An Updated Meta-Analysis of Randomized Controlled Trials Comparing Direct Oral Anticoagulants Against Warfarin for Left Ventricular Thrombus Resolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
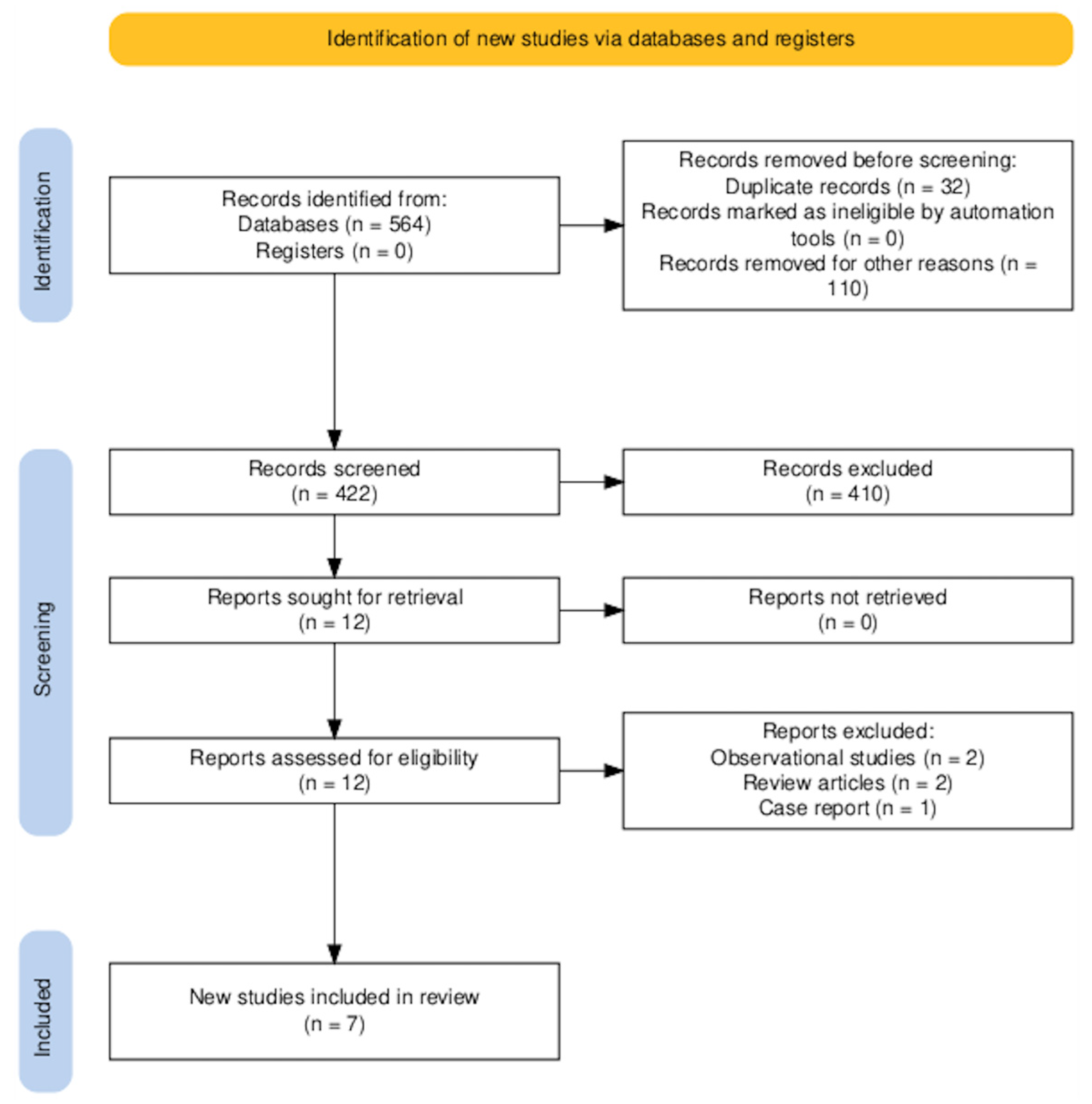
2.3. Study Selection
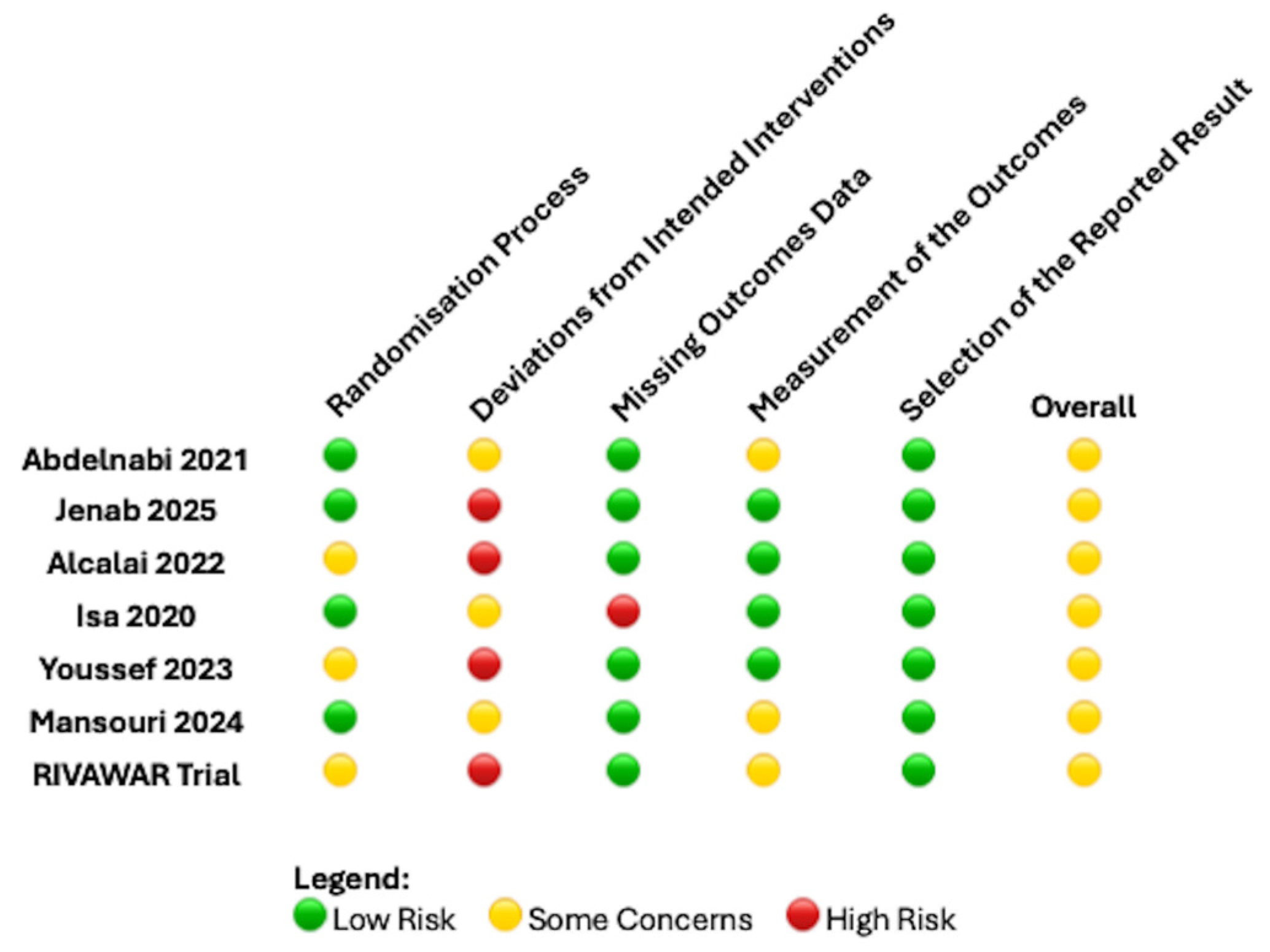
2.4. Data Extraction and Quality Assessment
| Study | Total Number of Patients and Country | RCT Type and Intervention | Inclusion and Exclusion Criteria | Follow-Up Duration | Endpoints |
|---|---|---|---|---|---|
| Isa et al. 2020 [17] | n = 27 Malaysia | Pilot, prospective, single-center, randomized, single-blinded outcome study Apixaban 5 mg BD or 2.5 mg BD for 12 weeks, based on recommendation Warfarin with initial heparin infusion, aiming for a target INR of 2–3 | Inclusion criteria:
Exclusion criteria:
| 15 weeks (Echocardiography 12 weeks) | Primary outcome: 12 weeks percentage of LVT mean size reduction or total resolution Secondary outcomes: All-cause mortality Ischemic stroke Worsening heart failure |
| Abdelnabi et al. 2021 [19] | n = 79 Egypt Bulgaria | Prospective, open-label, multi- center, RCT Rivaroxaban 20 mg daily Warfarin with initial enoxaparin until reaching target INR 2–3 | Inclusion criteria
Exclusion criteria
| 1, 3, 6 months | Primary outcomes: Presence or absence of LVT as assessed by 2D transthoracic echocardiography Secondary outcomes: Stroke or systemic embolism Major bleeding |
| Alcalai et al. 2022 [18] | n = 35 Israel | Multicentre, national, randomized open-label non-inferiority clinical trial Apixaban 5 mg BD or 2.5 mg BD for 3 months dose based on recommendations Warfarin targeting INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: Presence and dimensions of LVT as assessed by 2D echocardiography Secondary outcomes: Stroke or systemic embolism Major bleeding All-cause mortality |
| Youssef et al. 2023 [15] | n = 50 Saudi Arabia | Open-label RCT Apixaban 5 mg BD Warfarin targeting INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 1, 3, 6 months | Primary outcome: Resolution of LVT in 3 months Secondary outcomes: Resolution of LVT in 6 months Safety outcome: MACE or any relevant bleeding according to the BARC classification |
| Mansouri et al. 2024 [16] | n = 52 Iran | open- label non-inferiority RCT Rivaroxaban 20 mg daily Warfarin 5 mg loading dose aiming INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: Resolution of LVT on TTE in 3 months Secondary outcomes: Bleeding Systemic embolic events Rehospitalization MACE Echocardiographic measures focusing on changes in thrombus size, mobility, and morphology |
| Jenab et al. 2025 [11] | n = 50 Iran | Pilot, open-label, parallel-group RCT with a 1:1 allocation ratio, concealed allocation sequences, and blinded outcome assessments Rivaroxaban (15 mg daily) plus clopidogrel (75 mg daily) plus aspirin (80 mg daily, only during the first 7 days) Warfarin overlapping with enoxaparin, until reaching an INR goal of 2.0–2.5, plus clopidogrel (75 mg daily plus aspirin (80 mg daily, only during the first 7 days) | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: 3-month non-contrast 2D TTE-based complete LVT resolution Other outcomes: SSE at 3 months MACE) at 3 months All-cause death at 3 months Main safety outcomes: Major bleeding events based on ISTH definition at 3 months |
| RIVAWAR trial [12] | n = 261 Pakistan | Investigator initiated, single-center trial, open-label, RCT with a 2:1 allocation ratio Rivaroxaban 20 mg once daily Warfarin targeting INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: presence of LVT on TTE at 12 weeks post-randomization Secondary outcome: All-cause mortality Ischemic stroke Major bleeding |
2.5. Endpoints
2.6. Statistical Analysis
3. Results
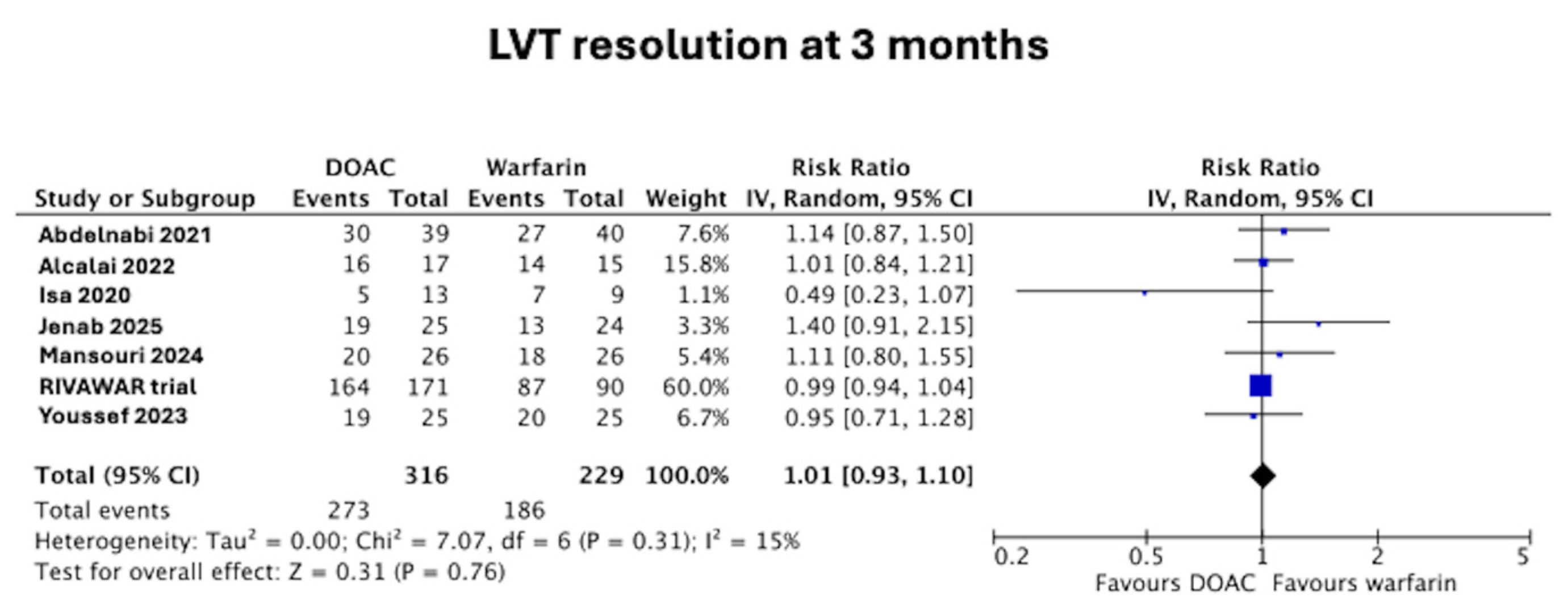
3.1. LVT Resolution at 3 Months
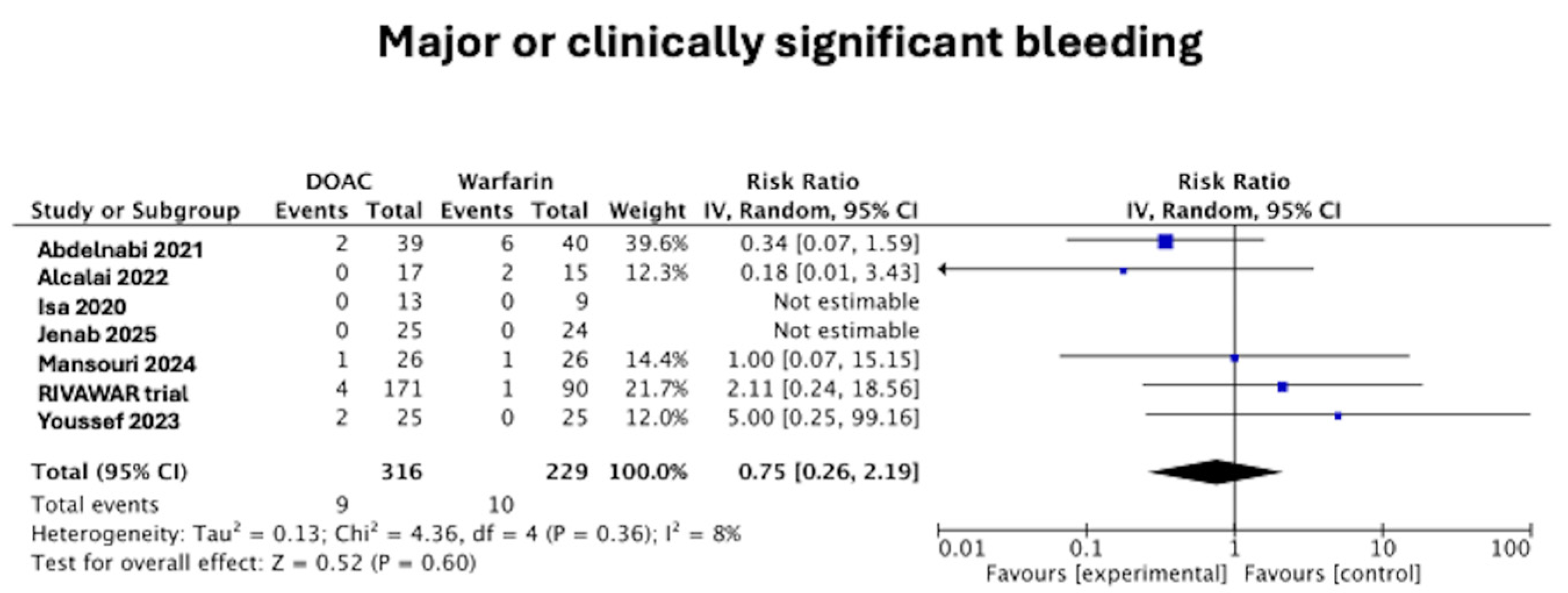
3.2. Major or Clinically Significant Bleeding
3.3. Stroke or Thromboembolic Complications
3.4. Apixaban Versus Rivaroxaban for LVT Resolution at 3 Months
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef]
- Holzknecht, M.; Reindl, M.; Tiller, C.; Lechner, I.; Perez Cabrera, R.; Mayr, A.; Brenner, C.; Klug, G.; Bauer, A.; Metzler, B.; et al. Clinical Risk Score to Predict Early Left Ventricular Thrombus After ST-Segment Elevation Myocardial Infarction. Cardiovasc. Imaging 2021, 14, 308–310. [Google Scholar] [CrossRef]
- Bulluck, H.; Chan, M.H.H.; Paradies, V.; Yellon, R.L.; Ho, H.H.; Chan, M.Y.; Chin, C.W.L.; Tan, J.W.; Hausenloy, D.J. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: A meta-analysis. J. Cardiovasc. Magn. Reson. 2018, 20, 72. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Gay, J.A.; VanVoorhees, L.; DiBianco, R.; Fletcher, R.D. Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: Assessment by 2-dimensional echocardiography. Am. J. Cardiol. 1983, 52, 1281–1285. [Google Scholar] [CrossRef]
- Ciaccheri, M.; Castelli, G.; Cecchi, F.; Nannini, M.; Santoro, G.; Troiani, V.; Zuppiroli, A.; Dolara, A. Lack of correlation between intracavitary thrombosis detected by cross sectional echocardiography and systemic emboli in patients with dilated cardiomyopathy. Br. Heart J. 1989, 62, 26–29. [Google Scholar] [CrossRef]
- Wu, H.S.; Dong, J.Z.; Du, X.; Hu, R.; Jia, C.Q.; Li, X.; Wu, J.H.; Ruan, Y.F.; Yu, R.H.; Long, D.Y.; et al. Risk Factors for Left Ventricular Thrombus Formation in Patients with Dilated Cardiomyopathy. Semin. Thromb Hemost 2023, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Adi, D.; Wu, Y.; Aizezi, A.; Li, Y.P.; Kerem, M.; Wei, X.; Liu, F.; Ma, X.; Ma, Y.T. A nomogram to predict ventricular thrombus in dilated cardiomyopathy patients. J. Thromb. Thrombolysis 2024, 57, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Velangi, P.S.; Choo, C.; Chen, K.A.; Kazmirczak, F.; Nijjar, P.S.; Farzaneh-Far, A.; Okasha, O.; Akcakaya, M.; Weinsaft, J.W.; Shenoy, C. Long-Term Embolic Outcomes After Detection of Left Ventricular Thrombus by Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Imaging: A Matched Cohort Study. Circulation. Cardiovasc. Imaging 2019, 12, e009723. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.; Anastasiou, V.; Papazoglou, A.S.; Daios, S.; Didagelos, M.; Kamperidis, N.; Moschovidis, V.; Papadopoulos, S.F.; Iatridi, F.; Sarafidis, P.; et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists for the Management of Left Ventricular Thrombus After Myocardial Infarction: A Meta-Analysis. Am. J. Cardiol. 2024, 232, 18–25. [Google Scholar] [CrossRef]
- Khalid, S.; Joseph, T.; Isa, W.Y.H.W.; Bulluck, H. Letter to the Editor Regarding “A Meta-Analysis of RCTs Comparing DOACs Against Warfarin for the Treatment of Left Ventricular Thrombus”. Heart Lung Circ. 2024, 33, e53–e54. [Google Scholar] [CrossRef] [PubMed]
- Jenab, Y.; Sadeghipour, P.; Mohseni-Badalabadi, R.; Kaviani, R.; Hosseini, K.; Pasebani, Y.; Khederlou, H.; Rafati, A.; Mohammadi, Z.; Jamalkhani, S.; et al. Direct oral anticoagulants or warfarin in patients with left ventricular thrombus after ST-elevation myocardial infarction: A pilot trial and a prespecified meta-analysis of randomised trials. EuroIntervention 2025, 21, 82–92. [Google Scholar] [CrossRef]
- Vranckx, P.; Halvorsen, S. Cracking the Clot: The RIVAWAR Trial Challenges Warfarin’s Reign in Left Ventricular Thrombus Post-Acute Coronary Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2025, 14, 243–244. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011, 343, d5928. [Google Scholar] [CrossRef]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients With Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2023, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, P.; Jazi, Z.A.; Mansouri, M.H.; Dehghan, H.; Zavar, R.; Hashemi, S.M.; Sattar, F.; Sadeghi, M.; Amirpour, A.; Abdar, M. Evaluation of the efficacy and safety of rivaroxaban compared to warfarin in patients with left ventricular apical thrombus: A randomized clinical trial. Thromb J. 2024, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Isa, W.Y.H.W.; Hwong, N.; Mohamed Yusof, A.K.; Yusof, Z.; Loong, N.S.; Wan-Arfah, N.; Naing, N.N. Apixaban versus Warfarin in Patients with Left Ventricular Thrombus: A Pilot Prospective Randomized Outcome Blinded Study Investigating Size Reduction or Resolution of Left Ventricular Thrombus. J. Clin. Prev. Cardiol. 2020, 9, 150–154. [Google Scholar] [CrossRef]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. warfarin in patients with left ventricular thrombus: A prospective multicentre randomized clinical trialdouble dagger. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, M.; Saleh, Y.; Fareed, A.; Nossikof, A.; Wang, L.; Morsi, M.; Eshak, N.; Abdelkarim, O.; Badran, H.; Almaghraby, A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 2021, 77, 1590–1592. [Google Scholar] [CrossRef]
- Yao, Z.; Gue, Y.; Lip, G.Y.H. Comparison of Direct Oral Anticoagulants and Vitamin K Antagonists for Left Ventricular Thrombus: A Global Retrospective Study. Am. J. Med. 2025, 138, 468–476. [Google Scholar] [CrossRef]
- Romualdi, E.; Ageno, W. Investigational factor Xa inhibitors for thrombosis and acute coronary syndromes. Expert. Opin. Investig. Drugs 2011, 20, 495–505. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdy, J.; He, M.; Arockiam, S.; Harada, N.; Wheatcroft, S.B.; Bulluck, H. An Updated Meta-Analysis of Randomized Controlled Trials Comparing Direct Oral Anticoagulants Against Warfarin for Left Ventricular Thrombus Resolution. J. Clin. Med. 2025, 14, 6735. https://doi.org/10.3390/jcm14196735
Magdy J, He M, Arockiam S, Harada N, Wheatcroft SB, Bulluck H. An Updated Meta-Analysis of Randomized Controlled Trials Comparing Direct Oral Anticoagulants Against Warfarin for Left Ventricular Thrombus Resolution. Journal of Clinical Medicine. 2025; 14(19):6735. https://doi.org/10.3390/jcm14196735
Chicago/Turabian StyleMagdy, Joseph, Maggie He, Sacchin Arockiam, Nanami Harada, Stephen B. Wheatcroft, and Heerajnarain Bulluck. 2025. "An Updated Meta-Analysis of Randomized Controlled Trials Comparing Direct Oral Anticoagulants Against Warfarin for Left Ventricular Thrombus Resolution" Journal of Clinical Medicine 14, no. 19: 6735. https://doi.org/10.3390/jcm14196735
APA StyleMagdy, J., He, M., Arockiam, S., Harada, N., Wheatcroft, S. B., & Bulluck, H. (2025). An Updated Meta-Analysis of Randomized Controlled Trials Comparing Direct Oral Anticoagulants Against Warfarin for Left Ventricular Thrombus Resolution. Journal of Clinical Medicine, 14(19), 6735. https://doi.org/10.3390/jcm14196735