Short-Term Repeated Transcutaneous Spinal Cord Stimulation Yields Sustained Orthostatic Benefits in Chronic Cervical SCI: A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Description
2.2. Study Design and Assessment Procedures
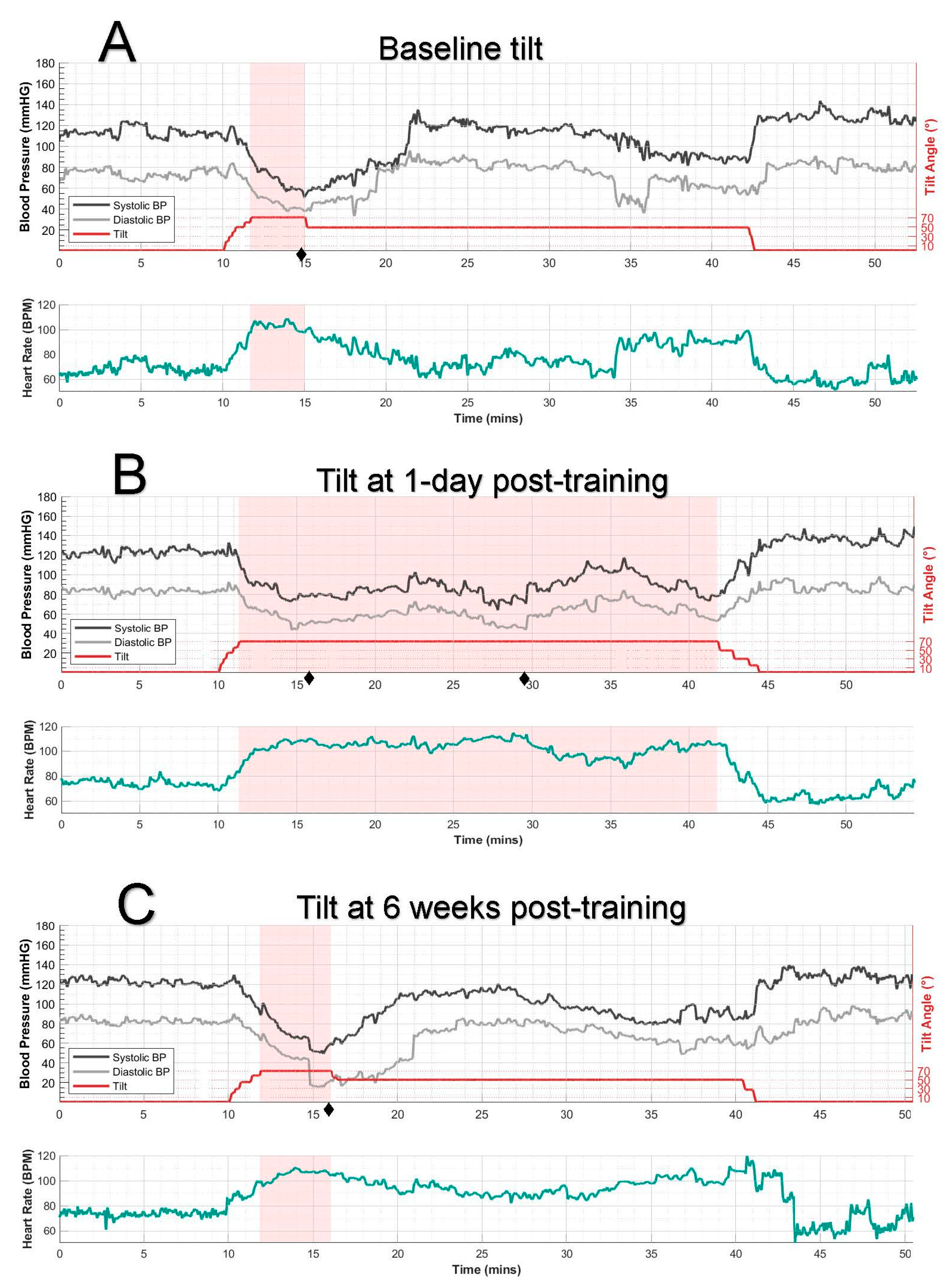
2.2.1. Tilt Testing
2.2.2. Cardiovascular Monitoring and Analysis
2.2.3. Stimulation Mapping
2.2.4. Training
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Autonomic dysreflexia |
| ADFSCI | Autonomic Dysfunction Following Spinal Cord Injury |
| BP | Blood pressure |
| AIS | American Spinal Injury Association (ASIA) Impairment Scale |
| CV | Cardiovascular |
| DBP | Diastolic blood pressure |
| HR | Heart rate |
| ISNCSCI | International Standards for Neurological Classification of SCI |
| OH | Orthostatic Hypotension |
| SBP | Systolic blood pressure |
| SCI | Spinal cord injury |
| ScES | Spinal cord epidural stimulation |
| ScES-CV | Spinal cord epidural stimulation focusing on cardiovascular function |
| ScTS | Spinal cord transcutaneous stimulation |
| ScTS-CV | Spinal cord transcutaneous stimulation focusing on cardiovascular function |
| sEMG | Surface electromyography |
References
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996, 46, 1470. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Wecht, J.M. Management of blood pressure disorders in individuals with spinal cord injury. Curr. Opin. Pharmacol. 2022, 62, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A.V.; Karlsson, A.K.; Wecht, J.M.; Wuermser, L.A.; Mathias, C.J.; Marino, R.J. Assessment of autonomic dysfunction following spinal cord injury: Rationale for additions to International Standards for Neurological Assessment. J. Rehabil. Res. Dev. 2007, 44, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sarafis, Z.K.; Monga, A.K.; Phillips, A.A.; Krassioukov, A.V. Is Technology for Orthostatic Hypotension Ready for Primetime? PM&R. 2018, 10, S249–S263. [Google Scholar] [CrossRef]
- Phillips, A.A.; Warburton, D.E.R.; Ainslie, P.N.; Krassioukov, A.V. Regional neurovascular coupling and cognitive performance in those with low blood pressure secondary to high-level spinal cord injury: Improved by alpha-1 agonist midodrine hydrochloride. J. Cereb. Blood Flow Metab. 2014, 34, 794–801. [Google Scholar] [CrossRef]
- Wecht, J.M.; Weir, J.P.; Katzelnick, C.G.; Wylie, G.; Eraifej, M.; Nguyen, N.; Dyson-Hudson, T.; Bauman, W.A.; Chiaravalloti, N. Systemic and cerebral hemodynamic contribution to cognitive performance in spinal cord injury. J. Neurotrauma 2018, 35, 2957–2964. [Google Scholar] [CrossRef]
- Cragg, J.J.; Noonan, V.K.; Krassioukov, A.; Borisoff, J. Cardiovascular disease and spinal cord injury: Results from a national population health survey. Neurology 2013, 81, 723–728. [Google Scholar] [CrossRef]
- Eigenbrodt, M.L.; Rose, K.M.; Couper, D.J.; Arnett, D.K.; Smith, R.; Jones, D. Orthostatic hypotension as a risk factor for stroke: The atherosclerosis risk in communities (ARIC) study, 1987-1996. Stroke 2000, 31, 2307–2313. [Google Scholar] [CrossRef]
- Rose, K.M.; Tyroler, H.A.; Nardo, C.J.; Arnett, D.K.; Light, K.C.; Rosamond, W.; Sharrett, A.R.; Szklo, M. Orthostatic hypotension and the incidence of coronary heart disease: The Atherosclerosis Risk in Communities study. Am. J. Hypertens. 2000, 13, 571–578. [Google Scholar] [CrossRef]
- Carlozzi, N.E.; Fyffe, D.; Morin, K.G.; Byrne, R.; Tulsky, D.S.; Victorson, D.; Lai, J.-S.; Wecht, J.M. Impact of Blood Pressure Dysregulation on Health-Related Quality of Life in Persons with Spinal Cord Injury: Development of a Conceptual Model. Arch. Phys. Med. Rehabil. 2013, 94, 1721–1730. [Google Scholar] [CrossRef]
- Krassioukov, A.; Eng, J.J.; Warburton, D.E.; Teasell, R. A Systematic Review of the Management of Orthostatic Hypotension After Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2009, 90, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.M.; Weir, J.P.; Katzelnick, C.G.; Chiaravalloti, N.D.; Kirshblum, S.C.; Dyson-Hudson, T.A.; Weber, E.; Bauman, W.A. Double-blinded, placebo-controlled crossover trial to determine the effects of midodrine on blood pressure during cognitive testing in persons with SCI. Spinal Cord. 2020, 58, 959–969. [Google Scholar] [CrossRef]
- Law, M.; Sachdeva, R.; Darrow, D.; Krassioukov, A. Cardiovascular Effects of Spinal Cord Stimulation: The Highs, the Lows, and the Don’t Knows. Neuromodulation 2024, 27, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Samejima, S.; Shackleton, C.; Malik, R.N.; Cao, K.; Bohorquez, A.; Nightingale, T.E.; Sachdeva, R.; Krassioukov, A.V. Spinal Cord Stimulation Prevents Autonomic Dysreflexia in Individuals with Spinal Cord Injury: A Case Series. J. Clin. Med. 2023, 12, 2897. [Google Scholar] [CrossRef]
- Sachdeva, R.; Nightingale, T.E.; Pawar, K.; Kalimullina, T.; Mesa, A.; Marwaha, A.; Williams, A.M.M.; Lam, T.; Krassioukov, A.V. Noninvasive Neuroprosthesis Promotes Cardiovascular Recovery After Spinal Cord Injury. Neurotherapeutics 2021, 18, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.J.; Wang, S.; Angeli, C.A.; Chen, Y.; Boakye, M.; Ugiliweneza, B.; Hirsch, G.A. Normalization of Blood Pressure with Spinal Cord Epidural Stimulation After Severe Spinal Cord Injury. Front. Hum. Neurosci. 2018, 12, 83. [Google Scholar] [CrossRef]
- Phillips, A.A.; Squair, J.W.; Sayenko, D.G.; Edgerton, V.R.; Gerasimenko, Y.; Krassioukov, A.V. An Autonomic Neuroprosthesis: Noninvasive Electrical Spinal Cord Stimulation Restores Autonomic Cardiovascular Function in Individuals with Spinal Cord Injury. J. Neurotrauma 2018, 35, 446–451. [Google Scholar] [CrossRef]
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J.; et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 2021, 590, 308–314. [Google Scholar] [CrossRef]
- Aslan, S.C.; Ditterline, B.E.L.; Park, M.C.; Angeli, C.A.; Rejc, E.; Chen, Y.; Ovechkin, A.V.; Krassioukov, A.; Harkema, S.J. Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits. Front. Physiol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Engel-Haber, E.; Bheemreddy, A.; Bayram, M.B.; Ravi, M.; Zhang, F.; Su, H.; Kirshblum, S.; Forrest, G.F. Neuromodulation in Spinal Cord Injury Using Transcutaneous Spinal Stimulation—Mapping for a Blood Pressure Response: A Case Series. Neurotrauma Rep. 2024, 5, 845–856. [Google Scholar] [CrossRef]
- Harkema, S.J.; Ditterline, B.L.; Wang, S.; Aslan, S.; Angeli, C.A.; Ovechkin, A.; Hirsch, G.A. Epidural Spinal Cord Stimulation Training and Sustained Recovery of Cardiovascular Function in Individuals with Chronic Cervical Spinal Cord Injury. JAMA Neurol. 2018, 75, 1569–1571. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Goldsmith, J.; Alazzam, A.; Trainer, R. Effects of percutaneously-implanted epidural stimulation on cardiovascular autonomic function and spasticity after complete spinal cord injury: A case report. Front. Neurosci. 2023, 17, 1112853. [Google Scholar] [CrossRef] [PubMed]
- Bloom, O.; Wecht, J.M.; Ditterline, B.E.L.; Wang, S.; Ovechkin, A.V.; Angeli, C.A.; Arcese, A.A.; Harkema, S.J. Prolonged Targeted Cardiovascular Epidural Stimulation Improves Immunological Molecular Profile: A Case Report in Chronic Severe Spinal Cord Injury. Front. Syst. Neurosci. 2020, 14, 72. [Google Scholar] [CrossRef]
- Boakye, M.; Ball, T.; Dietz, N.; Sharma, M.; Angeli, C.; Rejc, E.; Kirshblum, S.; Forrest, G.; Arnold, F.W.; Harkema, S. Spinal cord epidural stimulation for motor and autonomic function recovery after chronic spinal cord injury: A case series and technical note. Surg. Neurol. Int. 2023, 14, 87. [Google Scholar] [CrossRef]
- Hodgkiss, D.D.; Williams, A.M.M.; Shackleton, C.S.; Samejima, S.; Balthazaar, S.J.T.; Lam, T.; Krassioukov, A.V.; Nightingale, T.E. Ergogenic effects of spinal cord stimulation on exercise performance following spinal cord injury. Front. Neurosci. 2024, 18, 1435716. [Google Scholar] [CrossRef]
- International Standards for Neurological Classification of Spinal Cord Injury; American Spinal Injury Association: Richmond, VA, USA, 2019.
- Benditt, D.G.; Ferguson, D.W.; Grubb, B.P.; Kapoor, W.N.; Kugler, J.; Lerman, B.B.; Maloney, J.D.; Ravielle, A.; Ross, B.; Sutton, R.; et al. Tilt table testing for assessing syncope. J. Am. Coll. Cardiol. 1996, 28, 263–275. [Google Scholar] [CrossRef]
- Hubli, M.; Gee, C.M.; Krassioukov, A.V. Refined Assessment of Blood Pressure Instability After Spinal Cord Injury. Am. J. Hypertens. 2015, 28, 173–181. [Google Scholar] [CrossRef]
- West, C.R.; Phillips, A.A.; Squair, J.W.; Williams, A.M.; Walter, M.; Lam, T.; Krassioukov, A.V. Association of Epidural Stimulation With Cardiovascular Function in an Individual With Spinal Cord Injury. JAMA Neurol. 2018, 75, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Imholz, B.P.M.; Wieling, W.; Van Montfrans, G.A.; Wesseling, K.H. Fifteen years experience with finger arterial pressure monitoring: Assessment of the technology. Cardiovasc. Res. 1998, 38, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Momeni, K.; Ramanujam, A.; Garbarini, E.L.; Forrest, G.F. Multi-muscle electrical stimulation and stand training: Effects on standing. J. Spinal Cord. Med. 2019, 42, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Momeni, K.; Pilkar, R.; Ravi, M.; Bheemreddy, A.; Garbarini, E.; Forrest, G.F. Spinal Cord Transcutaneous Stimulation Enables Volitional Knee Extension in Motor-complete SCI. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Glasgow, UK, 11–15 July 2022; pp. 2373–2376. [Google Scholar] [CrossRef]
- Momeni, K.; Pilkar, R.; Ravi, M.; Forrest, G.F. Isolating Transcutaneous Spinal Cord Stimulation Artifact to Identify Motor Response during Walking. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Virtual, 1–5 November 2021; pp. 6569–6572. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Angeli, C.; Rejc, E.; Boakye, M.; Herrity, A.; Mesbah, S.; Hubscher, C.; Forrest, G.; Harkema, S. Targeted Selection of Stimulation Parameters for Restoration of Motor and Autonomic Function in Individuals with Spinal Cord Injury. Neuromodulation 2024, 27, 645–660. [Google Scholar] [CrossRef]
- Yi Lau, S.J.; Hang, P.Z.; Lijia, W.; Jing, C. Effective management of orthostatic hypotension in a patient with complete spinal cord injury using a portable transcutaneous spinal cord stimulation device: A case report. J. Spinal Cord Med. 2025, 1–5. [Google Scholar] [CrossRef]
- DiMarco, A.F.; Geertman, R.T.; Tabbaa, K.; Nemunaitis, G.A.; Kowalski, K.E. Restoration of cough via spinal cord stimulation improves pulmonary function in tetraplegics. J. Spinal Cord. Med. 2019, 43, 579. [Google Scholar] [CrossRef]
- DiMarco, A.F.; Kowalski, K.E.; Geertman, R.T.; Hromyak, D.R.; Frost, F.S.; Creasey, G.H.; Nemunaitis, G.A. Lower Thoracic Spinal Cord Stimulation to Restore Cough in Patients with Spinal Cord Injury: Results of a National Institutes of Health–Sponsored Clinical Trial. Part II: Clinical Outcomes. Arch. Phys. Med. Rehabil. 2009, 90, 726–732. [Google Scholar] [CrossRef]
- Richardson, R.R.; Cerullo, L.J.; Meyer, P.R. Autonomic hyper-reflexia modulated by percutaneous epidural neurostimulation: A preliminary report. Neurosurgery 1979, 4, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Samejima, S.; Shackleton, C.; Miller, T.; Moritz, C.T.; Kessler, T.M.; Krogh, K.; Sachdeva, R.; Krassioukov, A.V. Mapping the Iceberg of Autonomic Recovery: Mechanistic Underpinnings of Neuromodulation following Spinal Cord Injury. Neuroscientist 2024, 30, 378–389. [Google Scholar] [CrossRef]
- Solinsky, R.; Draghici, A.; Hamner, J.W.; Goldstein, R.; Taylor, J.A. High-intensity, whole-body exercise improves blood pressure control in individuals with spinal cord injury: A prospective randomized controlled trial. PLoS ONE 2021, 16, e0247576. [Google Scholar] [CrossRef] [PubMed]
| Tilt | Duration of 70° Tilt (min) | SBP, Avg ± SD | DBP, Avg ± SD | HR, Avg ± SD | Max Symptoms Total | |||
|---|---|---|---|---|---|---|---|---|
| Supine | 70° Tilt | Supine | 70° Tilt | Supine | 70° Tilt | |||
| Baseline | 3.3 | 112 ± 4 | 69 ± 10 | 74 ± 4 | 46 ± 6 | 68 ± 5 | 102 ± 7 | 13 |
| Δ = −43 | Δ = −28 | Δ = +34 | ||||||
| 1 day posttraining | 30.5 | 124 ± 3 | 88 ± 11 | 84 ± 2 | 61 ± 9 | 74 ± 3 | 100 ± 11 | 4 (0) * |
| Δ = −36 | Δ = −23 | Δ = +26 | ||||||
| 6 weeks posttraining | 4.2 | 121 ± 3 | 69 ± 14 | 82 ± 3 | 41 ± 17 | 74 ± 4 | 105 ± 4 | 12 |
| Δ = −52 | Δ = −41 | Δ = +31 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel-Haber, E.; Bheemreddy, A.; Bayram, M.B.; Ravi, M.; Snider, B.; Kirshblum, S.; Forrest, G.F. Short-Term Repeated Transcutaneous Spinal Cord Stimulation Yields Sustained Orthostatic Benefits in Chronic Cervical SCI: A Case Study. J. Clin. Med. 2025, 14, 6700. https://doi.org/10.3390/jcm14196700
Engel-Haber E, Bheemreddy A, Bayram MB, Ravi M, Snider B, Kirshblum S, Forrest GF. Short-Term Repeated Transcutaneous Spinal Cord Stimulation Yields Sustained Orthostatic Benefits in Chronic Cervical SCI: A Case Study. Journal of Clinical Medicine. 2025; 14(19):6700. https://doi.org/10.3390/jcm14196700
Chicago/Turabian StyleEngel-Haber, Einat, Akhil Bheemreddy, Mehmed Bugrahan Bayram, Manikandan Ravi, Brittany Snider, Steven Kirshblum, and Gail F. Forrest. 2025. "Short-Term Repeated Transcutaneous Spinal Cord Stimulation Yields Sustained Orthostatic Benefits in Chronic Cervical SCI: A Case Study" Journal of Clinical Medicine 14, no. 19: 6700. https://doi.org/10.3390/jcm14196700
APA StyleEngel-Haber, E., Bheemreddy, A., Bayram, M. B., Ravi, M., Snider, B., Kirshblum, S., & Forrest, G. F. (2025). Short-Term Repeated Transcutaneous Spinal Cord Stimulation Yields Sustained Orthostatic Benefits in Chronic Cervical SCI: A Case Study. Journal of Clinical Medicine, 14(19), 6700. https://doi.org/10.3390/jcm14196700






