Is Less More? A Meta-Analysis of Non-Intubated Versus Intubated VATS for Anatomic Resections in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Search and Articles Selection Strategy
- Original articles including more than 10 patients;
- Published between 1 January 2010, and 30 June 2025;
- Written in English;
- Involving human subjects;
- Comparing awake (non-intubated) VATS to conventional (intubated) VATS in patients undergoing pulmonary anatomical resection (defined as lobectomy ± segmentectomy) for lung cancer; wedge resections were excluded, as they are non-anatomical procedures with limited oncologic comparability, potentially introducing heterogeneity into the analysis; and
- Reporting at least one perioperative or oncologic outcome (e.g., operative time, chest tube duration, length of stay, complications, survival).
2.2. Data Extraction and Endpoints
2.3. Sensitivity Analysis on Survival Endpoints
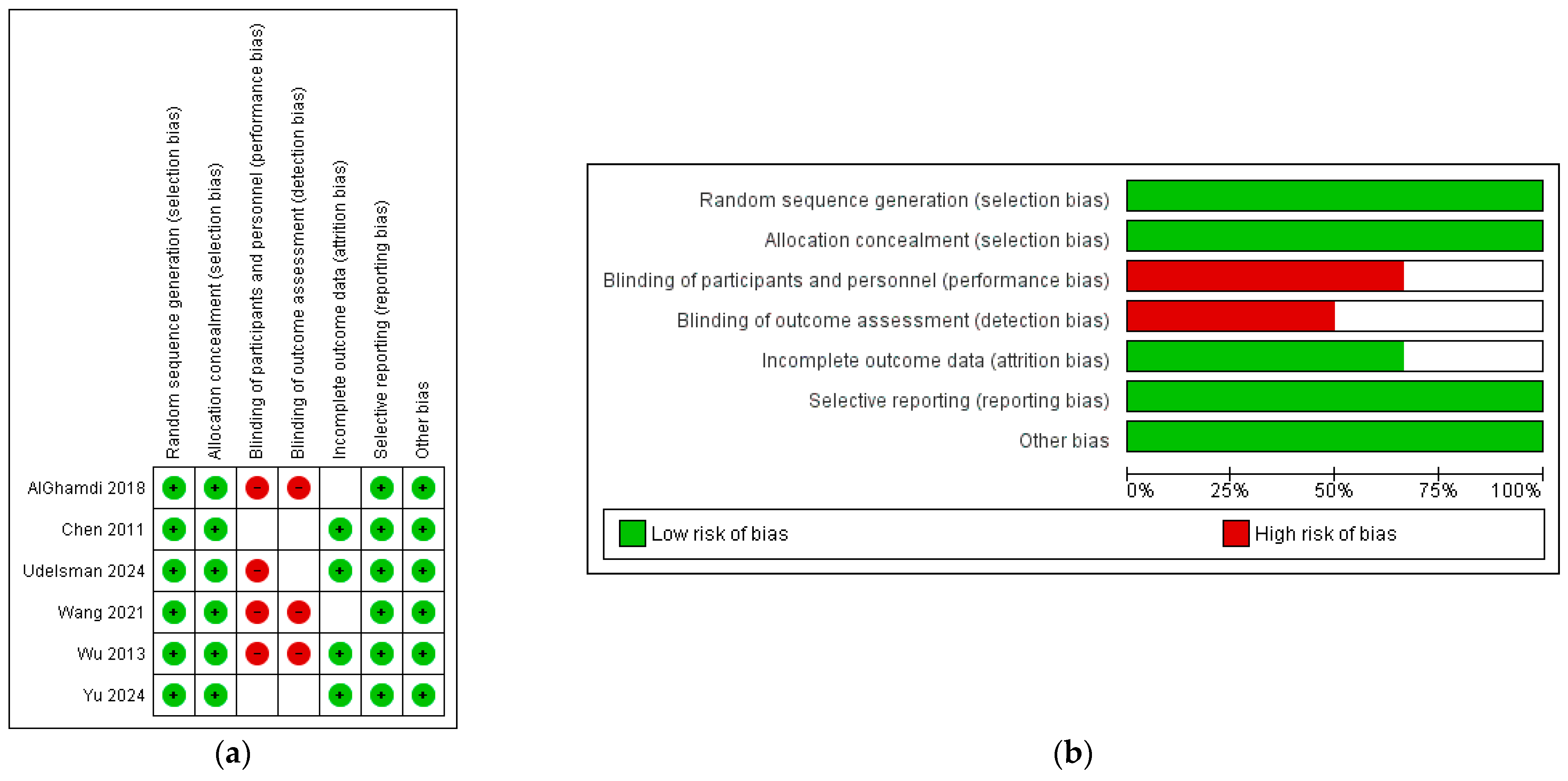
2.4. Quality and Publication Bias Assessment
3. Results
3.1. Search Strategy and Patient Demographics
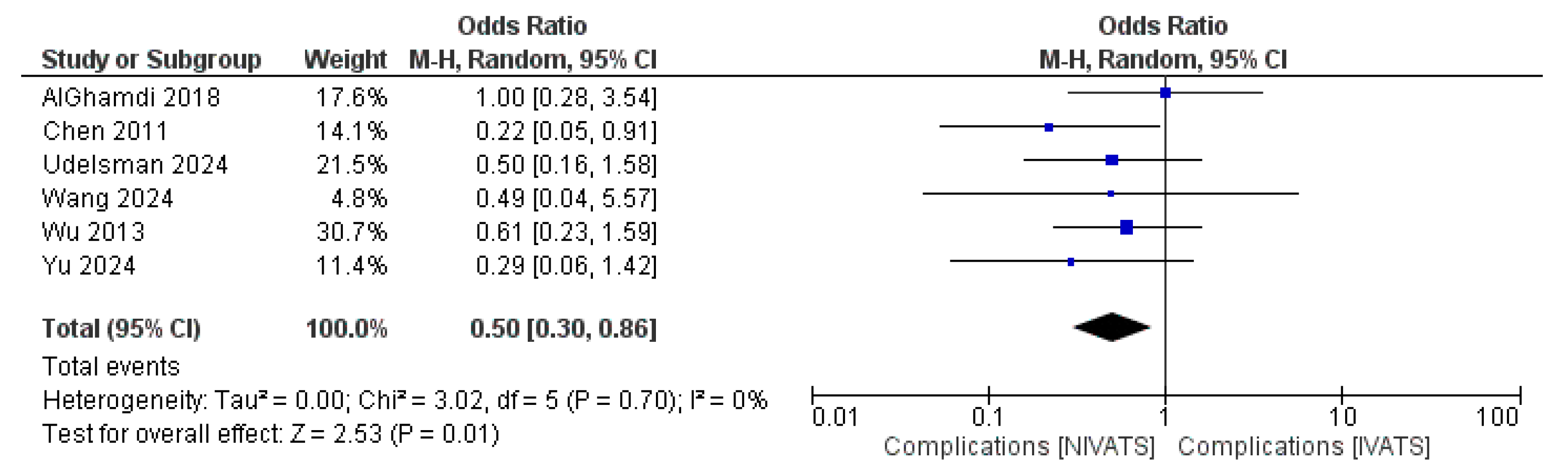
3.2. Primary Endpoints: Complications and Mortality
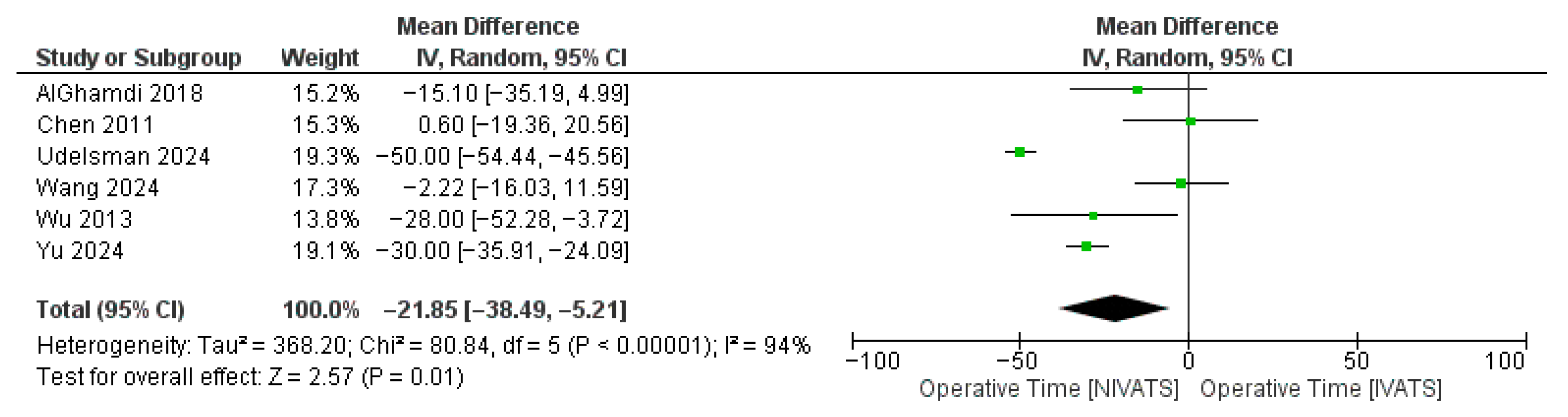
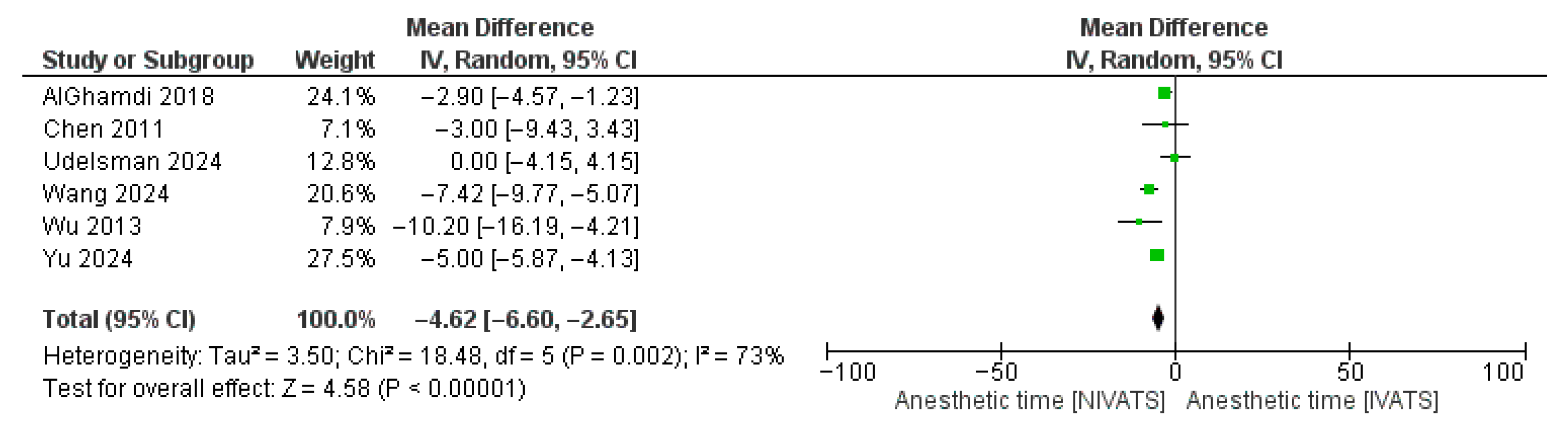
3.3. Secondary Endpoints
3.4. Sensitivity Analysis and Publication Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; DeCamp, M.; et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J. Natl. Compr. Canc Netw. 2023, 21, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Mangiameli, G.; Cioffi, U.; Alloisio, M.; Testori, A. The State of the Art in Thoracic Surgery: Treating Lung Cancer Between Tradition and Innovation. In Metastasis; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 3. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early-stage lung cancer: A randomized controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Anile, M.; Vannucci, J.; Ferrante, F.; Bruno, K.; De Paolo, D.; Bassi, M.; Pugliese, F.; Venuta, F.; NIVATS Interest Group. Non-Intubated Thoracic Surgery: Standpoints and Perspectives. Front. Surg. 2022, 9, 937633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Kim, M.; Park, B.; Park, Y.H.; Min, S.H. Feasibility of ventilator-assisted tubeless anesthesia for video-assisted thoracoscopic surgery. Medicine 2023, 102, e34220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocco, G.; Romano, V.; Accardo, R.; Tempesta, A.; La Manna, C.; La Rocca, A.; Martucci, N.; D′ Aiuto, M.; Polimeno, E. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann. Thorac. Surg. 2010, 89, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Elkhayat, H.; Gonzalez-Rivas, D. Non-intubated uniportal video-assisted thoracoscopic surgery. J. Thorac. Dis. 2019, 11 (Suppl. 3), S220–S222. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Cheng, Y.J.; Hung, M.H.; Tseng, Y.D.; Chen, K.C.; Lee, Y.C. Nonintubated thoracoscopic lobectomy for lung cancer. Ann. Surg. 2011, 254, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, H.G.; Wu, W.B.; Li, X.J.; Wu, Y.H.; Xu, J.N.; Jia, Y.B.; Zhang, J. Non-intubated video-assisted thoracoscopic surgery vs. intubated video-assisted thoracoscopic surgery for thoracic disease: A systematic review and meta-analysis of 1684 cases. J. Thorac. Dis. 2019, 11, 3556–3568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prisciandaro, E.; Bertolaccini, L.; Sedda, G.; Spaggiari, L. Non-intubated thoracoscopic lobectomies for lung cancer: An exploratory systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Pompeo, E. From Awake to Minimalist Spontaneous Ventilation Thoracoscopic Lung Surgery: An Ongoing Journey. J. Clin. Med. 2025, 14, 2475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.org (accessed on 30 June 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, Z.M.; Lynhiavu, L.; Moon, Y.K.; Moon, M.H.; Ahn, S.; Kim, Y.; Sung, S.W. Comparison of non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer. J. Thorac. Dis. 2018, 10, 4236–4243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Udelsman, B.V.; Jang, A.; Muniappan, A.; Zhan, P.L.; Bao, X.; Chen, T.; Gaissert, H.A. Perioperative morbidity and 3-year survival in non-intubated thoracoscopic surgery: A propensity matched analysis. J. Thorac. Dis. 2024, 16, 1180–1190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.L.; How, C.H.; Hung, M.H.; Huang, H.H.; Hsu, H.H.; Cheng, Y.J.; Chen, J.S. Long-term outcomes after nonintubated versus intubated thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: A propensity-matched analysis. J. Formos. Med. Assoc. 2021, 120, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Zhang, Y. Comparison of postoperative pulmonary complications and intraoperative safety in thoracoscopic surgery under non-intubated versus intubated anesthesia: A randomized, controlled, double-blind non-inferiority trial. Updates Surg. 2024, 76, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chen, J.S.; Lin, Y.S.; Tsai, T.M.; Hung, M.H.; Chan, K.C.; Cheng, Y.J. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann. Thorac. Surg. 2013, 95, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tantraworasin, A.; Laohathai, S. Non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer patients. Asian J. Surg. 2024, 47, 402–406. [Google Scholar] [CrossRef] [PubMed]

| Study ID, Year | Study Design | Patients, n | Females, % | FEV1 | Mean Age, y ± SD | Histology, % | Pathologic Staging | Neoadjuvant Tx | NOS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | NI | I | NI | I | |||||
| AlGhamdi 2018 [17] | R | 30 | 30 | 67 | 60 | 95 ± 9 | 95 ± 17 | 65 ± 11 | 66 ± 10 | ADC: 87 SCC: 13 | ADC: 83 SCC: 13 | I-II | NR | 6 |
| Chen 2011 [9] | R | 30 | 30 | 77 | 57 | 105 ± 18 | 104 ± 12 | 58 ± 10 | 57 ± 10 | ADC: 83 | ADC: 90 | I | E | 6 |
| Udelsman 2024 [18] | R-PSM | 67 | 134 | 33 | 33 | 90 | 98 | 68 ± 11 | 68 ± 12 | - | - | I | E | 8 |
| Wang 2021 [19] | R | 97 | 97 | 58 | 59 | 104 ± 7 | 114 ± 6 | 60 ± 11 | 62 ± 12 | ADC: 97 | ADC: 92 | IA-IB | E | 6 |
| Wang 2024 [20] | RCT | 60 | 60 | 70 | 60 | - | - | 52 ± 6 | 51 ± 8 | - | - | - | - | - |
| Wu 2013 [21] | R | 36 | 48 | 42 | 44 | 113 ± 26 | 106 ± 24 | 73 | 73 | ADC: 92 | ADC: 83 | I | E | 6 |
| Yu 2024 [22] | R | 54 | 78 | 63 | 51 | N/A | N/A | 65 | 65 | ADC: 89 SCC: 4 | ADC: 92 SCC: 8 | T1-T2 | Given if N2 | 8 |
| Categorical Outcomes | n | OR [95% CI] | p | Heterogeneity | |
| I2 | p | ||||
| Complications | 6 | 0.50 [0.30, 0.86] | 0.01 | 0% | 0.70 |
| 30-day mortality | 7 | Not estimable | - | - | - |
| Conversion to thoracotomy | 4 | 1.65 [0.30, 8.98] | 0.56 | 0% | 0.65 |
| Continuous outcomes | n | WMD (95% CI) | I2 | p | |
| MOT | 6 | −21.85 [−38.49, −5.21] | 0.01 | 94% | <0.01 |
| Anesthetic time | 6 | −4.62 [−6.60, −2.65] | <0.01 | 73% | <0.01 |
| Blood Loss | 4 | −24.36 [−30.67, −18.05] | <0.01 | 0% | 0.57 |
| Chest tube duration | 5 | −0.18 [−0.45, 0.10] | 0.21 | 50% | 0.09 |
| LOS | 6 | −0.57 [−1.17, 0.03] | 0.06 | 94% | <0.01 |
| Total Nodes Sampled | 6 | −0.84 [−2.29, 0.62] | 0.26 | 86% | <0.01 |
| Complication | NIVATS (%) | IVATS (%) |
|---|---|---|
| Prolonged air leak | 6.3 | 7.8 |
| Pneumonia | 3.0 | 3.9 |
| Re-intubation | 1.1 | 1.9 |
| Conversion to thoracotomy | 0.7 | 1.9 |
| Atrial fibrillation | 1.9 | 2.9 |
| Bleeding | 0.4 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magouliotis, D.E.; Karamolegkou, A.P.; Zotos, P.-A.; Minervini, F.; Cioffi, U.; Scarci, M. Is Less More? A Meta-Analysis of Non-Intubated Versus Intubated VATS for Anatomic Resections in Non-Small Cell Lung Cancer. J. Clin. Med. 2025, 14, 6731. https://doi.org/10.3390/jcm14196731
Magouliotis DE, Karamolegkou AP, Zotos P-A, Minervini F, Cioffi U, Scarci M. Is Less More? A Meta-Analysis of Non-Intubated Versus Intubated VATS for Anatomic Resections in Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2025; 14(19):6731. https://doi.org/10.3390/jcm14196731
Chicago/Turabian StyleMagouliotis, Dimitrios E., Anna P. Karamolegkou, Prokopis-Andreas Zotos, Fabrizio Minervini, Ugo Cioffi, and Marco Scarci. 2025. "Is Less More? A Meta-Analysis of Non-Intubated Versus Intubated VATS for Anatomic Resections in Non-Small Cell Lung Cancer" Journal of Clinical Medicine 14, no. 19: 6731. https://doi.org/10.3390/jcm14196731
APA StyleMagouliotis, D. E., Karamolegkou, A. P., Zotos, P.-A., Minervini, F., Cioffi, U., & Scarci, M. (2025). Is Less More? A Meta-Analysis of Non-Intubated Versus Intubated VATS for Anatomic Resections in Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 14(19), 6731. https://doi.org/10.3390/jcm14196731









