Critical Care and Surgical Management of Vascular Complications in Minimally Invasive Urological Reconstructive Surgery
Abstract
1. Introduction
- (a)
- Access to the sacral promontory: a peritoneal incision is made over the promontory, followed by dissection of the pelvic spaces, including the vesicovaginal and rectovaginal compartments, as shown in Figure 1.
- (b)
- Mesh placement: polypropylene mesh is sutured to the anterior and posterior vaginal walls and vaginal dome.
- (c)
- Mesh fixation: the mesh is elevated and redirected toward the sacral promontory.
- (d)
- Peritoneal closure: the mesh is covered to ensure its integration into the retroperitoneal space.

2. Materials and Methods
3. Results
| Study | Design | N | Surgical Approach | Vascular Injury (n) | Transfusion Rate (n) | Bowel Injury (n) | Ureteral Injury (n) | Bladder Injury (n) |
|---|---|---|---|---|---|---|---|---|
| Nosti et al. [2] | R | 535 | RSC and LSC | 1 | 0 | 4 | 0 | 10 |
| Siddiqui et al. [7] | R | 125 | RSC | 1 | 1 | 0 | NA | 2 |
| Anger JT et al. [8] | RCT | 78 | RSC and LSC | 2 | NA | 2 | NA | NA |
| Unger et al. [9] | R | 370 | RSC and LSC | 3 | 2 | 6 | 0 | 4 |
| Gutzeit, O. et al. [10] | CR | 1 | LSC | 1 | 0 | NA | NA | NA |
| Zhao and Martin [13] | R | 47 | RSC | 0 | 1 | 0 | 1 | 2 |
4. Discussion
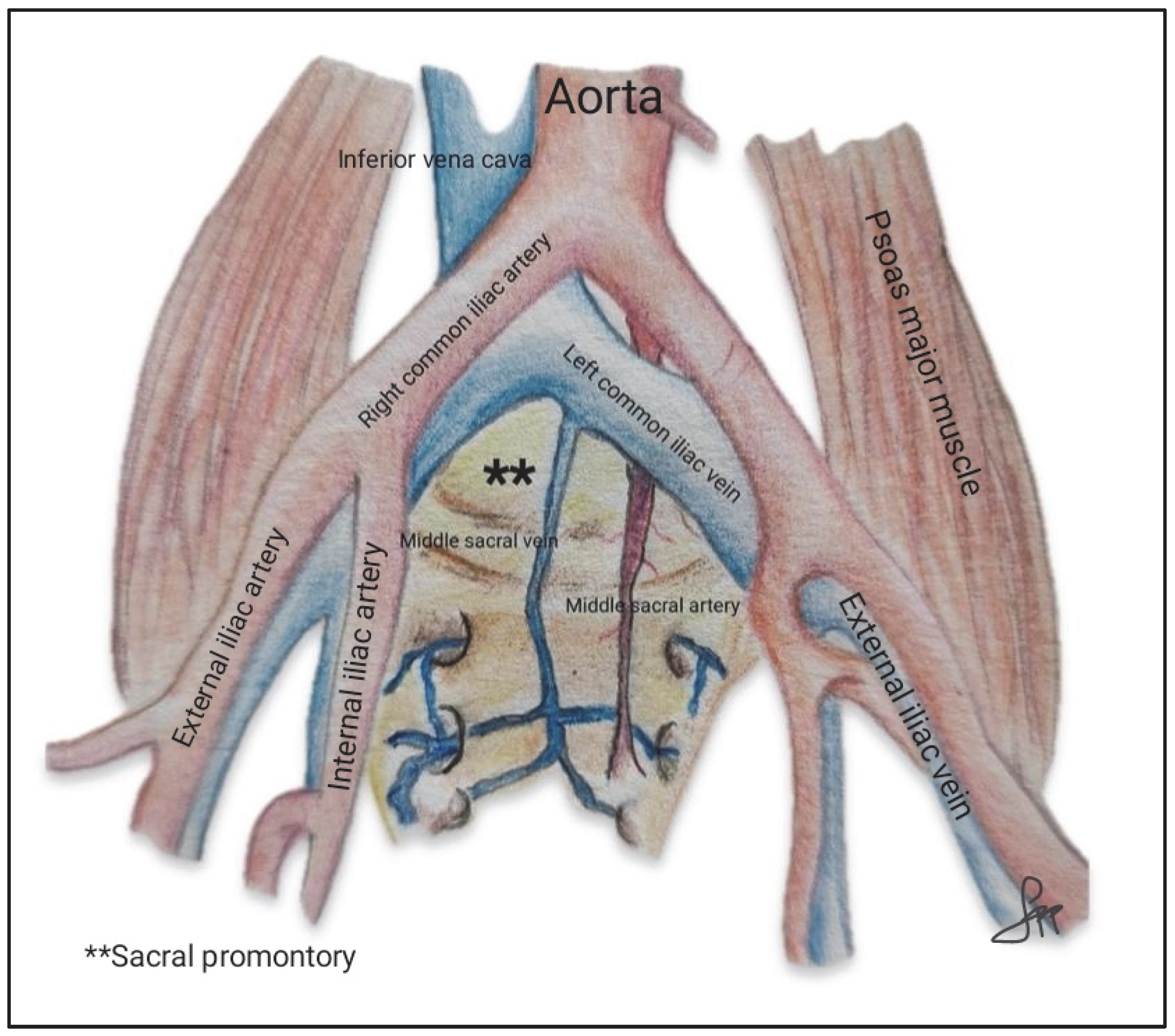
- Sacral promontory: anatomical and risk factor considerations.
- Preventing Perioperative Bleeding: surgical approaches and alternative strategies.
- Management of complications.
- Other reconstructive surgeries.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SC | Sacrocolpopexy |
| RSC | Robotic sacrocolpopexy |
| LSC | Laparoscopic sacrocolpopexy |
| AUS | Artificial urinary sphincter |
| ICG | Indocyanine green |
| IVC | Inferior vena cava |
| 3D | Three dimensions |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| NA | Not available |
| LLS | Laparoscopic lateral suspension |
| R | Restrospective study |
| CR | Case report |
| RCT | Randomized controlled trial |
| OP | Observational prospective |
| SR | Systematic review |
References
- Paraiso, M.F.R.; Jelovsek, J.E.; Frick, A.; Chen, C.C.G.; Barber, M.D. Laparoscopic Compared with Robotic Sacrocolpopexy for Vaginal Prolapse. Obstet. Gynecol. 2011, 118, 1005–1013. [Google Scholar] [CrossRef]
- Nosti, P.A.; Umoh-Andy, U.; Kane, S.; White, D.E.; Harvie, H.S.; Lowenstein, L.; Gutman, R.E. Outcomes of Abdominal and Minimally Invasive Sacrocolpopexy. Female Pelvic Med. Reconstr. Surg. 2014, 20, 33–37. [Google Scholar] [CrossRef]
- Ferrando, C.A.; Paraiso, M.F.R. A Prospective Randomized Trial Comparing Restorelle Y Mesh and Flat Mesh for Laparoscopic and Robotic-Assisted Laparoscopic Sacrocolpopexy. Female Pelvic Med. Reconstr. Surg. 2019, 25, 83–87. [Google Scholar] [CrossRef]
- Antosh, D.D.; Grotzke, S.A.; McDonald, M.A.; Shveiky, D.; Park, A.J.; Gutman, R.E.; Sokol, A.I. Short term outcomes of robotic versus conventional laparoscopic sacral colpopexy. Female Pelvic Med. Reconstr. Surg. 2012, 18, 158–161. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Zhang, X.; Wang, Z.; Zuo, X.; Gao, L.; Hong, L. Robotic and laparoscopic sacrocolpopexy for pelvic organ prolapse: A systematic review and meta-analysis. Ann. Transl. Med. 2021, 9, 449. [Google Scholar] [CrossRef]
- Nygaard, I.E.; McCreery, R.; Brubaker, L.; Connolly, A.; Cundiff, G.; Weber, A.M.; Zyczynski, H. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: A comprehensive review. Obstet. Gynecol. 2004, 104, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.Y.; Geller, E.J.; Visco, A.G. Symptomatic and anatomic 1-year outcomes after robotic and abdominal sacrocolpopexy. Am. J. Obstet. Gynecol. 2012, 206, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Anger, J.T.; Mueller, E.R.; Tarnay, C.; Smith, B.; Stroupe, K.; Rosenman, A.; Brubaker, L.; Bresee, C.; Kenton, K. Robotic compared with laparoscopic Sacrocolpopexy. Obstet. Gynecol. 2014, 123, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.A.; Paraiso, M.F.R.; Jelovsek, J.E.; Barber, M.D.; Ridgeway, B. Perioperative adverse events after minimally invasive abdominal sacrocolpopexy. Am. J. Obstet. Gynecol. 2014, 211, e1–e8. [Google Scholar] [CrossRef]
- Gutzeit, O.; Lauterbach, R.; Loberman, Z.; Sachner, R.; Karram, T.; Lowenstein, L. Laparoscopic sacrocolpopexy complication: Ilio-femoral deep vein thrombosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 247, 270–271. [Google Scholar] [CrossRef]
- Mahoney, C.; Scott, G.; Dwyer, L.; Reid, F.; Ward, K.; Smith, A.; Kearney, R. Laparoscopic sacrocolpopexy posthysterectomy: Intraoperative feasibility and safety in obese women compared with women of normal weight. Int. Urogynecol. J. 2019, 30, 2041–2048. [Google Scholar] [CrossRef]
- Turner, L.; Lavelle, E.; Lowder, J.L.; Shepherd, J.P. The impact of obesity on intraoperative complications and prolapse recurrence after minimally invasive Sacrocolpopexy. Female Pelvic Med. Reconstr. Surg. 2016, 22, 317–323. [Google Scholar] [CrossRef]
- Zhao, Y.; St-Martin, B. Robotic-assisted laparoscopic sacrocolpopexy: Initial Canadian experience. Can. Urol. Assoc. J. 2020, 14, E257–E263. [Google Scholar] [CrossRef]
- Barakat, B.; Franke, K.; Hijazi, S.; Schakaki, S.; Gauger, U.; Hasselhof, V.; Vögeli, T.A. A systematic review and meta-analysis of clinical and functional outcomes of artificial urinary sphincter implantation in women with stress urinary incontinence. Arab. J. Urol. 2020, 18, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Vincendeau, S.; Tondut, L.; Bensalah, K.; Damphousse, M.; Manunta, A. Artificial urinary sphincter implantation in women with stress urinary incontinence: Preliminary comparison of robot-assisted and open approaches. Int. Urogynecol. J. 2016, 27, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Yip, M.J.; Jhamb, A.; Goad, J.R. Case report of deep vein thrombosis caused by artificial urinary sphincter reservoir compressing right external iliac vein. Urol Ann. 2015, 7, 112–114. [Google Scholar] [CrossRef]
- Lazarou, G.; Rahimi, S.; Cui, N.; Zormpa, M. Variant iliocaval confluence discovered during sacrocolpopexy. Obstet. Gynecol. 2011, 117 Pt 2, 436–437. [Google Scholar] [CrossRef]
- Giraudet, G.; Protat, A.; Cosson, M. The anatomy of the sacral promontory: How to avoid complications of the sacrocolpopexy procedure. Am. J. Obstet. Gynecol. 2018, 218, e1–e3. [Google Scholar] [CrossRef]
- Wieslander, C.K.; Rahn, D.D.; McIntire, D.D.; Marinis, S.I.; Wai, C.Y.; Schaffer, J.I.; Corton, M.M. Vascular anatomy of the presacral space in unembalmed female cadavers. Am. J. Obstet. Gynecol. 2006, 195, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Abramowitch, S.; Moalli, P.A. 3D vascular anatomy of the presacral space: Impact of age and adiposity. Int. Urogynecol. J. 2019, 30, 401–407. [Google Scholar] [CrossRef]
- Carracedo-Calvo, D.; Pereira-Rodriguez, N.; Moscatiello, P.; Jerez-Izquierdo, T.; Meilán-Hernández, E.; Toledo-Jimenez, M.; Hernández- Bermejo, I.; Gimbernat-Diaz, H.; Sánchez- Encinas, M. Robotic sacrocolpopexy for the treatment of pelvic organ prolapse in elderly women: Comparative analysis of safety and efficacy versus younger women. Actas Urol. Esp. 2024, 48, 611–617. [Google Scholar] [CrossRef]
- Crisp, C.C.; Herfel, C.V.; Pauls, R.N.; Westermann, L.B.; Kleeman, S.D. Critical Anatomy Relative to the Sacral Suture: A Postoperative Imaging Study After Robotic Sacrocolpopexy. Female Pelvic Med. Reconstr. Surg. 2016, 22, 33–36. [Google Scholar] [CrossRef]
- Degirmenci, Y.; Schepers, M.; Steetskamp, J.; Hasenburg, A.; Skala, C. Three-dimensional vs. two-dimensional endoscopic approach in urogynecology: A retrospective cohort study of laparoscopic sacrocolpopexy. J. Obs. Gynaecol. Res. 2023, 49, 1028–1035. [Google Scholar] [CrossRef]
- Jun, H.S.; Lee, N.; Gil, B.; Jang, Y.; Yu, N.K.; Jung, Y.W.; Yun, B.S.; Kim, M.K.; Won, S.; Seong, S.J. Intraoperative Fluorescent Navigation of the Ureters, Vessels, and Nerves during Robot-Assisted Sacrocolpopexy. J. Pers. Med. 2024, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Klyushnikov, I.; Fedorov, A.; Koval, A.; Tyurina, S.; Idashkin, A. Sacrocolpopexy: Anatomical landmarks, clinical appliance and 3-year outcomes. Gynecol. Pelvic Med. 2022, 5, 5. [Google Scholar] [CrossRef]
- Dubuisson, J.B.; Chapron, C. Laparoscopic iliac colpo-uterine suspension for the treatment of genital prolapse using two meshes: A new operative laparoscopic approach. J. Gynecol. Surg. 1998, 14, 153–159. [Google Scholar] [CrossRef]
- Kumbasar, S.; Salman, S.; Sogut, O.; Gencer, F.K.; Bacak, H.B.; Tezcan, A.D.; Timur, G.Y. Uterine-sparing laparoscopic lateral suspension in the treatment of pelvic organ prolapse. J. Obs. Gynaecol. Res. 2023, 49, 341–349. [Google Scholar] [CrossRef]
- Miele, G.M.; Marra, P.M.; Cefalì, K.; Venturella, R.; Di Carlo, C.; Zullo, F. A New Combined Laparoscopic-Vaginal Lateral Suspension Procedure for the Treatment of Pelvic Organ Prolapse. Urology 2021, 149, 263. [Google Scholar] [CrossRef]
- Dällenbach, P. Laparoscopic Lateral Suspension (LLS) for the Treatment of Apical Prolapse: A New Gold Standard? Front. Surg. 2022, 9, 898392. [Google Scholar] [CrossRef]
- Boukerrou, M.; Orazi, G.; Nayama, M.; Boodhun, R.; Crépin, G.; Cosson, M. Promontofixation procedure: Use of non-absorbable sutures or Tackers? J. Gynecol. Obstet. Biol. La Reprod. 2003, 32, 524–528. [Google Scholar]
- Papp, S.B.; Gaitonde, S.; Baig, S.; Zimmern, P.E. Hemorrhage Occluder™ Pin to control life-threatening bleeding during the removal of an infected sacrocolpopexy mesh: A case report. Case Rep. Womens Health. 2023, 39, e00533. [Google Scholar] [CrossRef]
- Hokenstad, E.D.; Occhino, J.A. Management of presacral bleeding. Int. Urogynecol. J. 2020, 31, 215–217. [Google Scholar] [CrossRef]
- Panico, G.; Mastrovito, S.; Arrigo, D.; Riccetti, C.; Campagna, G.; Scambia, G.; Ercoli, A. Laparoscopic management of presacral retroperitoneal haematoma after sacrocolpopexy. Facts Views Vis Obgyn. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; O’Connor, E.; Khavari, R.; Capon, G.; Manunta, A.; Allue, M.; Hascoet, J.; Nitti, V.W.; Gamé, X.; Gilleran, J.; et al. AMS-800 Artificial urinary sphincter in female patients with stress urinary incontinence: A systematic review. Neurourol Urodyn. 2019, 38 (Suppl. 4), S28–S41. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sun, W.; Guo, X.; Zhou, L. Artificial Urinary Sphincter Is Better Than Slings for Moderate Male Stress Urinary Incontinence with Acceptable Complication Rate: A Systematic Review and Meta-Analysis. Front. Surg. 2022, 9, 841555. [Google Scholar] [CrossRef] [PubMed]
- Frazier, R.L.; Jones, M.E.; Hofer, M.D. Artificial Urinary Sphincter Complications: A Narrative Review. J. Clin. Med. 2024, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- DeLay, K.J.; Haney, N.M.; Chiang, J.; Stewart, C.; Yafi, F.A.; Angermeier, K.; Wood, H.; Boone, T.; Kavanagh, A.G.; Gretzer, M.; et al. Comparison of adjuvant radiation therapy before or after artificial urinary sphincter placement: A multi-institutional, retrospective analysis. Urology 2018, 113, 160–165. [Google Scholar] [CrossRef]
- Mamane, J.; Sanchez, S.; Lellouch, A.G.; Gaillard, V.; Poussot, B.; Tricard, T.; Saussine, C.; Brierre, T.; Game, X.; Beraud, F.; et al. Impact of radiation therapy on artificial urinary sphincter implantation in male patients: A multicenter study. Neurourol. Urodyn. 2022, 41, 332–339. [Google Scholar] [CrossRef]
- Henry, G.; Hsiao, W.; Karpman, E.; Bella, A.J.; Carrion, R.; Jones, L.; Christine, B.; Eisenhart, E.; Cleves, M.A.; Kramer, A. A guide for inflatable penile prosthesis reservoir placement: Pertinent anatomical measurements of the retropubic space. J. Sex. Med. 2014, 11, 273–278. [Google Scholar] [CrossRef]
- Cayetano-Alcaraz, A.A.; Yassin, M.; Desai, A.; Tharakan, T.; Tsampoukas, G.; Zurli, M.; Minhas, S. Penile implant surgery-managing complications. Fac. Rev. 2021, 10, 73. [Google Scholar] [CrossRef]
- Khouri, R.K.; Ortiz, N.M.; Dropkin, B.M.; Joice, G.A.; Baumgarten, A.S.; Morey, A.F.; Hudak, S.J. Artificial Urinary Sphincter Complications: Risk Factors, Workup, and Clinical Approach. Curr. Urol. Rep. 2021, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; Buchner, A.; Grabbert, M.; Stief, C.G.; Pavlicek, M.; Bauer, R.M. Risk factors for artificial urinary sphincter failure. World J. Urol. 2016, 34, 595–602. [Google Scholar] [CrossRef] [PubMed]
| Clinical Scenario | Treatment Options | Considerations/Risks |
|---|---|---|
| Hemodynamically stable with visible bleeding vessel | Direct hemostasis using bipolar energy Surgical clip placement | Risk of exacerbated bleeding due to vessel retraction during coagulation |
| Hemodynamically stable with inaccessible vessel | Orthopedic tacks Bone wax Sutures | Mechanical alternatives to direct coagulation |
| Minor bleeding or controlled lesion | Conservative management with topical hemostatic agents (Surgicel® (Ethicon, Inc., Somerville, NJ, USA), Fibrillar™ (Ethicon, Inc., Somerville, NJ, USA), FloSeal® (Baxter Healthcare Corporation, Deerfield, IL, USA)) | Effective in cases where invasive intervention is not required |
| Major vascular injury or hemodynamic instability | Hemostatic sutures Conversion to open surgery | Requires more aggressive surgical intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polanco-Pujol, L.; Caño-Velasco, J.; Duarte Pedrosa, R.M.; Fernandes, C.; López-Fando, L. Critical Care and Surgical Management of Vascular Complications in Minimally Invasive Urological Reconstructive Surgery. J. Clin. Med. 2025, 14, 6740. https://doi.org/10.3390/jcm14196740
Polanco-Pujol L, Caño-Velasco J, Duarte Pedrosa RM, Fernandes C, López-Fando L. Critical Care and Surgical Management of Vascular Complications in Minimally Invasive Urological Reconstructive Surgery. Journal of Clinical Medicine. 2025; 14(19):6740. https://doi.org/10.3390/jcm14196740
Chicago/Turabian StylePolanco-Pujol, Lucía, Jorge Caño-Velasco, Rui Miguel Duarte Pedrosa, Claudia Fernandes, and Luis López-Fando. 2025. "Critical Care and Surgical Management of Vascular Complications in Minimally Invasive Urological Reconstructive Surgery" Journal of Clinical Medicine 14, no. 19: 6740. https://doi.org/10.3390/jcm14196740
APA StylePolanco-Pujol, L., Caño-Velasco, J., Duarte Pedrosa, R. M., Fernandes, C., & López-Fando, L. (2025). Critical Care and Surgical Management of Vascular Complications in Minimally Invasive Urological Reconstructive Surgery. Journal of Clinical Medicine, 14(19), 6740. https://doi.org/10.3390/jcm14196740








