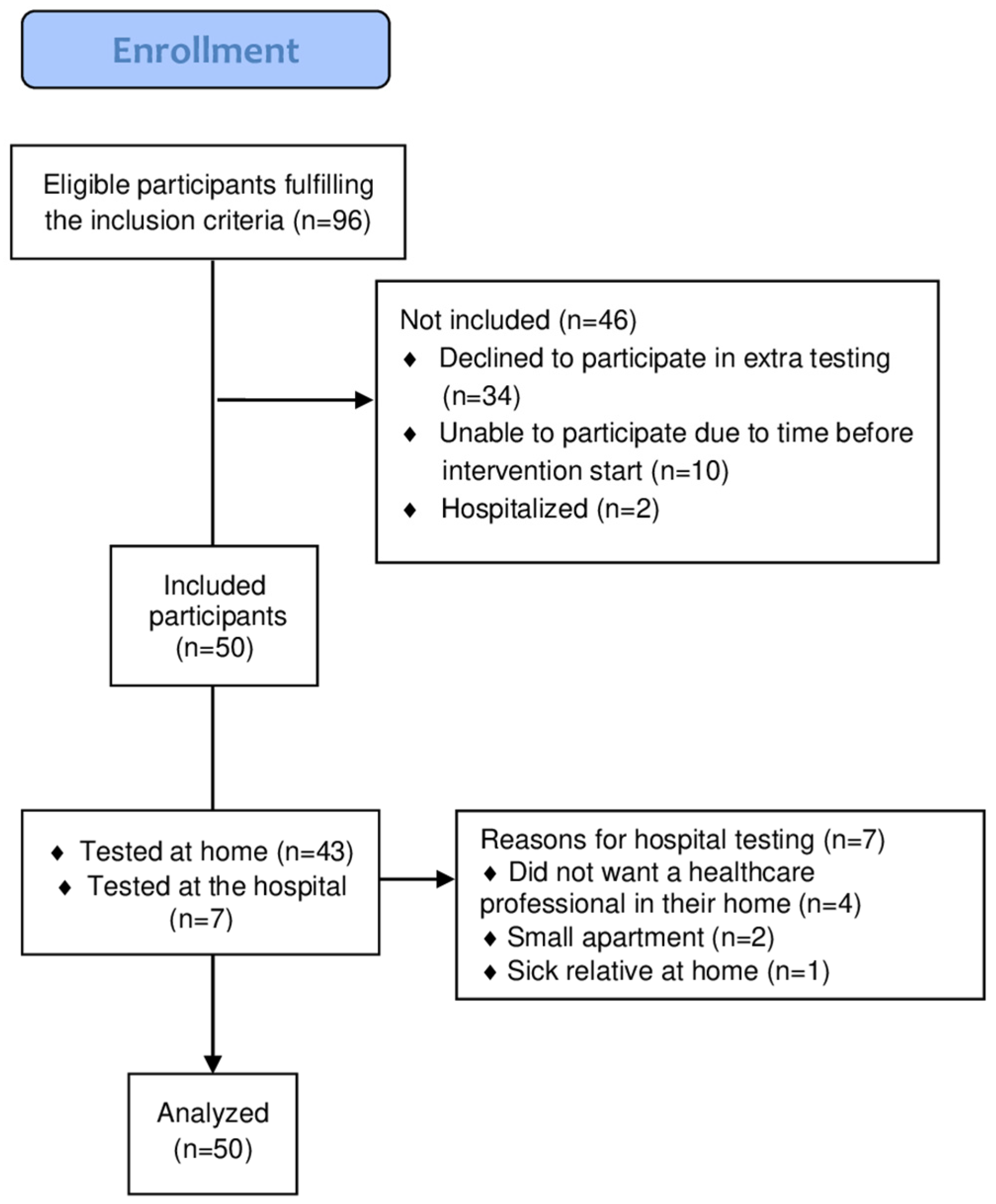
2.2. Participants
2.3. Study Settings and Raters
2.4. Test Procedures
2.5. Other Variables
2.6. Statistical Analyses
3. Results
3.1. Participants Characteristics
3.2. Intra-Rater Reproducibility for 1MSTS
3.3. Inter-Rater Reproducibility
4. Discussion
4.1. MSTS Intra-Rater and Inter-Rater Reliability
4.2. Handgrip Strength
4.3. SPPB—Inter-Rater Reliability
4.4. Strengths
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mccarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2015, CD003793. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; ZuWallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining modern pulmonary rehabilitation: An official American thoracic society workshop report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef]
- Diaba-Nuhoho, P.; Amponsah-Offeh, M. Reproducibility and research integrity: The role of scientists and institutions. BMC Res. Notes 2021, 14, 451. [Google Scholar] [CrossRef]
- Munafò, M.R.; Nosek, B.A.; Bishop, D.V.M.; Button, K.S.; Chambers, C.D.; Sert, P.D.; Simonsohn, U.; Wagenmakers, E.-J.; Ware, J.J.; Ioannidis, J.P.A. A manifesto for reproducible science. Nat. Hum. Behav. 2017, 1, 0021. [Google Scholar] [CrossRef]
- Kottner, J.; Audige, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. Int. J. Nurs. Stud. 2011, 48, 661–671. [Google Scholar] [CrossRef]
- Labadessa, I.G.; Arcuri, J.F.; Sentanin, A.C.; da Costa, J.N.F.; Pessoa, B.V.; Di Lorenzo, V.A.P. Should the 6-minute walk test be compared when conducted by 2 different assessors in subjects with COPD? Respir. Care 2016, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Reychler, G.; Boucard, E.; Peran, L.; Pichon, R.; Le Ber-Moy, C.; Ouksel, H.; Liistro, G.; Chambellan, A.; Beaumont, M. One minute sit-to-stand test is an alternative to 6MWT to measure functional exercise performance in COPD patients. Clin. Respir. J. 2018, 12, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Crouch, R. 1-Minute Sit-To-Stand Test: Systematic review of procedures, performance, and clinimetric properties. J. Cardiopulm. Rehabil. Prev. 2019, 39, 2–8. [Google Scholar] [CrossRef]
- Larrateguy, S.; Otto-Yáñez, M.; Bogado, J.; Larrateguy, L.; Barros-Poblete, M.; Mazzucco, G.; Blanco, I.; Gimeno-Santos, E.; Torres-Castro, R. Agreement and Reliability between Tele-assessment and in-person assessment of the One-minute sit-to-stand test in patients with Chronic Respiratory Diseases. J. Clin. Med. 2025, 14, 5049. [Google Scholar] [CrossRef]
- Rehman, S.T.; Qureshi, M.A.K.; Qureshi, M.A. Correlation of Sit to Stand Test with Six Minute Walk Test in Chronic Obstructive Pulmonary Disease Patients. Int. J. Med. Res. Health Sci. 2019, 8, 86–91. Available online: https://www.ijmrhs.com/medical-research/correlation-of-sit-to-stand-test-with-six-minute-walk-test-in-chronic-obstructive-pulmonary-disease-patients.pdf (accessed on 8 December 2024).
- Ozalevli, S.; Ozden, A.; Itil, O.; Akkoclu, A. Comparison of the Sit-to-Stand Test with 6 min walk test in patients with chronic obstructive pulmonary disease. Respir. Med. 2007, 101, 286–293. [Google Scholar] [CrossRef]
- Mellaerts, P.; Demeyer, H.; Blondeel, A.; Vanhoutte, T.; Breuls, S.; Wuyts, M.; Coosemans, I.; Claes, L.; Vandenbergh, N.; Beckers, K. The one-minute sit-to-stand test: A practical tool for assessing functional exercise capacity in patients with COPD in routine clinical practice. Chron. Respir. Dis. 2024, 21, 14799731241291530. [Google Scholar] [CrossRef]
- Crook, S.; Büsching, G.; Schultz, K.; Lehbert, N.; Jelusic, D.; Keusch, S.; Wittmann, M.; Schuler, M.; Radtke, T.; Frey, M.; et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur. Respir. J. 2017, 49, 1601871. [Google Scholar] [CrossRef]
- Holden, M.; Fyfe, M.; Poulin, C.; Bethune, B.; Church, C.; Hepburn, P.; Afreixo, V.; Brooks, D.; Oliveira, A. Handgrip Strength in People With Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab057. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kang, H.K.; Song, P.; Park, H.K.; Jung, H.; Lee, S.S.; Koo, H.-K. Hand grip strength in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, C.; Savva, C.; Korakakis, V.; Matheou, I.; Adamide, T.; Georgiou, A.; Xanthos, T. Test–Retest Reliability of Handgrip Strength in Patients with Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mirapeix, F.; Bernabeu-Mora, R.; Llamazares-Herrán, E.; Sánchez-Martínez, M.P.; García-Vidal, J.A.; Escolar-Reina, P. Interobserver Reliability of Peripheral Muscle Strength Tests and Short Physical Performance Battery in Patients with Chronic Obstructive Pulmonary Disease: A Prospective Observational Study. Arch. Phys. Med. Rehabil. 2016, 97, 2002–2005. [Google Scholar] [CrossRef]
- Osadnik, C.R.; Brighton, L.J.; Burtin, C.; Cesari, M.; Lahousse, L.; Man, W.D.C.; Marengoni, A.; Sajnic, A.; Singer, J.P.; Beek, L.T.; et al. European Respiratory Society statement on frailty in adults with chronic lung disease. Int. J. COPD 2023, 62, 1247–1256. [Google Scholar] [CrossRef]
- Maddocks, M.; Brighton, L.J.; Alison, J.A.; ter Beek, L.; Bhatt, S.P.; Brummel, N.E.; Burtin, C.; Cesari, M.; Evans, R.A.; Ferrante, L.E.; et al. Rehabilitation for People with Respiratory Disease and Frailty: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2023, 20, 767–780. [Google Scholar] [CrossRef]
- Nielsen, C.I.; Godtfredsen, N.; Molsted, S.; Ulrik, C.; Kallemose, T.; Hansen, H. Supervised pulmonary tele-rehabilitation and individualized home-based pulmonary rehabilitation for patients with COPD, unable to participate in center-based programs. The protocol for a multicenter randomized controlled trial-the REPORT study. PLoS ONE 2025, 20, e0312742. Available online: https://pubmed.ncbi.nlm.nih.gov/39774509/ (accessed on 12 May 2025). [CrossRef]
- Mokkink, L.B.; de Vet, H.C.W.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1171. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.E.; Li, M.A. A guideline of selecting and reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- de Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength. Cond. Res. 2005, 19, 231–240. Available online: https://pubmed.ncbi.nlm.nih.gov/15705040/ (accessed on 12 May 2025). [PubMed]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef]
- Thu, H.N.T.; Khac BLe Poncin, W. Reliability of the 1-minute sit-to-stand test in chronic obstructive pulmonary disease. Ann. Phys. Rehabil. Med. 2024, 67, 10–13. [Google Scholar] [CrossRef]
- Laursen, J.; Christensen, A.; Egsgaard, S.; Søndergaard, K.; Mechlenburg, I.; Brincks, J. A study of the reliability and construct validity of the 1-minute sit-to-stand test for individuals with systemic sclerosis. Physiother. Theory Pract. 2024, 41, 836–843. [Google Scholar] [CrossRef]
- Tremblay Labrecque, P.F.; Harvey, J.; Nadreau, É.; Maltais, F.; Dion, G.; Saey, D. Validation and Cardiorespiratory Response of the 1-Min Sit-to-Stand Test in Interstitial Lung Disease. Med. Sci. Sports Exerc. 2020, 52, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, J.; Meng, S.; Li, J.; Yu, Y.; Zhang, T.; Tsang, R.C.C.; El-Ansary, D.; Han, J.; Jones, A.Y.M. Reliability and validity of sit-to-stand test protocols in patients with coronary artery disease. Front. Cardiovasc. Med. 2022, 9, 841453. [Google Scholar] [CrossRef]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef]
- Stoffels, A.A.; Brandt, J.D.; Meys, R.; Hees, H.W.V.; Vaes, A.W.; Klijn, P.; Burtin, C.; Franssen, F.M.; Borst, B.V.B.; Sillen, M.J.; et al. Short Physical Performance Battery: Response to Pulmonary Rehabilitation and Minimal Important Difference Esti-mates in Patients With Chronic Obstructive Pulmonary Disease. Arch. Phys. Med. Rehabil. 2021, 102, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- de Villar, L.O.P.; Martínez-Olmos, F.J.; Junqué-Jiménez, A.; Amer-Cuenca, J.J.; Martínez-Gramage, J.; Mercer, T.; Segura-Ortí, E. Test-retest reliability and minimal detectable change scores for the short physical performance battery, one-legged standing test and timed up and go test in patients undergoing hemodialysis. PLoS ONE 2018, 13, e0201035. [Google Scholar]

| 1 | Participant history—only obtained at baseline test. Introduction to the tests, performed while seated. Measurement seated: resting blood pressure, resting heart rate, resting SpO2, resting dyspnea (10 min). |
| 2 | Introduction and performing: 1MSTS. Measurement seated: end-heart rate, end-SpO2, end-dyspnea (10 min). |
| 3 | Introduction and performing: HGS (10 min). |
| 4 | Seated rest while completing questionnaires (CAT, HADS, EQ5D-3L) (15 min). |
| 5 | Seated: resting blood pressure, resting heart rate, resting SpO2, resting dyspnea (5 min). |
| 6 | Introduction and performing: 1MSTS. Measurement seated: end-heart rate, end-SpO2, end-dyspnea (5 min). |
| 7 | Seated rest while completing questionnaires (PSQI, MFI, BPI) (15 min). |
| 8 | Introduction and performing: SPPB (10 min). |
| 9 | Assessment session completed: total time approximately 75–90 min. |
| Variables | Patients Included in Reproducibility Study | Patients NOT Included in Reproducibility Study |
|---|---|---|
| Sex, male/female (n) | 21/29 (50) | 15/31 (46) |
| Age, years, mean (SD) | 71.3 (±7.7) | 70.0 (±8.9) |
| Body mass index, kg/m2, mean (SD) | 25.9 (±5.9) | 25.4 (±6.1) |
| FEV1, % predicted, mean (SD) | 37.4 (±14.1) | 34.5 (±12.0) |
| FEV1/FVC, mean (SD) | 43.5 (±10.8) | 41.9 (±10.3) |
| GOLD I/II/III/IV, % | 0/22/40/38 | 0/15/46/39 |
| A/B/C/D, % | 0/50/2/48 | 0/28/11/61 |
| MRC dyspnea scale, median (range) | 4.0 (2–5) | 4.0 (2–5) |
| CAT symptoms, mean (SD) | 19.4 (±6.5) | 21.3 (±5.8) |
| BODS index points, mean (SD) | 5.2 (±2.1) | 5.8 (±1.7) |
| Charlson index, 0/1/2 ≥ 3, % | 2/30/42/26 | 0/54/17/29 |
| LTOT, n (%) | 6 (12) | 9 (20) |
| Walking aid, stick/walker n (%) | 23 (46) | 23 (50) |
| Walking aid during SPPB gait test, n (%) | 5 (22) | 4 (17) |
| Highest 1MSTS, (SD) | 15.2 (7.6) | 12.7 (8.6) |
| Highest HGS, (SD) | 26.7 (10.1) | 24.8 (8.9) |
| Highest SPPB point, (SD) | 8.6 (2.5) | 8.3 (2.8) |
| Variables | Test One | Test Two | Difference | Floor Effect (n) (%) | ICCI.I (LL95) | SEM (SEM%) | SEM95 (SEM95%) | SRD (SRD%) | SRD95 (SRD95%) |
|---|---|---|---|---|---|---|---|---|---|
| 1MSTS | 14.4 (± 7.5) | 14.3 (±7.6) | −0.14 [−0.84; 0.56] | 4 (8) | 0.95 (0.91) | 1.7 (12) | 3.3 (23) | 2.4 (17) | 4.7 (33) |
| End SpO2, (after STS) | 88.9 (±13.8) | 89.7 (±14.0) | 0.77 [0.06; 1.48] * | NA | 0.98 (0.97) | 1.9 (2) | 3.7 (4) | 2.7 (3) | 5.3 (6) |
| End HR (after STS) | 98.6 (±22.1) | 99.5 (±22.4) | 0.88 [−1.72; 3.39] | NA | 0.92 (0.87) | 6.1 (6) | 12.0 (12) | 8.6 (8) | 16.7 (17) |
| End dyspnea score (after STS) | 4.9 (±2.0) | 5.2 (±2.0) | 0.27 [−0.07; 0.61] | NA | 0.83 (0.72) | 0.8 (16) | 1.5 (30) | 1.1 (22) | 2.2 (44) |
| Variables | Rater T1 | Rater T2 | Difference | Floor (n) (%) | ICCI.I (LL95) | SEM (SEM%) | SEM95 (SEM95%) | SRD (SRD%) | SRD95% (SRD95%) |
|---|---|---|---|---|---|---|---|---|---|
| 1MSTS | 15.2 (±7.8) | 15.7 (±8.9) | 0.48 [−2.87; 3.83] | 4 (8) | 0.91 (0.84) | 2.52 (16) | 4.9 (31) | 3.5 (22) | 6.9 (44) |
| End SpO2, (after STS) | 89.4 (±13.8) | 87.3 (±19.2) | −2.1 [−8.89; 4.64] | NA | 0.66 (0.46) | 9.6 (11) | 18.8 (21) | 13.6 (15) | 26.6 (30) |
| End HR (after STS) | 99.5 (±22.4) | 98.6 (±22.5) | −0.39 [−10.70; 9.90] | NA | 0.63 (0.43) | 10.1 (10) | 19.8 (20) | 14.3 (14) | 12.3 (12) |
| End dyspnea score (after STS) | 5.1 (±2.0) | 4.9 (±2.5) | −0.28 [−1.22; 0.65] | NA | 0.63 (0.42) | 1.4 (28) | 2.7 (54) | 1.9 (38) | 3.9 (78) |
| Highest HGS, dominant hand, kg. | 26.7 (±10.1) | 29.3 (±11.0) | 2.5 [−1.67; 6.71] | NA | 0.84 (0.74) | 4.2 (15) | 8.2 (29) | 5.9 (21) | 11.6 (41) |
| SPPB, total score | 8.6 (±2.5) | 8.9 (±2.6) | 0.28 [−0.72; 1.28] | NA | 0.86 (0.77) | 0.9 (10) | 1.8 (20) | 1.3 (15) | 2.5 (29) |
| SPPB balance, score | 3.48 (±0.9) | 3.46 (±0.9) | 0.02 [−0.39; 0.35] | NA | 0.66 (0.47) | 0.5 (14) | 0.9 (26) | 0.7 (20) | 1.4 (40) |
| SPPB 3-m gait, score | 2.6 (±0.9) | 2.8 (±0.9) | 0.20 [−0.16; 0.56] | NA | 0.79 (0.65) | 0.4 (15) | 0.8 (30) | 0.6 (23) | 1.2 (45) |
| SPPB 5-times-STS, score | 2.6 (±1.5) | 2.7 (±1.5) | 0.10 [−0.49; 0.69] | 8 (16) | 0.84 (0.74) | 0.6 (23) | 1.1 (41) | 0.8 (30) | 1.5 (56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, C.; Godtfredsen, N.; Molsted, S.; Ulrik, C.; Hansen, H. Intra- and Inter-Rater Reproducibility of Measures of Physical Performance in Patients with COPD. J. Clin. Med. 2025, 14, 6755. https://doi.org/10.3390/jcm14196755
Nielsen C, Godtfredsen N, Molsted S, Ulrik C, Hansen H. Intra- and Inter-Rater Reproducibility of Measures of Physical Performance in Patients with COPD. Journal of Clinical Medicine. 2025; 14(19):6755. https://doi.org/10.3390/jcm14196755
Chicago/Turabian StyleNielsen, Christina, Nina Godtfredsen, Stig Molsted, Charlotte Ulrik, and Henrik Hansen. 2025. "Intra- and Inter-Rater Reproducibility of Measures of Physical Performance in Patients with COPD" Journal of Clinical Medicine 14, no. 19: 6755. https://doi.org/10.3390/jcm14196755
APA StyleNielsen, C., Godtfredsen, N., Molsted, S., Ulrik, C., & Hansen, H. (2025). Intra- and Inter-Rater Reproducibility of Measures of Physical Performance in Patients with COPD. Journal of Clinical Medicine, 14(19), 6755. https://doi.org/10.3390/jcm14196755







