Photobiomodulation Therapy in Hypertension Management—Evidence from a Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Outcomes
2.3. Search Strategy
2.4. Selection
2.5. Data Extraction
2.6. Assessment of Risk of Bias
2.7. Data Analysis and Synthesis
2.8. Summary of Findings and Assessment of the Certainty of the Evidence
3. Results
3.1. Characteristics of Randomized Controlled Trials
3.2. Characteristics of Experimental Studies
3.3. Outcomes—Randomized Controlled Trials
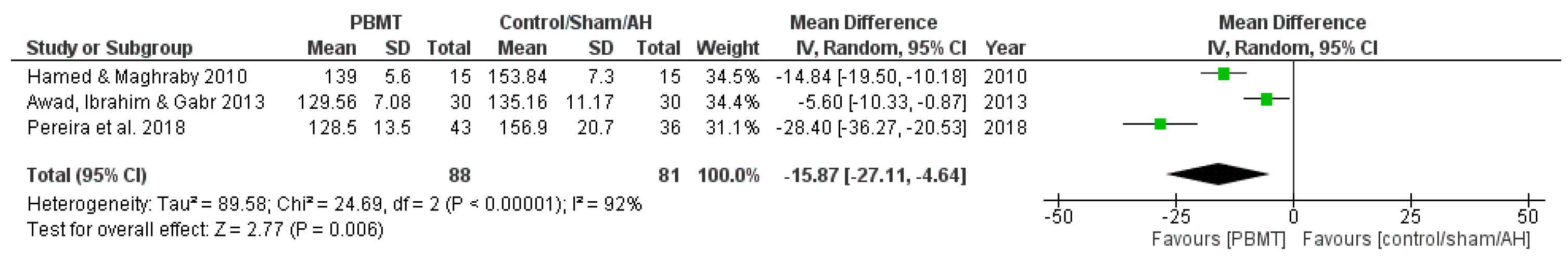
3.3.1. Systolic Blood Pressure
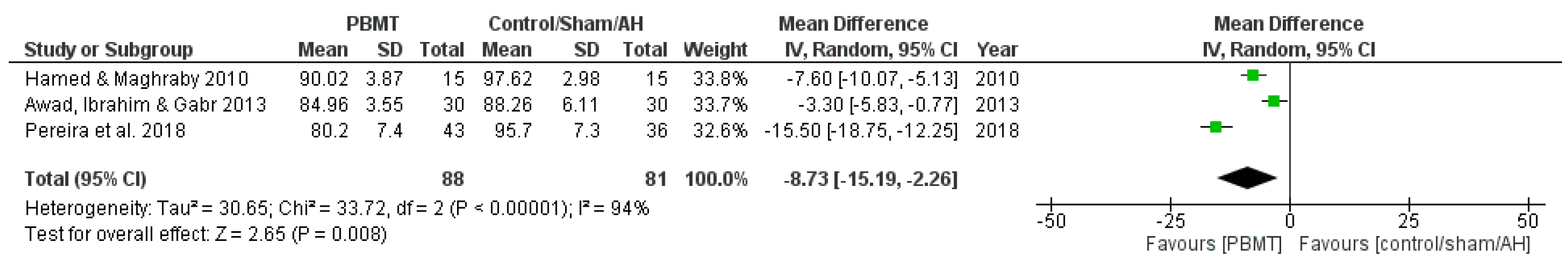
3.3.2. Diastolic Blood Pressure
3.3.3. Heart Rate
3.3.4. Adverse Effects
3.4. Outcomes—Experimental Studies
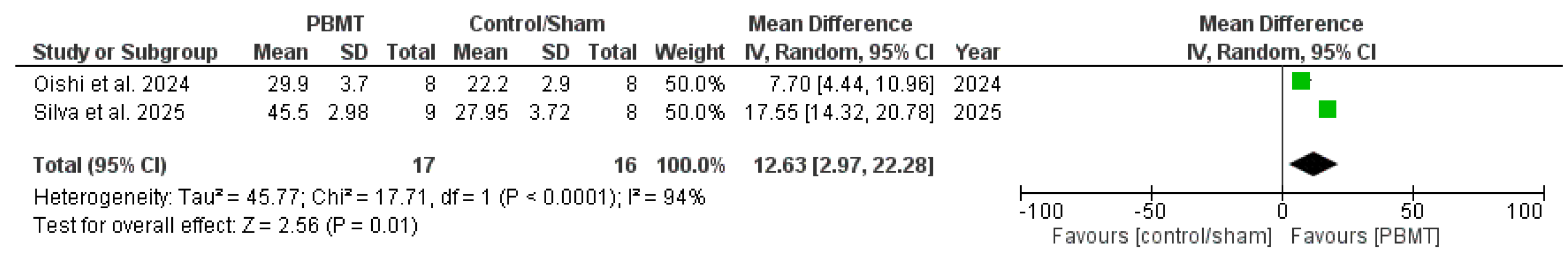
3.4.1. Systolic Blood Pressure
3.4.2. Diastolic Blood Pressure
3.4.3. Mean Arterial Pressure
3.4.4. Heart Rate
3.4.5. Nitric Oxide (Nitrite and Nitrate)
3.4.6. Adverse Effects
3.5. Photobiomodulation Therapy Protocols
3.5.1. Randomized Controlled Trials
3.5.2. Experimental Studies
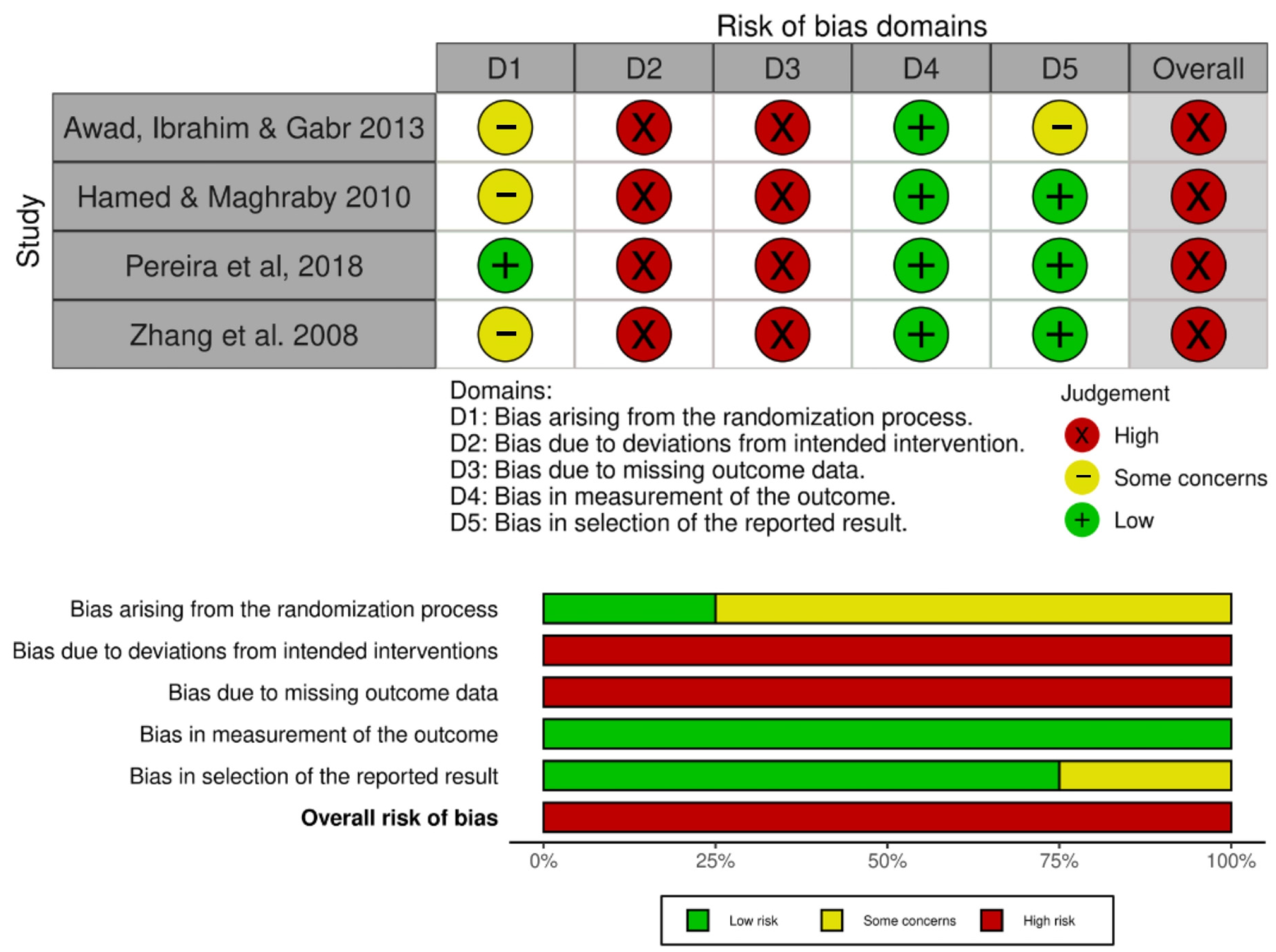
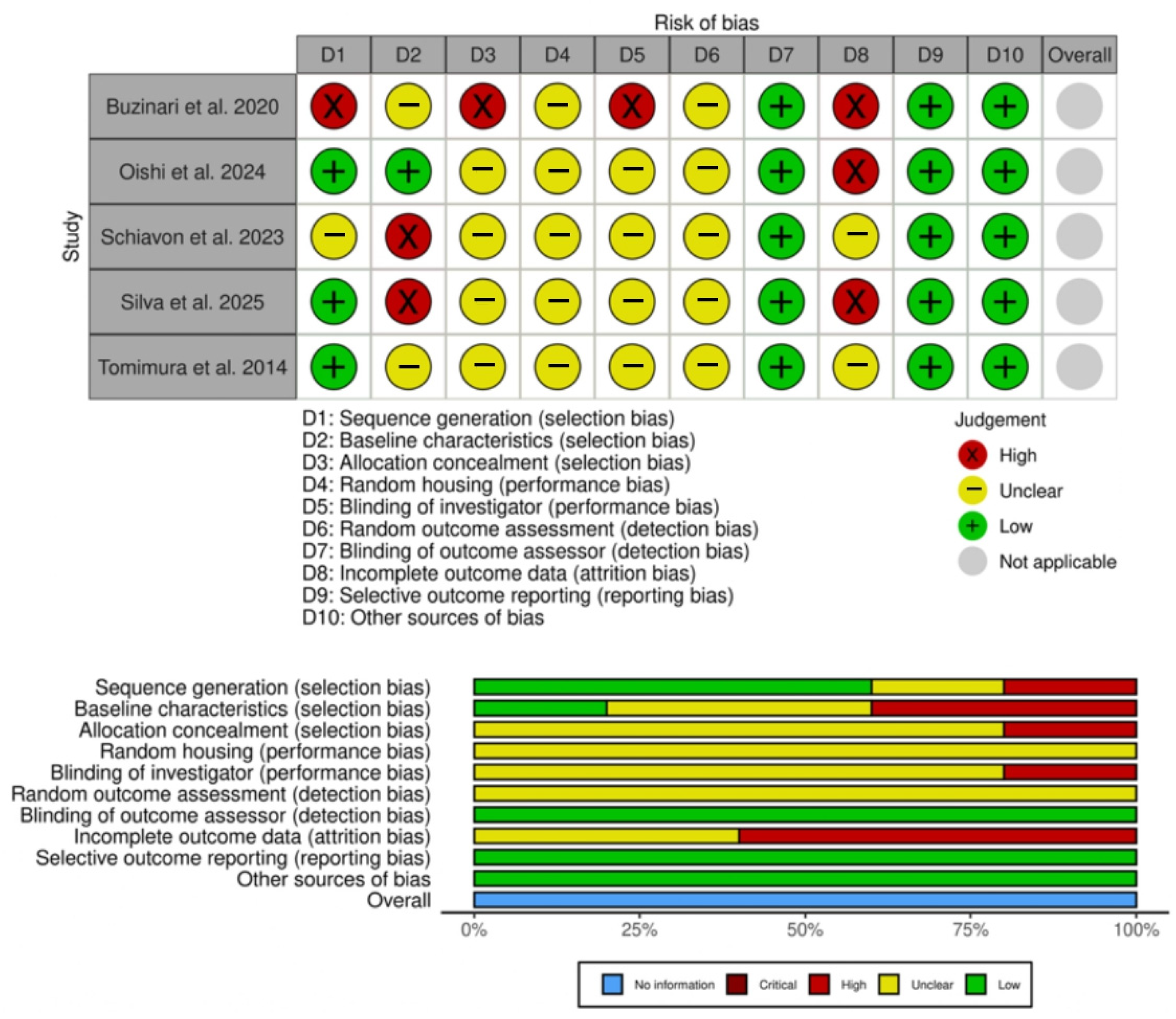
3.6. Risk of Bias
3.7. Quality of Evidence
Additional Information
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| DBP | Diastolic blood pressure |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluations |
| HR | Heart rate |
| MAP | Mean arterial pressure |
| NO | Nitric oxide |
| PBM TREATMENTS | Photobiomodulation therapy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized controlled trial |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
Appendix A
Appendix A.1
| Article | Participants | Study Design | Outcomes and Data Collection | Main Findings |
|---|---|---|---|---|
| Awad, Ibrahim & Gabr (2013) [17] Effect of Laser Acupuncture on Reducing Postmenopausal Hypertension | Postmenopausal women (50–65 years old) BP ranged from 140/90 to 170/105 mmHg | Group A—Antihypertensive drug only (n = 30); Group B—Antihypertensive drug + LA (n = 30) Chronic treatment (3 times a week for 6 weeks) | BP measured by sphygmomanometer | LA reduced SBP and DBP, compared to the group that received antihypertensive only treatment |
| Hamed & Maghraby (2010) [29] Effectiveness of Laser Acupoint Therapy and Exercise Program on Oxidative Stress and Antioxidant Response in Mild Essential Hypertensive Patients | Male participants (40–60 years old) with mild essential hypertension Resting BP ranged between 140/90 and 160/100 mmHg | Laser therapy group (n = 15); Exercise group—Moderate treadmill program (n = 15); Control group—No treatment (n = 15) Chronic treatment (3 times a week for 4 weeks) | BP and HR measured by digital upper-arm BP monitor (Model UA-767, Advanced System Technologies Ltd., Surrey, UK) | LA reduced SBP, DBP, and HR, compared to control group |
| Pereira et al. (2018) [15] Laser acupuncture protocol for essential systemic arterial hypertension: randomized clinical trial | Participants of both sexes (30–75 years old) with SAH at any stage Undergoing drug treatment for SAH with difficulty to control BP (>140/90 mmHg) | Intervention group (LA) (n = 51); Control group (simulation) (n = 51) Chronic treatment (once a week for 6 weeks) | BP measured using the indirect oscillometric method, with a precision digital monitor | LA group presented reduced SBP and DBP |
| Zhang et al. (2008) [28] Effect of laser acupoint treatment on blood pressure and body weight—a pilot study | Participants of both sexes (2 dropout) (25 ± 5 years old) with mild hypertension (SBP 125–160 mmHg; DBP 81–110 mmHg) | Control group (sham LLLT) (n = 22); Experimental group (n = 23) Chronic treatment (2 times a week for 12 weeks) | BP measured by Biopac manual blood pressure monitor | LA group presented reduced SBP and DBP |
Appendix A.2
| Article | Animals | Groups, Acute or Chronic | Outcomes and Data Collection | Main Findings |
|---|---|---|---|---|
| Buzinari et al. (2020) [8] Photobiomodulation induces hypotensive effect in spontaneously hypertensive rats | Male spontaneously hypertensive rats 180–200 g Age of 9 to 10 weeks | Sham group (laser off for the same amount of time); Laser group n = 26 (crossover study) Acute application | SBP, DBP, MAP, and HR collected by an intra-arterial cannula NO measured by NO/ozone chemiluminescence Outcomes collected for 1 h | PBMT reduced SBP, DBP, and MAP in a percentage of the animals for about 25 min. HR was not altered. PBMT increased NO levels only in responsive animals. |
| Oishi et al. (2024) [30] Long-term effects of photobiomodulation therapy on blood pressure in obese rats induced by a high-fat diet | Male Wistar rats, obesity hypertension model 250–300 g Standard or high-fat diet | Control group (standard diet) (n = 8); High-fat diet (without PBMT) (n = 8); High-fat diet + PBM (n = 8) Chronic application (3 times a week for 12 weeks) | SBP measured by tail-cuff plethysmography Serum nitrite and nitrate (NOx) analyzed by NO/ozone chemiluminescence | PBMT prevented increases in SBP induced by HFDiet and normalized NO. |
| Schiavon et al. (2023) [7] Chronic red laser treatment induces hypotensive effect in two-kidney one-clip model of renovascular hypertension in rat. | Male spontaneously hypertensive rats 180–200 g Age of 9 to 10 weeks | Placebo 2K (laser application with the device turned off) (n = 6); 2K+PBM (n = 6); Placebo 2K-1C (laser application with the device turned off) (n = 8); 2K-1C+PBM (n = 22) Chronic application (4 weeks—twice a week) | SBP measured by tail plethysmography Serum nitrite and nitrate (NOx) measured by chemiluminescence | PBMT induced a hypotensive effect in a percentage of the animals but did not elevate NO. |
| Silva et al. (2025) [31] Chronic treatment with photobiomodulation decrease blood pressure and improves endothelial function in ovariectomized rats | Female ovariectomized Wistar rats 200–250 g | Ovariectomized (n = 8); Ovariectomized treated with PBM (n = 9); Sham group (n = 9). Chronic application (2 weeks—twice a week) | SBP measured by tail plethysmography Serum nitrite and nitrate (NOx) measured using NO Analyzer 280i | PBMT decreased SBP and increased NO from ovariectomized rats when compared to ovariectomized rats with no treatment. |
| Tomimura et al. (2014) [10] Hemodynamic effect of laser therapy in spontaneously hypertensive rats | Male spontaneously hypertensive rats | Sham Group (n = 8); Laser Group (n = 8). Chronic application (7 weeks—three times a week) | SBP, DBP, MAP, and HR measured by an arterial cannula attached to an electromagnetic transducer | PBMT decreased DBP, MAP, and HR when compared to sham group. SBP values presented no differences |
Appendix B
| Article | Source of Light | Wavelength (nm) | Energy (J) | Energy Density (J/cm2) | Power (mW) | Power Density (W/cm2) | Spot Size (cm2) | Irradiation Time (s) and Number of Points | Irradiation Technique and Number of Sessions |
|---|---|---|---|---|---|---|---|---|---|
| Randomized Controlled Trials | |||||||||
| Awad, Ibrahim & Gabr (2013) [17] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 240 per point 3 acupuncture points | 3 acupuncture points (LI 4, LI 11, and Spleen 6), 4 min per point, 3 sessions a week for 6 weeks |
| Hamed & Maghraby (2010) [29] | The Laser Therapy Unit, BTL-5110 Laser, USA | 904 | Not reported | 2 | Average peak power of 5 mW Frequency of 5000 Hz | Not reported | Not reported | 120 2 acupuncture points | Acupuncture points (LI 4 and LI 11) in the upper arm through pressure contact technique with a laser probe 3 sessions a week for 4 weeks |
| Pereira et al. (2018) [15] | GaAlAs low-power infrared laser-acupuncture equipment | Infrared | Not reported | Not reported | Frequency of “6 MW” | Not reported | Not reported | 1440 (total duration) 11 acupuncture points | Acupoints located in the head (frontal and occipital regions), upper (hands and arms), and lower (feet) limbs 1 session a week for 6 weeks |
| Zhang et al. (2008) [28] | Insight 40 Infrared Laser (USA Laser, Richmond, VA) | Infrared | 16 (energy of the treatment) | Not reported | Frequency of 10 kHz | Not reported | Not reported | 240 per point 2 points | Laser acupuncture to the LI 4 (Hegu) and LI 11 (Quchi) 2 sessions a week for 12 weeks |
| Experimental Studies | |||||||||
| Buzinari et al. (2020) [8] | AlGaAs diode laser device Photon Lase III (DMC Equipment) | 660 | 5.6 per point (calculated) | 96 | 100 | 1.71 | 0.0586 | 56 per point (6 points) | Irradiation on the abdominal region at six different points, with contact at a 90° angle One session Continuous mode |
| Oishi et al. (2024) [30] | AlGaAs diode laser device (DMC Equipment) | 660 | 5.6 per point | 190.4 | 100 | 3.4 | 0.0295 | 56 (6 simultaneous spots) | Irradiation in contact with the abdominal region, with an angle of 90° and 6 simultaneous spots Three times a week for 12 weeks Continuous mode |
| Schiavon et al. (2023) [7] | GaAlAs diode laser (DMC Equipment) | 660 | 5.6 per point | 190 | 100 | 3.40 | 0.0295 | 56 per point (3 points) | Irradiation in animal’s tail, at 3 different points simultaneously, in contact Twice a week for 4 weeks Continuous mode |
| Silva et al. (2025) [31] | Low-level laser therapy | 660 | 5.6 per point | Not reported | 100 | Not reported | Not reported | 6 points | Irradiation on the abdominal region Twice a week for 2 weeks |
| Tomimura et al. (2014) [10] | Laser diode (MMOptics) | 780 | 3.6 per point (calculated) | Informed: 30 Calculated: 90 | 40 | 1 | 0.04 | 90 (1 point) | Transcutaneous irradiation on the rats’ tails. Three times a week for 7 weeks |
References
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion Júnior, D.; et al. Brazilian Guidelines of Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Durao, S.; Toews, I.; Bavuma, C.M.; Hohlfeld, A.; Nury, E.; Meerpohl, J.J.; Kredo, T. Screening strategies for hypertension. Cochrane Database Syst. Rev. 2020, 5, Cd013212. [Google Scholar] [CrossRef]
- Jones, D.W.; Ferdinand, K.C.; Taler, S.J.; Johnson, H.M.; Shimbo, D.; Abdalla, M.; Altieri, M.M.; Bansal, N.; Bello, N.A.; Bress, A.P.; et al. 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2025, 82, e212–e316. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Dos Santos Carvalho Schiavon, M.; de Moraes, L.H.O.; de Moraes, T.F.; Buzinari, T.C.; Neto, J.; Rodrigues, G.J. Chronic red laser treatment induces hypotensive effect in two-kidney one-clip model of renovascular hypertension in rat. Lasers Med. Sci. 2023, 38, 252. [Google Scholar] [CrossRef]
- Buzinari, T.C.; de Moraes, T.F.; Cárnio, E.C.; Lopes, L.A.; Salgado, H.C.; Rodrigues, G.J. Photobiomodulation induces hypotensive effect in spontaneously hypertensive rats. Lasers Med. Sci. 2020, 35, 567–572. [Google Scholar] [CrossRef]
- De Moraes, T.F.; Filho, J.C.C.; Oishi, J.C.; Almeida-Lopes, L.; Parizotto, N.A.; Rodrigues, G.J. Energy-dependent effect trial of photobiomodulation on blood pressure in hypertensive rats. Lasers Med. Sci. 2020, 35, 1041–1046. [Google Scholar] [CrossRef]
- Tomimura, S.; Silva, B.P.; Sanches, I.C.; Canal, M.; Consolim-Colombo, F.; Conti, F.F.; De Angelis, K.; Chavantes, M.C. Hemodynamic effect of laser therapy in spontaneously hypertensive rats. Arq. Bras. Cardiol. 2014, 103, 161–164. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Ganipineni, V.D.P.; Gutlapalli, S.D.; Ajay Sai Krishna Kumar, I.; Monica, P.; Vagdevi, M.; Samuel Sowrab, T. Exploring the Potential of Energy-Based Therapeutics (Photobiomodulation/Low-Level Laser Light Therapy) in Cardiovascular Disorders: A Review and Perspective. Cureus 2023, 15, e37880. [Google Scholar] [CrossRef]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra269. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.Y.; de Sousa, M.V.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3-6 h. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef]
- Pereira, R.D.M.; Alvim, N.A.T.; Pereira, C.D.; Gomes Junior, S. Laser acupuncture protocol for essential systemic arterial hypertension: Randomized clinical trial. Rev. Lat. Am. Enferm. 2018, 26, e2936. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose Response 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Awad, M.A.; Ibrahim, D.A.; Gabr, A.A. Effect of Laser Acupuncture on Reducing Postmenopausal Hypertension. Bull. Fac. Phys. Ther. 2013, 18, 109–117. [Google Scholar]
- Higgins, J.T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023). 2023. Available online: https://www.training.cochrane.org/handbook (accessed on 4 March 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- de Vries, R.B.M.; Hooijmans, C.R.; Langendam, M.W.; van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid.-Based Preclin. Med. 2015, 2, e00007. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- PEDro. PEDro Scale. Available online: https://pedro.org.au/english/resources/pedro-scale/ (accessed on 8 April 2024).
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Neumann, I.; Brennan, S.; Meerpohl, J.; Davoli, M.; Coello, P.A.; Akl, E.; Dahm, P.; Skoetz, N.; Xia, J.; Brozek, J.; et al. Overview of the GRADE approach. In The GRADE Book Version 1.0; 2024; Available online: https://book.gradepro.org (accessed on 4 March 2024).
- Zhang, J.; Marquina, N.; Oxinos, G.; Sau, A.; Ng, D. Effect of laser acupoint treatment on blood pressure and body weight-a pilot study. J. Chiropr. Med. 2008, 7, 134–139. [Google Scholar] [CrossRef]
- Hamed, H.M.; Maghraby, M.A.A. Effectiveness of Laser Acupoint Therapy and Exercise Program on Oxidative Stress and Antioxidant Response in Mild Essential Hypertensive Patients. Bull. Egypt. Soc. Physiol. Sci. 2010, 30, 229–244. [Google Scholar] [CrossRef]
- Oishi, J.C.; de Moraes, L.H.O.; Filho, J.C.C.; de Moraes, T.F.; Terroni, B.; de Castro, C.A.; Almeida-Lopes, L.; Rodrigues, G.J. Long-term effects of photobiomodulation therapy on blood pressure in obese rats induced by a high-fat diet. Lasers Med. Sci. 2024, 39, 20. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Moraes, L.H.O.; Sabadini, C.P.; Alcântara, R.C.C.; Dias, P.C.; Rodrigues, G.J. Chronic treatment with photobiomodulation decreases blood pressure and improves endothelial function in ovariectomized rats. Lasers Med. Sci. 2025, 40, 144. [Google Scholar] [CrossRef]
- Madani, A.; Ahrari, F.; Fallahrastegar, A.; Daghestani, N. A randomized clinical trial comparing the efficacy of low-level laser therapy (LLLT) and laser acupuncture therapy (LAT) in patients with temporomandibular disorders. Lasers Med. Sci. 2020, 35, 181–192. [Google Scholar] [CrossRef]
- Law, D.; McDonough, S.; Bleakley, C.; Baxter, G.D.; Tumilty, S. Laser acupuncture for treating musculoskeletal pain: A systematic review with meta-analysis. J. Acupunct. Meridian Stud. 2015, 8, 2–16. [Google Scholar] [CrossRef]
- Calò, L.A.; Pagnin, E.; Davis, P.A.; Sartori, M.; Ceolotto, G.; Pessina, A.C.; Semplicini, A. Increased expression of regulator of G protein signaling-2 (RGS-2) in Bartter’s/Gitelman’s syndrome. A role in the control of vascular tone and implication for hypertension. J. Clin. Endocrinol. Metab. 2004, 89, 4153–4157. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Caldieraro, M.A.; Norton, R.; Mischoulon, D.; Trinh, N.H.; Nyer, M.; Dording, C.; Hamblin, M.R.; Campbell, B.; Iosifescu, D.V. Reported Side Effects, Weight and Blood Pressure, After Repeated Sessions of Transcranial Photobiomodulation. Photobiomodul. Photomed. Laser Surg. 2019, 37, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Damush, T.; Myers, L.; Anderson, J.; Yu, Z.; Ofner, S.; Schmid, A.; Williams, L. Abstract TP415: Implementation of a Secondary Stroke Prevention Program: Effect on Medication Adherence. Stroke 2013, 44, ATP415. [Google Scholar] [CrossRef]
- de Freitas, V.H.; Mariano, I.M.; Amaral, A.L.; Rodrigues, M.L.; Carrijo, V.H.V.; Puga, G.M. Effects of light-emitting diode therapy on cardiovascular and salivary nitrite responses in postmenopausal women submitted to a single bout of high-intensity interval training. Lasers Med. Sci. 2022, 37, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, L.H.O.; Terroni, B.; da Silva Mayer, N.F.; Rodrigues, G.J. Multidrug-resistant protein inhibitor and phosphodiesterase inhibitor potentiate the vasodilator effect induced by photobiomodulation in isolated aortic rings. Lasers Med. Sci. 2022, 37, 1209–1216. [Google Scholar] [CrossRef]
- Fierro, G.; Armentano, R.; Silveira, F. Evaluation of transit time-based models in wearable central aortic blood pressure estimation. Biomed. Phys. Eng. Express 2020, 6, 035006. [Google Scholar] [CrossRef]
- Goncalves, M.L.Z.V.M.L.L.; Da Silva, C.D.; Barbosa Filho, V.F.; Lourenco, M.F.; Bussadori, S.K.; Deana, A.M. Evaluation of photobiomodulation in salivar production of patients with anti-hypertensive drug-induced xerostomia. Lasers Med. Sci. 2020, 35, 280. [Google Scholar]
- Hirschberg, R.E. Light therapy in treatment of chronic renal disease. In Proceedings of the 28th International Congress Laser Medicine and IALMS Courses, Laser Florence, Florence, Italy, 5–7 November 2015. [Google Scholar] [CrossRef]
- Isabella, A.P.J.; Silva, J.T.C.; da Silva, T.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Motta, L.J.; Bussadori, S.K.; Pavani, C.; Silva, D.F.T.D. Effect of irradiation with intravascular laser on the hemodynamic variables of hypertensive patients: Study protocol for prospective blinded randomized clinical trial. Medicine 2019, 98, e15111. [Google Scholar] [CrossRef]
- Semplicini, A.; Lenzini, L.; Sartori, M.; Papparella, I.; Calò, L.A.; Pagnin, E.; Strapazzon, G.; Benna, C.; Costa, R.; Avogaro, A.; et al. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J. Hypertens. 2006, 24, 1115–1124. [Google Scholar] [CrossRef]
- Syed, S.B.; Ahmet, I.; Chakir, K.; Morrell, C.H.; Arany, P.R.; Lakatta, E.G. Photobiomodulation therapy mitigates cardiovascular aging and improves survival. Lasers Surg. Med. 2023, 55, 278–293. [Google Scholar] [CrossRef]
- Valverde, A.; Mitrofanis, J. Photobiomodulation for Hypertension and Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2022, 90, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Bäumer, A.T.; Strehlow, K.; van Eickels, M.; Grohé, C.; Ahlbory, K.; Rösen, R.; Böhm, M.; Nickenig, G. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation 2001, 103, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, X.; Liu, J.; Sun, J.; Sun, H.; Zhao, J.; Jiang, T.; Li, Y. A4894 Correlation Analysis Between Peripheral Blood Monocyte Subsets and Left Ventricular Remodeling in Spontaneously Hypertensive Rats. J. Hypertens. 2018, 36, e38. [Google Scholar] [CrossRef]
- Buzinari, T.C.; de Moraes, T.F.; Conceição-Filho, J.C.; Cárnio, E.C.; Almeida-Lopes, L.; Salgado, H.C.; Rodrigues, G.J. Nitric oxide storage levels modulate vasodilation and the hypotensive effect induced by photobiomodulation using an aluminum gallium arsenide (AlGaAs) diode laser (660 nm). Lasers Med. Sci. 2022, 37, 2753–2762. [Google Scholar] [CrossRef]
- Canal, M.; Conti, F.; Pinto, N.; Pinto, M.; Silva, B.; Sanches, I.; Duarte, I.; De Angelis, K. Hemodynamic changes post low level laser therapy in elderly obese rats: An experimental study. In Proceedings of the 33rd Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS, Boston, MA, USA, 3–7 April 2013. [Google Scholar] [CrossRef]
- Elmahy, R.M.A.; Mohamed, G.S.; Rashed, L.A. Influence of Laser Puncture on Endothelial Dysfunction on Hypertensive Patients. Trends Med. Res. 2016, 11, 107–112. [Google Scholar] [CrossRef]
- Madi, O.; Tomimura, S.; Pinto, N.; Duarte, I.; Lopes, H.; Colombo, F.; Chavantes, M.C. The immediate hemodynamic response post laser therapy in hypertensive and normotensive pregnant woman. In Proceedings of the 36th Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS, Boston, MA, USA, 30 March–3 April 2016. [Google Scholar] [CrossRef]
- Oishi, J.C.; De Moraes, T.F.; Buzinari, T.C.; Cárnio, E.C.; Parizotto, N.A.; Rodrigues, G.J. Hypotensive acute effect of photobiomodulation therapy on hypertensive rats. Life Sci. 2017, 178, 56–60. [Google Scholar] [CrossRef]
- Silva, B.; Tomimura, S.; Sanches, I.; Canal, M.; Pinto, N.; Madi, O.; Conti, F.; Angelis, K.; Colomb, F.; Chavantes, M.C. Low Level Laser Therapy Improves Cardiovascular Autonomic Activity in Spontaneously Hypertensive Rats. Lasers Surg. Med. 2015, 47, 382–383. [Google Scholar]
- Kovalenko, Y.L.; Melekhovets, O.K.; Orlovskyi, V.F.; Melekhovets, Y.V. Correction of functional capacity of myocardium in arterial hypertension associated with hyperuricemia and poikilocytosis comorbidities. Zaporozhye Med. J. 2019, 21, 420–427. [Google Scholar] [CrossRef]
- Achilov, A.A.; Lebedeva, O.D.; Bulatetskaia, L.S.; Usmonzoda, D.U.; Belov, A.S.; Kotov, S.A.; Achilova, S.hA.; Rykov, S.V. Potentials of combined non-medication therapy of arterial hypertension associated with ischemic heart disease. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2010, 6, 12–15. (In Russian) [Google Scholar]
- Kucheriavyĭ, A.M.; Ponomarenko, G.N.; Kovlen, D.V. Magnetolaser therapy of patients with bronchial asthma in combination with essential hypertension. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2007, 4–7. (In Russian) [Google Scholar]
- Antoniuk, M.V.; Kantur, T.A.; Karaman, I.uK.; Zhukova, N.V. Combined application of magnetolaserotherapy and polyunsaturated fatty acids for the treatment of patients with hypertensive disease. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2011, 9–13. (In Russian) [Google Scholar]
- Ucero, A.C.; Sabban, B.; Benito-Martin, A.; Carrasco, S.; Joeken, S.; Ortiz, A. Laser therapy in metabolic syndrome-related kidney injury. Photochem. Photobiol. 2013, 89, 953–960. [Google Scholar] [CrossRef]
- Pandey, R.; Lutle, L.; Mishra, A. Low Level Laser Therapy (Lllt) For the Treatment of Hypertension. Int. J. Eng. Res. Dev. 2013, 6, 35–38. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataglia Espósito, L.M.; Costa da Rocha, F.; Arany, P.R.; Ferraresi, C. Photobiomodulation Therapy in Hypertension Management—Evidence from a Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6716. https://doi.org/10.3390/jcm14196716
Bataglia Espósito LM, Costa da Rocha F, Arany PR, Ferraresi C. Photobiomodulation Therapy in Hypertension Management—Evidence from a Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(19):6716. https://doi.org/10.3390/jcm14196716
Chicago/Turabian StyleBataglia Espósito, Lara Maria, Francisco Costa da Rocha, Praveen R. Arany, and Cleber Ferraresi. 2025. "Photobiomodulation Therapy in Hypertension Management—Evidence from a Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 19: 6716. https://doi.org/10.3390/jcm14196716
APA StyleBataglia Espósito, L. M., Costa da Rocha, F., Arany, P. R., & Ferraresi, C. (2025). Photobiomodulation Therapy in Hypertension Management—Evidence from a Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(19), 6716. https://doi.org/10.3390/jcm14196716







