Abstract
Background: Childhood obesity has reached epidemic levels in developed countries and is an emerging concern in developing regions. Children with excess weight are more likely to maintain this condition over time into adulthood and face a higher risk of developing metabolic disorders such as type 2 diabetes, hypertension, metabolic dysfunction-associated liver disease, and dyslipidemia. Early identification of obesity risk is, therefore, a key public health challenge. Methods: This is an observational, prospective, single-center cohort pilot study in 66 mother–infant dyads recruited at the Gynecology and Obstetrics Service of the Virgen de la Arrixaca University Hospital (Murcia, Spain). The primary objective is to identify early-life, non-invasive biomarkers associated with increased adiposity by integrating multi-omics approaches and analyzing maternal–infant interactions. Pregnant women will be enrolled during the third trimester and will undergo a baseline visit at 38 weeks of gestation for clinical and anthropometric assessment. Buccal swabs and fecal samples will be collected at baseline and in the peripartum period for epigenetic (DNA methylation), metagenomic, and metabolomic analyses. Infants will be evaluated at birth and followed at 6 months, 1 year, 2 years, and 3 years. Each visit will include detailed anthropometric measurements, along with collection of buccal swabs and fecal samples for multi-omics profiling. Conclusions: This multidisciplinary study aims to assess how maternal factors influence infant epigenetic and microbial patterns, and their relation to adiposity development. Early identification of such biomarkers may guide personalized prevention strategies and reduce the long-term burden of obesity-related comorbidities.
1. Introduction
Childhood obesity has reached epidemic levels in developed countries and is becoming a major cause for concern in the developing world. According to the World Health Organization (WHO), in 2020, over 39 million children under 5 years of age were affected by overweight or obesity [1,2]. In Europe, nowadays, obesity represents a major public health challenge, with a significant socio-economic burden associated with its management [3]. In fact, children with excess weight are more likely to maintain this condition over time and are at increased risk for long-term metabolic diseases, such as insulin resistance, type 2 diabetes mellitus, hypertension, metabolic dysfunction-associated liver disease, obstructive sleep apnea, and dyslipidemia [4,5,6,7,8]. The causes of childhood obesity are complex and multifactorial, including the interaction between genetics, environmental and developmental factors, and gut microbiota [9,10,11,12]. In addition, the risk of developing long-term diseases such as obesity is significantly higher in children born to both women with obesity at the beginning of pregnancy and those with excessive weight gain during gestation [13,14,15].
In recent years, the number of studies focused on identifying early-life predictors of obesity risk has increased. Currently, the most widely accepted predictors include child size, growth velocity (mainly in the first months of life), and neonatal adiposity. Evidence indicates that high neonatal adiposity, greater body size, and rapid weight or fat gain during the early postnatal period are all associated with an increased risk of long-term obesity [16,17,18], as well as a higher likelihood of developing other metabolic disorders [19,20], hypertension [21], and asthma [22,23].
Although the genetic factors involved in the development of childhood obesity are only partially understood, several studies have associated different epigenetic changes during the first months of life and the risk of long-term obesity. Within these epigenetic changes, the analysis of modifications in DNA methylation is the most studied in relation to increased adiposity [24]. In the case of adiposity in newborns, these epigenetic changes are mainly influenced by maternal environmental factors, such as obesity at the time of conception or lifestyle during pregnancy [25,26,27]. For example, it has been described that the methylation of the cg11531579 (CHFR) site is related to rapid weight gain at early ages and the development of long-term obesity [24].
On the other hand, in recent years there has been increasing evidence about the role of the gut microbiota and its derived metabolites as epigenetic regulators [28]. Such epigenetic modifications can lead to profound reprogramming in the host genome, thus altering the transcriptional machinery of cells and sensitizing them to different stimuli that affect both physiological processes [29] and the development of metabolic diseases, including obesity [30]. The establishment of the gut microbiota is a complex process that occurs in a stepwise manner and involves a dynamic evolution in abundance and diversity [31]. Although the first 2 years of life are essential for the establishment of the microbiota, there are two critical moments that affect intestinal microbial colonization, which are the first month of life, and at weaning after the introduction of new foods [32,33,34,35,36]. These represent the periods of maximum immune and homeostatic reorganization and have a great influence on the growth and the development of the individual [37]. In fact, several studies have linked alterations during the first years of live in the gut microbiota ant its derived metabolites such as short-chain fatty acids (SCFA), branched-chain amino acids (BCAA), and bile acids, to the development of various diseases, including obesity [38,39,40,41,42]. However, the molecular mechanisms by which the gut microbiota and its derived metabolites directly or indirectly influence epigenetic changes involved in the development of obesity during this critical early-life period remain largely unknown.
In this study, we aim to identify potential non-invasive biomarkers related to adiposity increase during early life by integrating various omics approaches. To achieve this, non-invasive sample collection methods will be used, which is particularly relevant when referring to early infancy, as it may provide an effective tool for the prediction and/or prevention of the development of childhood obesity and associated metabolic diseases.
2. Materials and Methods
2.1. Study Design
A single-center, observational, prospective cohort pilot study will be conducted with maternal–infant dyads at the Gynecology and Obstetrics Service and the Pediatrics Service of the Virgen de la Arrixaca University Hospital (HCUVA) in Murcia, Spain. Healthy pregnant women attending the third-trimester ultrasound appointment (32–36 weeks) at the Gynecology and Obstetrics Service, and who meet the inclusion criteria, will be recruited. Their child will be followed for the first 3 years of life.
2.2. Study Objectives
The main objective of this study is to identify potential non-invasive biomarkers related to adiposity during early life by integrating various omics approaches.
To achieve this, three specific aims are defined:
- To determine DNA methylation patterns, gut microbiota composition, and metabolomic profiles at different early-life stages to identify potential biomarkers related to increased adiposity.
- To establish the relationship between changes in epigenetic, microbial, and metabolomic profiles during the first years of life.
- To evaluate the influence of maternal pregestational epigenetic, microbial, and metabolomic patterns on changes in neonatal adiposity and to establish links with corresponding neonatal patterns.
2.3. Study Settings
Volunteers will be recruited at the Gynecology and Obstetrics Service of the HCUVA. Each pregnant volunteer will be assigned a consecutive numerical code upon inclusion in the study. Likewise, the corresponding offspring samples will be assigned the same numerical code, followed by a letter.
2.4. Eligibility Criteria
2.4.1. Inclusion Criteria
Pregnant women meeting the following criteria will be eligible:
- Age between 18 and 45 years of age.
- Within the third trimester of gestation.
2.4.2. Exclusion Criteria
Participants (mothers and/or children) who fulfill any of the following criteria will be excluded from the study:
- Toxic habits during pregnancy.
- Volunteers who suffer from autoimmune disease.
- Volunteers who suffer or have suffered from cancer.
- Severe malformation of the fetus.
- Multiple pregnancy.
- Pregestational diabetes.
- Preeclampsia.
- Altered mental state that prevents understanding of the informed consent process and/or completion of the study.
- Birthweight below the third percentile.
- Children requiring hospitalization by pediatrics immediately after birth.
- Antibiotic consumption.
- Consumption of probiotics at least within the month prior to sample collection.
- Any condition that, in the judgement of the investigators, could interfere with the study objectives or participant safety.
2.5. Consent
Informed consent will be obtained from all women who wish to participate in the study by the medical specialist or research nurse at the Gynecology and Obstetrics Service. A member of the research team will provide volunteers with detailed information about the objectives and the methodology of the study and will give them a written Information Sheet (Supplementary Material). Similarly, both parents will be informed of the objectives and methodology of the study for the inclusion of the child and the informed consent form must be signed by both parents (when applicable). The Declaration of Helsinki will be followed at all stages of the study.
2.6. Withdrawal
The subjects participating in the study may withdraw or revoke consent at any time without giving explanation and without liability or prejudice to them. Individuals who withdraw from the study will not be further followed up and will not be replaced by new participants. The researchers/clinicians may withdraw a subject from the study if they consider that the subject can no longer comply with all the requirements of the study or if any of the procedures are considered as potentially harmful to the subject or the child. Data already collected on withdrawn subjects will be retained and used for analysis, but no new data will be collected after withdrawal. Participants (mothers and children) who meet any of these criteria will be withdrawn from the study:
- Presence of adverse events that at the discretion of the investigator implies the withdrawal of the subject.
- Deviation from the protocol that affects the interpretation of the results of the study and its scientific validity.
- Medical decision.
- Resignation of the individual to continue in the study.
- Loss of follow-up.
2.7. Safety Assessment During Childbirth
Both vaginal and cesarean deliveries will be supervised and assisted by experienced professionals from the Gynecology and Obstetrics Service of the HCUVA following the standard protocol of the service, and without any influence of the study participation on clinical decision-making. Nevertheless, the safety of the volunteers and their offspring will be carefully monitored at all times, both during their admission to the hospital and their subsequent discharge, as well as during the various post-delivery follow-up visits to the hospital. This procedure will be routinely carried out by the staff of both the Gynecology and Obstetrics Service and the Pediatrics Service of the HCUVA.
2.8. Research Ethics Approval
This study will be carried out in the HCUVA in Murcia, Spain, in accordance with the current Spanish legislation that regulates biomedical research projects. The study has already obtained written authorization from the Research Ethics Committee (internal code 2023-2-13-HCUVA). All procedures comply with the ethical principles of Good Clinical Practice (GCP), the Declaration of Helsinki and all applicable regulations and the current legislation governing biomedical research, as well as with the current national and European data protection regulations, including General Data Protection Regulation (GDPR, EU 2016/679).
2.9. Protocol Amendments
Except in the case of emergency situations, no changes or deviations from the study protocol will be permitted without prior documented approval by the Research Ethics Committee. The committee must be informed of any proposed modification and must provide written approval for any changes or deviations that could adversely affect the rights of the volunteer or the integrity of the research. This requirement does not apply to changes intended solely to minimize inconvenience or prevent risks to participants, or to modifications related to the administrative aspects of the study.
2.10. Outcomes
The primary outcome of this study is to determine whether the dynamics of changes in the epigenome, metabolome, and microbiome in mother–infant dyads are associated with increased adiposity during the first years of life, and whether these changes can serve as early biomarkers for future metabolic risk. Secondary outcomes include the detailed characterization of the epigenetic profile using buccal swab samples, analysis of microbiota composition, diversity, and richness from stool samples, and targeted metabolomic profiling of bile acids, SCFAs and BCAAs in stool samples.
2.11. Participant Timeline
Eligible volunteers who agree to participate will be enrolled and evaluated by a gynecologist. The mother will be assessed in week 38 of pregnancy and on the day of delivery, and the child will be evaluated at birth, and followed up at 1 month, 6 months, 1 year, 2 years, and 3 years after birth (Figure 1). A detailed assessment schedule is provided in Table 1.
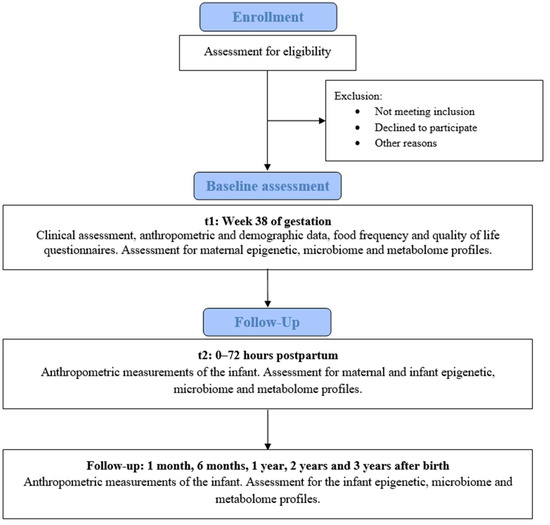
Figure 1.
Flowchart of participant inclusion and follow-up.

Table 1.
Schedule of assessments and follow-up visits.
2.12. Sample Size
This is a pilot observational study aiming to explore potential associations between dynamic changes in the epigenome, metabolome, and microbiome of mother–infant dyads and subsequent increases in infant adiposity. Based on different previous studies on the association of both the microbiome and epigenetic changes with adiposity [24,43,44], to reach a significance level of 0.05 and a statistical power (β) of 0.8, we established an n = 51 of mother–child pairs. A loss rate of around 30% is expected; 66 mother–child pairs will be included in the study. This pilot study will provide preliminary data to inform the design and sample size calculation for a larger study, which will be powered to detect statistically significant associations.
2.13. Data and Sample Collection and Analysis of Variables
2.13.1. Data Collection
Data and biological samples from participating mother–infant dyads will be collected at multiple time points throughout the study (Figure 2). Maternal data and samples will be obtained during the third trimester of pregnancy (gestational week 36) and on the day of delivery. Offspring data and samples will be collected at birth (within 0–48 h postpartum) and during follow-up visits at 1 month, 6 months, 1 year, 2 years, and 3 years of age.
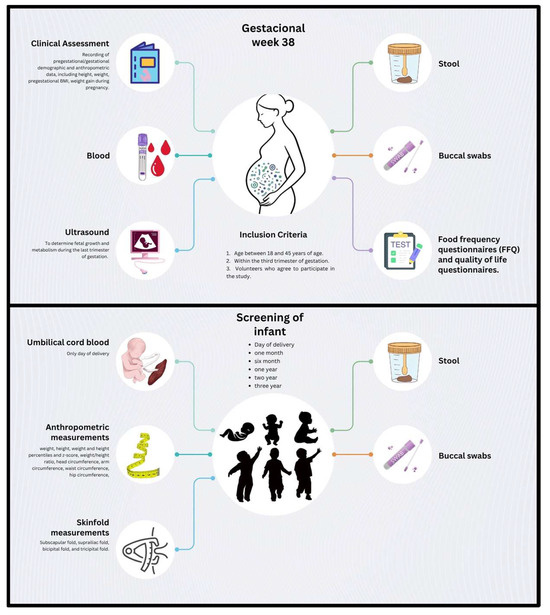
Figure 2.
Overview of the data and sample collection in the study protocol. Schematic representation of the data and biological samples to be collected throughout the study. Maternal blood, stool, and buccal swab samples will be collected at week 38 of gestation (t = 1). During this visit, an ultrasound, clinical assessment, and food frequency questionnaires will also be conducted. Offspring stool and buccal swab samples, along with anthropometric measurements, will be collected at birth (t = 2), 1 month (t = 3), 6 months (t = 4), 1 year (t = 5), 2 years (t = 6), and 3 years (t = 7) of age.
At the maternal third-trimester visit, a complete clinical evaluation will be conducted. This will include the collection of sociodemographic information, obstetric history, and anthropometric measurements (height, weight, pregestational BMI, and gestational weight gain). Nutritional status will be assessed through a validated Food Frequency Questionnaire (FFQ) [45,46], maternal well-being will be evaluated using a standardized quality of life questionnaire, and eating behavior and weight-related attitudes will be evaluated with the Pregnancy Eating Attitudes-Questionnaire (PEA-Q) [47]. Additionally, all participants will undergo an ultrasound examination between 36 + 0 and 37 + 6 weeks of gestation, including fetal biometry for growth assessment, Doppler evaluation of the fetal and placental compartments, and measurement of fetal metabolic and adiposity indicators.
For the infant participants, detailed anthropometric data will be recorded at each time point. Measurements will include weight, height/length, corresponding percentiles and z-scores, weight-for-height ratio, head circumference, mid-upper arm circumference, waist circumference, hip circumference, and skinfold thickness at the subscapular, suprailiac, bicipital, and tricipital sites. These measurements will be conducted by trained personnel using standardized procedures. Total fat mass will be estimated using the skinfold data, measured with a Holtain skinfold caliper (Holtain Ltd., Bryberian, UK), and calculated using validated equations [48].
2.13.2. Sample Collection and Storage
Biological samples collected from mothers will include saliva, blood, and stool. From infants, saliva and stool samples will be collected at scheduled time points (Figure 2).
All samples will be collected using sterile techniques. Stool samples will be transferred into sterile containers, kept at 4 °C during short-term handling, and subsequently stored at −80 °C. Buccal swabs will be collected at delivery and follow-up visits using the Buccal Swab Collection & Stabilization Kit (Canvax Biotech, Valladolid, Spain) and stored at −80 °C. Blood samples will be collected into EDTA tubes, processed, and immediately frozen at −80 °C.
All biospecimens will be stored at the Biomedical Research Institute of Murcia (IMIB) Pascual Parrilla Biobank, which is registered in the Spanish Biobank Registry of the Carlos III Health Institute (reference number PT17/0015/0038), ensuring compliance with national biobanking standards and ethical guidelines.
2.14. Epigenetic Changes
DNA methylation levels at CpG sites of target genes will be performed on DNA obtained buccal swab samples. Buccal swabs will be collected using the Buccal Swab Collection & Stabilization Kit (Canvax, Valladolid, Spain) and DNA will be extracted using the HigherPurity™ Buccal Swab DNA Extraction Kit (Canvax) following manufacturer’s instructions. DNA (50 ng) will be subjected to bisulfite treatment using the EZ DNA methylation kit (Zymo Research, Orange, CA, USA), according to the manufacturer’s instructions. The conversion cycling conditions will be as follows: 95 °C for 30 s and 40 °C for 60 min, repeated for 16 cycles. DNA will be then eluted in 10 µL of elution buffer. A total of 25 ng of bisulfite-treated DNA will be used for PCR amplification in a final reaction volume of 25 µL, using the PyroMark PCR kit (Qiagen, Aarhus, Denmark) and gene-specific oligonucleotides. PCR products (2 µL) will be checked on 2% agarose gel prepared in Tris-acetate-EDTA (TAE) buffer to verify the presence of a single band of the expected size for each amplicon. Pyrosequencing will be carried out in duplicate using the PyroMark Q24 system (Qiagen) and PyroMark Gold Q24 reagents (Qiagen) at the Genomic Platform of the IMIB, targeting the CpG-rich regions within the amplified sequences. Pyrosequencing run files (*.pyrorun) will be automatically analyzed using the PyroMark Q24 Software (v.2.0.8), which calculates the methylation percentage at each CpG position and provides a quality assessment of each site.
2.15. Analysis of Microbiota Composition
Analysis of microbiota composition will be performed on stool samples from the mother and the infant collected at different time points. Microbiota profiling will be conducted using 16S rRNA gene sequencing. Bacterial DNA will be extracted using the NZY Microbial gDNA Isolation Kit (NZYtech), according to the manufacturer’s protocol. DNA concentration and integrity will be assessed using the Agilent 2200 TapeStation system (Agilent Technologies, Santa Clara, CA, USA).
For library preparation, DNA samples (5 ng/µL) will be amplified using primers targeting the hypervariable regions V3-V4 regions of the bacterial 16S rRNA gene with the 16S Metagenomics Kit (Thermo Fisher, Waltham, MA, USA). Resulting amplicons will be purified using AMPure® XP magnetic beads (Beckman Coulter, Pasadena, CA, USA). Libraries will be constructed with the Ion Plus Fragment Library Kit and barcoded using the Ion Xpress Barcode Adapters 1–16 Kit (Thermo Fisher). Final libraries will be purified with AMPure® XP beads and quantified using fluorometric methods. Sequencing will be performed using the Illumina MiSeq system (Illumina, San Diego, CA, USA). Microbial composition, diversity, and richness metrics will be analyzed using QIIME2 software (version 2025.4), and predictive functional profiling will be conducted using the PICRUSt package (version v2.6.2).
2.16. Targeted Metabolomic Analysis
The analysis of bile acid profiles will be conducted on fecal samples collected at the same time points as the metagenomics samples. This will be performed by UHPLC (Infinity 1290; Agilent, Santa Clara, CA, USA) coupled to a high-resolution mass spectrometer with a quadrupole time-of-flight mass analyzer (6550 iFunnel Q-TOF LC/MS; Agilent) with an Agilent Jet Stream (AJS) electrospray (ESI), following their established protocols [49]. Raw data analysis will be performed using Profinder 10.0 software (Version B.10.0, Agilent Technologies) with a batch-targeted feature extraction method based on an internal bile acid database. Following data processing, the identified bile acids will be analyzed using Mass Hunter Qualitative 10.0 software (Version B.10.0, Agilent Technologies).
The analysis of SCFAs and BCAAs will be performed on stool samples mixed with an aqueous NaOH solution (5 mM) containing the internal standard (D3-caproic acid). The samples will then be centrifuged, and the supernatant will be derivatized. The samples will then undergo a two-step extraction process with the sequential addition of n-hexane, followed by centrifugation. The upper n-hexane layers containing the derivatives will be collected for analysis using gas chromatography-mass spectrometry (GC-MS). The quantification of SCFA, including acetic acid, propionic acid, butyric acid, isobutyric acid, methyl-butyric acid, valeric acid, isovaleric acid, caproic acid, and heptanoic acid, will be performed using an Agilent 7890B GC coupled to an Agilent 5977A mass spectrometer in selected ion monitoring (SIM) mode. The separations will be achieved using an HP-5MS capillary column (30 m × 250 µm i.d., 0.25 µm film thickness). Derivatized sample extracts will be injected in split mode with a 10:1 ratio, and the solvent delay will be set at 2.36 min. The temperature program will start at 50 °C for 2 min, increase to 70 °C at a rate of 10 °C per minute, to 85 °C at a rate of 3 °C per minute, to 110 °C at a rate of 5 °C per minute, to 290 °C at a rate of 30 °C per minute, and will then be held at 290 °C for 8 min. Helium will be used as the carrier gas at a constant flow rate of 1 mL per minute through the column. The temperatures of the front inlet, transfer line, and electron impact (EI) ion source will be set at 260 °C, 290 °C, and 230 °C, respectively, with an electron energy of −70 eV. Compound quantification will be carried out using calibration curves based on serial dilutions of analytical standard mixtures.
2.17. Statistical Analysis
To assess the distribution of continuous variables, the Kolmogorov–Smirnov test will be used for cross-sectional analyses and the Shapiro–Wilk test for longitudinal data. Descriptive statistics will summarize mother–infant characteristics at each time point, with continuous variables reported as mean ± standard deviation or median and interquartile range, and categorical variables as frequencies and percentages. Longitudinal analyses will be performed using mixed-effects models to evaluate changes in adiposity and related outcomes over time, accounting for within-subject correlations and adjusting for relevant covariates such as maternal BMI, mode of delivery, and type of feeding. In addition, stratified analyses will be planned for these factors in order to explore their potential modifying effect on primary outcomes. To explore the associations between omics-derived variables (including microbiota, epigenetic, and metabolomic data), correlation analyses (Pearson or Spearman, as appropriate), and multivariable regression models (linear or logistic) will be conducted. Unsupervised and supervised multivariate approaches, such as principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and hierarchical clustering, will be used to identify patterns and variability within high-dimensional omics datasets. Data integration techniques, including canonical correlation analysis (CCA) and machine-learning methods like random forest algorithms, will be employed to uncover potential predictive biomarkers of increased adiposity. Missing data will be addressed using multiple imputation techniques or mixed models that inherently account for missingness. All statistical analyses will be conducted using SPSS version 28.0 (IBM Corp., Armonk, NY, USA) and R (version v.4.5.1, including packages such as lme4, vegan, and mixOmics).
2.18. Data Management
An electronic Data Collection Notebook will be created in which all variables relevant to the study will be recorded. A formulary will be developed for the collection of information in SPSS v.22.0 software (SPSS Inc., Chicago, IL, USA).
The variables to be collected in the record will be the following:
- Identification data of the physician responsible:
- Name.
- Work center.
- Administrative data of the intervention:
- c.
- Date of ultrasound.
- d.
- Date of delivery.
- e.
- Date of the first follow-up visit of the newborn (1 month).
- f.
- Date of the second follow-up visit of the newborn (6 months).
- g.
- Date of the third follow-up visit of the newborn (1 year).
- h.
- Date of the fourth follow-up visit of the newborn (2 years).
- i.
- Date of the fifth follow-up visit of the newborn (3 years).
- Demographic data:
- j.
- Age.
- k.
- Ethnicity.
- l.
- Gender of the child.
- Maternal antepartum data:
- m.
- Physical examination: pregestational and gestational anthropometric data.
- n.
- Quality of life questionnaire.
- o.
- Nutritional questionnaire.
- p.
- Personal and medical history, including arterial hypertension, diabetes mellitus, and cardiovascular, hepatic, respiratory or renal diseases, as well as chronic consumption of toxic substances (alcohol, tobacco), or chronic consumption of drugs (including anti-inflammatory drugs, immunosuppressants, etc.).
- q.
- Ultrasound data: fetal growth assessment, hemodynamic evaluation of the maternal-fetal compartment, and evaluation of fetal adiposity.
- Maternal data after childbirth:
- r.
- Type of delivery.
- Data of the neonate after delivery:
- s.
- Anthropometric data obtained by physical examination.
- Follow-up data of the neonate:
- t.
- Anthropometric data obtained by physical examination at 1 month, 6 months, 1 year, 2 years and 3 years.
- u.
- Type of nutrition; breast milk, formula, or combined.
- v.
- Adverse events.
All documentation related to the study will remain stored at the participating center under the custody of the Principal Investigator until the end of the study. Once the study is completed, the documentation will be indexed and passed to the general archive of the research center, complying with the recommendations established with respect to the Good Clinical Practice Guidelines.
Data collected from participants that withdraw from the study will be retained following the guidelines from the Office for Human Research Protections. No financial incentives will be provided to any of the participants.
2.19. Study Limitations
This project involves a longitudinal observational clinical study spanning over 3 years (from 1 month before to 3 years after delivery). Given the extended follow-up period, some participants may face challenges in completing all scheduled visits. Despite the expected high dropout rate (estimated at 30%), the annual birth rate at the HCUVA exceeds 6500 deliveries, ensuring feasibility in recruiting the proposed cohort of 66 mother–infant dyads. Close monitoring by our clinical collaborators is anticipated to mitigate any loss to follow-up and promote participant compliance. Also, this is a single-center study and, therefore, the findings may not be generalizable to populations with different exposomes or genetic backgrounds. In addition, the observational nature of the study may make it difficult to establish causality among the variables determined. However, the prospective design, the well-characterized cohort, and the longitudinal data collection will enhance the reliability of temporal associations between potential biomarkers and dynamic changes over time related to obesity risk.
Although the initial 3-year follow-up might not be sufficient to evaluate long-term health outcomes, it covers critical developmental windows for adiposity programming and metabolic risk, which could offer valuable information related to the early pathophysiology of obesity. Finally, while the sample size is sufficient for exploratory biomarker discovery, it may not allow detection of small size effect. However, this study could generate valuable information that will guarantee further large-scale investigations.
3. Discussion
This study aims to identify early non-invasive biomarkers during the first 3 years of life that could help to predict the rise in adiposity and, therefore, an increased metabolic risk later in life. Childhood obesity has become one of the major health concerns worldwide due to its significant implications for long-term metabolic health [3]. Although Health Systems are developing strategies to manage this issue, early prediction and prevention remain challenging due to the complex and multifactorial nature of this disease [50]. In this regard, prenatal and postnatal environmental factors have been described to influence epigenetic modification that affects the metabolic state of the offspring and, therefore, to promote the development of different metabolic conditions later in life [51,52,53,54,55]. Similarly, alterations in gut microbiome colonization and its associated metabolites, such as SCFAs or bile acids, have also emerged as critical regulators of host metabolism and immune development [56,57,58,59,60]. Given their role in modulating epigenetic marks, the study of microbiome–metabolome–epigenome interactions may help to generate novel information about pathways linking alterations in early microbial colonization to later adiposity. This study protocol proposes a comprehensive, longitudinal, multi-omics approach in a mother–infant cohort. The recruitment within the third trimester of pregnancy and the subsequent follow-up of the offspring will allow us to explore both prenatal and postnatal exposures. Moreover, the evaluation of maternal profiles offers the opportunity to identify transgenerational patterns that could provide a potent tool to generate prevention strategies against obesity development even before birth, as well as to understand how maternal metabolic, microbial, and epigenetic status influences offspring metabolic state and related health trajectories.
Summarizing, this study will generate novel insights into the early-life determinants of adiposity by combining epigenetic, metagenomic, and metabolomic data. The identification of predictive biomarkers may enable earlier and more effective prevention strategies, ultimately reducing the burden of obesity and related metabolic diseases later in life.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14196694/s1, Supplementary Material S1: Information Sheet.
Author Contributions
Conceptualization: M.S.-S., J.E.B.-C., A.J.R.-A. and M.Á.N.-S.; Methodology: M.S.-C., M.S.-S., J.E.B.-C., B.R.-M., A.J.R.-A. and M.Á.N.-S.; Investigation: M.S.-C., A.J.-P., A.M.-R., J.A.d.C., M.Á.C.-M., V.E.F.-R., A.J.-M., P.M.P.-M., S.R.-C., M.S.-S. and J.E.B.-C.; Data curation: M.S.-C., A.J.-P. and M.Á.N.-S.; Formal analysis: M.S.-C., A.J.-P., B.R.-M., A.J.R.-A. and M.Á.N.-S.; Project administration: A.J.R.-A. and M.Á.N.-S.; Resources: M.Á.N.-S.; Supervision: M.S.-S., J.E.B.-C., A.J.R.-A. and M.Á.N.-S.; Validation: M.S.-S., J.E.B.-C., A.J.R.-A. and M.Á.N.-S.; Visualization: M.Á.N.-S.; Funding acquisition: M.Á.N.-S.; Writing—Original draft preparation: M.S.-C., A.J.R.-A. and M.Á.N.-S.; Writing—Review and editing: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Comunidad Autónoma de la Región de Murcia through the call for Grants for Projects for the Generation of New Scientific Leadership “Young Research Leaders” (22080/JLI/22), included in the Regional Program for the Promotion of Scientific and Technical Research (Action Plan 2022) of the Fundación Séneca—Science and Technology Agency of the Region de Murcia. M.Á.N.-S. is supported by the Miguel Servet program (CP23/00051) funded by the Carlos III Health Institute and co-funded by the European Union.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Virgen de la Arrixaca University Hospital (code 2023-2-13-HCUVA, 20 January 2023).
Informed Consent Statement
Informed consent will be obtained from all subjects involved in the study.
Acknowledgments
The authors would like to thank all the midwives and gynecologists at the delivery ward of the HCUVA for their invaluable support and commitment to this project, as their collaboration is essential to the success of the study. We would also wish to thank all the volunteers for their generosity in participating in the study. The authors thank the Genomics Core Facility platform at the IMIB for pyrosequencing support. The authors will also like to thank the collaboration of Biobank Network of the Region of Murcia, BIOBANC-MUR, registered on the Registro Nacional de Biobancos with registration number B.0000859. BIOBANC-MUR is supported by “Instituto de Salud Carlos III (proyecto PT20/00109), by IMIB, and by “Consejería de Salud de la Comunidad Autónoma de la Región de Murcia”.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the writing of the manuscript; or in the decision to publish the protocol.
Abbreviations
The following abbreviations are used in this manuscript:
| HCUVA | Virgen de la Arrixaca University Hospital |
| SCFA | Short-chain fatty acids |
| BCAA | Branched-chain amino acids |
| FFQ | Food frequency questionnaires |
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 May 2025).
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.; Dee, A.; Perry, I.J. The lifetime costs of overweight and obesity in childhood and adolescence: A systematic review. Obes. Rev. 2018, 19, 452–463. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Wong, A.H.C.; Hon, K.L. Childhood Obesity: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 2–26. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Juonala, M.; Viikari, J.S. Obesity in childhood and vascular changes in adulthood: Insights into the Cardiovascular Risk in Young Finns Study. Int. J. Obes. 2005, 29 (Suppl. S2), S101–S104. [Google Scholar] [CrossRef]
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, M.M.; Pyle, L.; Hilkin, A.; Severn, C.D.; Utzschneider, K.; Van Pelt, R.E.; Nadeau, K.J.; Zeitler, P.S. The Impact of Obesity On Insulin Sensitivity and Secretion During Pubertal Progression: A Longitudinal Study. J. Clin. Endocrinol. Metab. 2020, 105, e2061–e2068. [Google Scholar] [CrossRef]
- Maximova, K.; Kuhle, S.; Davidson, Z.; Fung, C.; Veugelers, P.J. Cardiovascular risk-factor profiles of normal and overweight children and adolescents: Insights from the Canadian Health Measures Survey. Can. J. Cardiol. 2013, 29, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Lv, Q.; Chen, Y.J.; Yan, L.B.; Xiong, X. Association between childhood obesity and gut microbiota: 16S rRNA gene sequencing-based cohort study. World J. Gastroenterol. 2024, 30, 2249–2257. [Google Scholar] [CrossRef]
- Sarni, R.O.S.; Kochi, C.; Suano-Souza, F.I. Childhood obesity: An ecological perspective. J. Pediatr. 2022, 98 (Suppl. S1), S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Robiou-du-Pont, S.; Anand, S.S.; Morrison, K.M.; McDonald, S.D.; Atkinson, S.A.; Teo, K.K.; Meyre, D. Parental and child genetic contributions to obesity traits in early life based on 83 loci validated in adults: The FAMILY study. Pediatr. Obes. 2018, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Munthali, R.J.; Sahibdeen, V.; Kagura, J.; Hendry, L.M.; Norris, S.A.; Ong, K.K.; Day, F.R.; Lombard, Z. Genetic risk score for adult body mass index associations with childhood and adolescent weight gain in an African population. Genes Nutr. 2018, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.M.; Renault, K.M.; Norgaard, K.; Nilas, L.; Jensen, J.E.; Hyldstrup, L.; Michaelsen, K.F.; Cortes, D.; Pryds, O. Newborn regional body composition is influenced by maternal obesity, gestational weight gain and the birthweight standard score. Acta Paediatr. 2014, 103, 939–945. [Google Scholar] [CrossRef]
- Renault, K.M.; Carlsen, E.M.; Norgaard, K.; Nilas, L.; Pryds, O.; Secher, N.J.; Cortes, D.; Jensen, J.E.; Olsen, S.F.; Halldorsson, T.I. Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. Am. J. Clin. Nutr. 2015, 102, 1475–1481. [Google Scholar] [CrossRef]
- Patel, N.; Godfrey, K.M.; Pasupathy, D.; Levin, J.; Flynn, A.C.; Hayes, L.; Briley, A.L.; Bell, R.; Lawlor, D.A.; Oteng-Ntim, E.; et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int. J. Obes. 2017, 41, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Koontz, M.B.; Gunzler, D.D.; Presley, L.; Catalano, P.M. Longitudinal changes in infant body composition: Association with childhood obesity. Pediatr. Obes. 2014, 9, e141–e144. [Google Scholar] [CrossRef]
- Entringer, S.; Rasmussen, J.; Cooper, D.M.; Ikenoue, S.; Waffarn, F.; Wadhwa, P.D.; Buss, C. Association between supraclavicular brown adipose tissue composition at birth and adiposity gain from birth to 6 months of age. Pediatr. Res. 2017, 82, 1017–1021. [Google Scholar] [CrossRef]
- Moore, B.F.; Harrall, K.K.; Sauder, K.A.; Glueck, D.H.; Dabelea, D. Neonatal Adiposity and Childhood Obesity. Pediatrics 2020, 146, e20200737. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lee, Y.S.; Wang, J.P.; Jiang, Y.Y.; Li, H.; An, Y.L.; Hu, Y.H.; Lee, K.O.; Li, G.W. Longitudinal study of childhood adiposity and the risk of developing components of metabolic syndrome-the Da Qing children cohort study. Pediatr. Res. 2011, 70, 307–312. [Google Scholar] [CrossRef]
- Kumaran, K.; Lubree, H.; Bhat, D.S.; Joshi, S.; Joglekar, C.; Yajnik, P.; Bhave, S.; Pandit, A.; Fall, C.H.D.; Yajnik, C.S. Birth weight, childhood and adolescent growth and diabetes risk factors in 21-year-old Asian Indians: The Pune Children’s Study. J. Dev. Orig. Health Dis. 2021, 12, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Liu, G.; Wang, P.; Ye, T.; Tian, Z.; Liu, E.; Yu, Z.; Yang, X.; Klebanoff, M.; Yeung, E.; et al. Birth weight, postnatal weight change, and risk for high blood pressure among chinese children. Pediatrics 2011, 127, e1272–e1279. [Google Scholar] [CrossRef]
- Tsai, H.J.; Wang, G.; Hong, X.; Yao, T.C.; Ji, Y.; Radovick, S.; Ji, H.; Cheng, T.L.; Wang, X. Early Life Weight Gain and Development of Childhood Asthma in a Prospective Birth Cohort. Ann. Am. Thorac. Soc. 2018, 15, 1197–1204. [Google Scholar] [CrossRef]
- Taveras, E.M.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Gold, D.R.; Litonjua, A.A.; Oken, E.; Weiss, S.T.; Gillman, M.W. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J. Allergy Clin. Immunol. 2008, 121, 1161–1166.e3. [Google Scholar] [CrossRef]
- Robinson, N.; Brown, H.; Antoun, E.; Godfrey, K.M.; Hanson, M.A.; Lillycrop, K.A.; Crozier, S.R.; Murray, R.; Pearce, M.S.; Relton, C.L.; et al. Childhood DNA methylation as a marker of early life rapid weight gain and subsequent overweight. Clin. Epigenetics 2021, 13, 8. [Google Scholar] [CrossRef]
- Lesseur, C.; Armstrong, D.A.; Paquette, A.G.; Li, Z.; Padbury, J.F.; Marsit, C.J. Maternal obesity and gestational diabetes are associated with placental leptin DNA methylation. Am. J. Obstet. Gynecol. 2014, 211, 654.e1–654.e9. [Google Scholar] [CrossRef]
- Vahamiko, S.; Laiho, A.; Lund, R.; Isolauri, E.; Salminen, S.; Laitinen, K. The impact of probiotic supplementation during pregnancy on DNA methylation of obesity-related genes in mothers and their children. Eur. J. Nutr. 2019, 58, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, J.; Renault, K.M.; Garcia-Calzon, S.; Perfilyev, A.; Estampador, A.C.; Norgaard, K.; Lind, M.V.; Vaag, A.; Hjort, L.; Michaelsen, K.F.; et al. Lifestyle Intervention in Pregnant Women With Obesity Impacts Cord Blood DNA Methylation, Which Associates With Body Composition in the Offspring. Diabetes 2021, 70, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Lund, R.; Laiho, A.; Lundelin, K.; Ley, R.E.; Isolauri, E.; Salminen, S. Gut microbiota as an epigenetic regulator: Pilot study based on whole-genome methylation analysis. mBio 2014, 5, e02113-14. [Google Scholar] [CrossRef]
- Joyce, S.A.; Gahan, C.G. The gut microbiota and the metabolic health of the host. Curr. Opin. Gastroenterol. 2014, 30, 120–127. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Fulde, M.; Hornef, M.W. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev. 2014, 260, 21–34. [Google Scholar] [CrossRef]
- Wang, X.A.; Li, J.P.; Lee, M.S.; Yang, S.F.; Chang, Y.S.; Chen, L.; Li, C.W.; Chao, Y.H. A common trajectory of gut microbiome development during the first month in healthy neonates with limited inter-individual environmental variations. Sci. Rep. 2024, 14, 3264. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Neumann, C.J.; Mohammadzadeh, R.; Woh, P.Y.; Kobal, T.; Pausan, M.R.; Shinde, T.; Haid, V.; Mertelj, P.; Weiss, E.C.; Kolovetsiou-Kreiner, V.; et al. First-year dynamics of the anaerobic microbiome and archaeome in infants’ oral and gastrointestinal systems. mSystems 2025, 10, e0107124. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.B.; Alexeev, D.G.; Boytsov, S.A. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016, 32, 620–627. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Mei, H.; Chang, X.; He, P.; Sun, L.; Xiao, H.; Wang, S.; Li, R. Association between gut microbiota and short-chain fatty acids in children with obesity. Sci. Rep. 2025, 15, 483. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.N.Y.; Paul, A.; Guleria, S.; O’Sullivan, J.M.; Toldi, G. Short-chain fatty acids-a key link between the gut microbiome and T-lymphocytes in neonates? Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- van Best, N.; Rolle-Kampczyk, U.; Schaap, F.G.; Basic, M.; Olde Damink, S.W.M.; Bleich, A.; Savelkoul, P.H.M.; von Bergen, M.; Penders, J.; Hornef, M.W. Bile acids drive the newborn’s gut microbiota maturation. Nat. Commun. 2020, 11, 3692. [Google Scholar] [CrossRef]
- Francis, E.C.; Hunt, K.J.; Grobman, W.A.; Skupski, D.W.; Mani, A.; Hinkle, S.N. Maternal Obesity and Differences in Child Urine Metabolome. Metabolites 2024, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Sakwinska, O.; Soh, S.E.; Ngom-Bru, C.; Bruck, W.M.; Berger, B.; Brussow, H.; Lee, Y.S.; Yap, F.; Chong, Y.S.; et al. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. mBio 2015, 6, e02419-14. [Google Scholar] [CrossRef]
- Raspini, B.; Vacca, M.; Porri, D.; De Giuseppe, R.; Calabrese, F.M.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; et al. Early Life Microbiota Colonization at Six Months of Age: A Transitional Time Point. Front. Cell. Infect. Microbiol. 2021, 11, 590202. [Google Scholar] [CrossRef]
- Hernandez-Morante, J.J.; Larque, E.; Lujan, J.A.; Zamora, S.; Garaulet, M. N-6 from different sources protect from metabolic alterations to obese patients: A factor analysis. Obesity 2009, 17, 452–459. [Google Scholar] [CrossRef]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- Call, C.C.; Jouppi, R.J.; Tavernier, R.L.E.; Grace, J.L.; Sweeney, G.M.; Conlon, R.P.K.; Ferguson, E.A.; Levine, M.D. Pregnancy Eating Attitudes-Questionnaire (PEA-Q): Exploratory factor analysis and psychometric performance in a pregnant community sample with body mass index ≥ 25. Appetite 2025, 206, 107828. [Google Scholar] [CrossRef]
- van Beijsterveldt, I.; Dorrepaal, D.J.; de Fluiter, K.S.; de Ridder, M.A.J.; Hokken-Koelega, A.C.S. Skinfold-based-equations to assess longitudinal body composition in children from birth to age 5 years. Clin. Nutr. 2023, 42, 1213–1218. [Google Scholar] [CrossRef]
- Bullich-Vilarrubias, C.; Romani-Perez, M.; Lopez-Almela, I.; Rubio, T.; Garcia, C.J.; Tomas-Barberan, F.A.; Sanz, Y. Nav1.8-expressing neurons control daily oscillations of food intake, body weight and gut microbiota in mice. Commun. Biol. 2024, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Kapoor, N.; Senapati, S.; Singh, O.; Bhadoria, A.S.; Khetarpal, P.; Kumar, S.; Bansal, K.; Ranjan, R.; Kakkar, R.; et al. Comprehending the Epidemiology and Aetiology of Childhood Obesity: Integrating Life Course Approaches for Prevention and Intervention. Diabetes Ther. 2025, 16, 1177–1206. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Mitsui, T.; Nobumoto, E.; Hiramatsu, Y. The Effects of High-Fat Diet Exposure In Utero on the Obesogenic and Diabetogenic Traits Through Epigenetic Changes in Adiponectin and Leptin Gene Expression for Multiple Generations in Female Mice. Endocrinology 2015, 156, 2482–2491. [Google Scholar] [CrossRef]
- Franzago, M.; Borrelli, P.; Di Nicola, M.; Cavallo, P.; D’Adamo, E.; Di Tizio, L.; Gazzolo, D.; Stuppia, L.; Vitacolonna, E. From Mother to Child: Epigenetic Signatures of Hyperglycemia and Obesity during Pregnancy. Nutrients 2024, 16, 3502. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Ling, D.; Cheng, Y.; Akbar, R.; Jin, L.; Ren, J.; Wu, H.; Chen, B.; Zhou, Y.; Zhu, H.; et al. Gestational high-fat diet impaired demethylation of Pparalpha and induced obesity of offspring. J. Cell. Mol. Med. 2021, 25, 5404–5416. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.; Wang, L.; Liu, H.; Zheng, Y.; Drong, A.; Joyce, B.T.; Wang, J.; Li, W.; Leng, J.; Shen, Y.; et al. Prenatal gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics 2021, 16, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Symons, L.; Vanautgaerden, E.L.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; et al. The Influence of the Duration of Breastfeeding on the Infant’s Metabolic Epigenome. Nutrients 2019, 11, 1408. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- White, P.J.; Newgard, C.B. Branched-chain amino acids in disease. Science 2019, 363, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; Herisson, F.M.; Keane, J.M.; Garcia-Gonzalez, N.; Rossini, V.; Pinhiero, J.; Daly, J.; Bustamante-Garrido, M.; Hueston, C.M.; Patel, S.; et al. Microbial bile salt hydrolase activity influences gene expression profiles and gastrointestinal maturation in infant mice. Gut Microbes 2022, 14, 2149023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).