Evaluating the Sequelae of Mastoidectomy for Acute Mastoiditis: A Long-Term Follow-Up Study of Mastoid Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Review of Medical Records and Examination
2.4. Sensory Testing
2.5. Chronic Otitis Media Questionnaire 12
2.6. Audiometry
2.7. Middle Ear Impedance Testing
2.8. Distortion-Product Otoacoustic Emissions
2.9. Ultrasound Examination
2.10. Non-Invasive Assessment of Mastoid Function
2.11. Data Analysis
3. Results
3.1. Characteristics of Patients Undergoing Mastoidectomy
3.2. Chronic Otitis Media Questionnaire 12
3.3. Tactile Sensitivity Testing of the Auricle
3.4. Otomicroscopic Examination
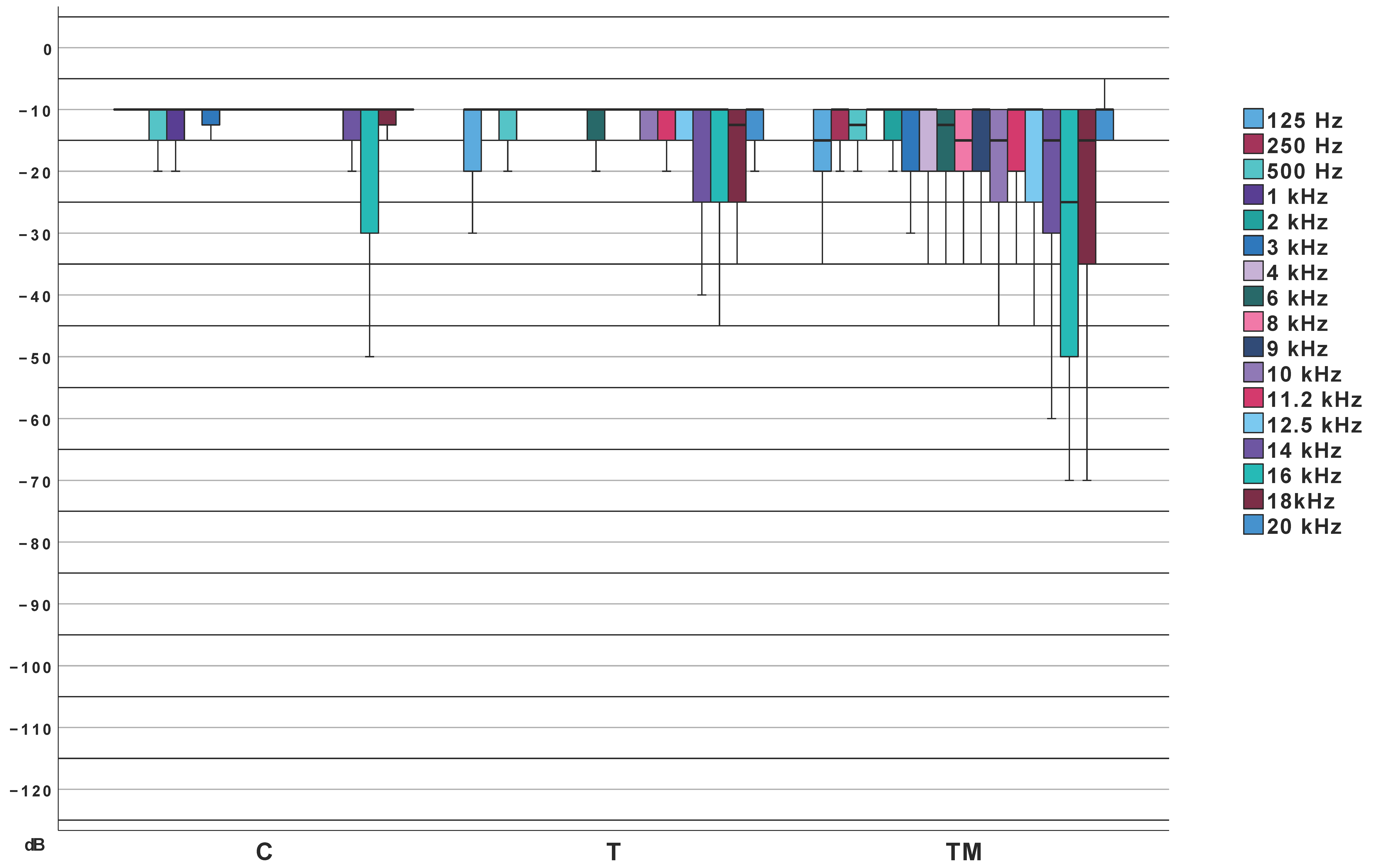
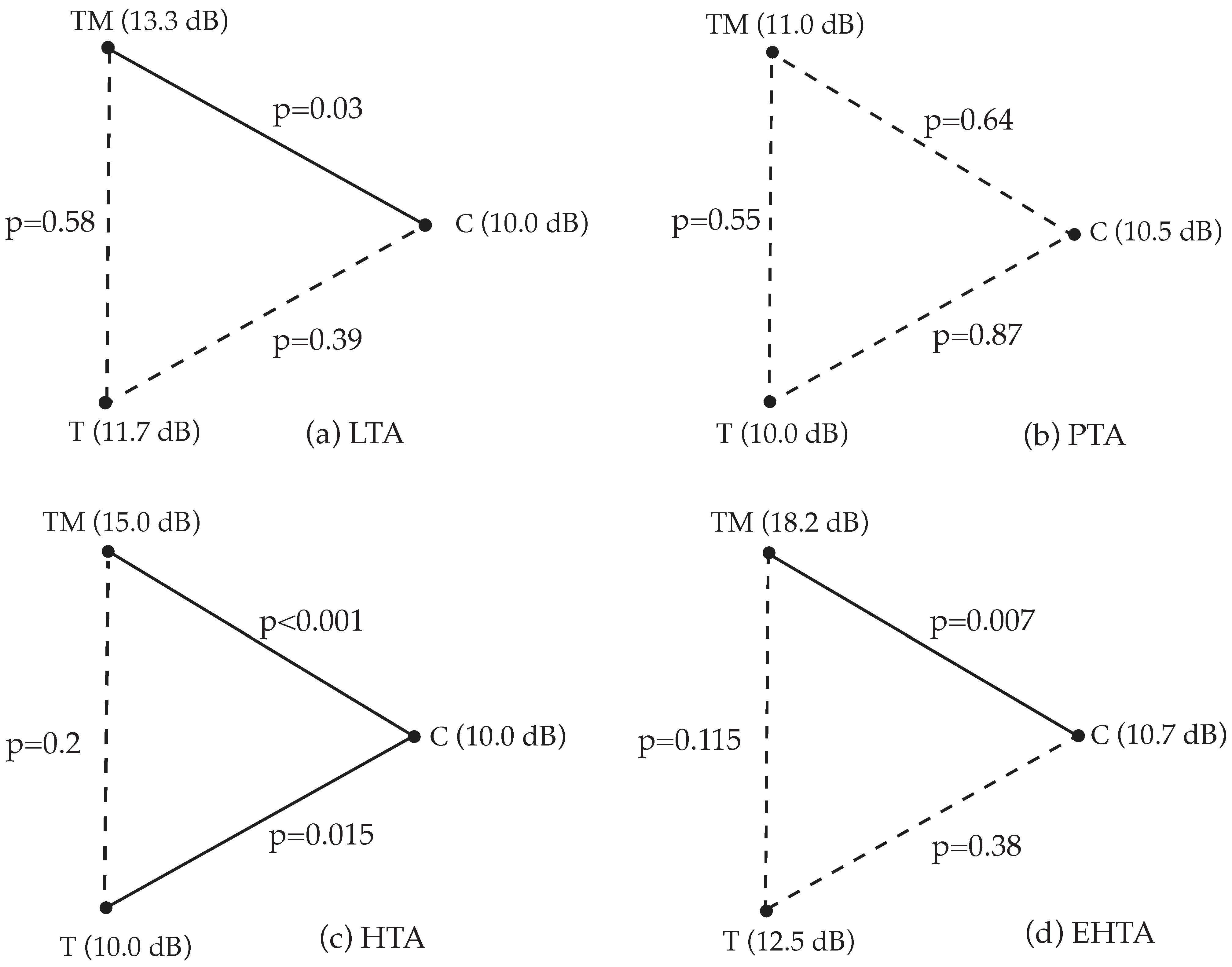
3.5. Extended High-Frequency Pure-Tone Audiometry
3.6. Middle Ear Impedance Testing
3.7. Distortion Product Otoacoustic Emissions
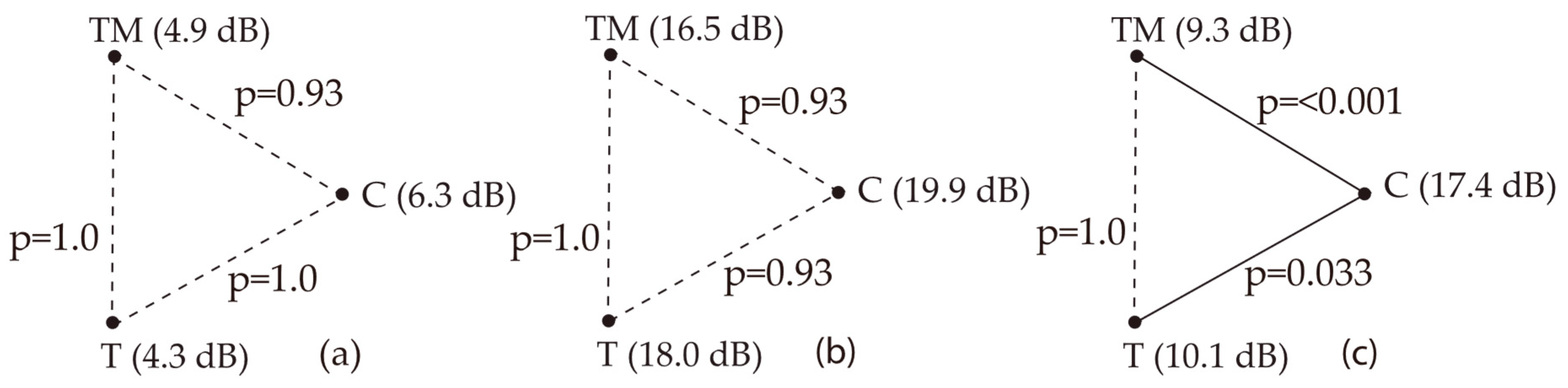
3.8. Ultrasound Examination of the Mastoid
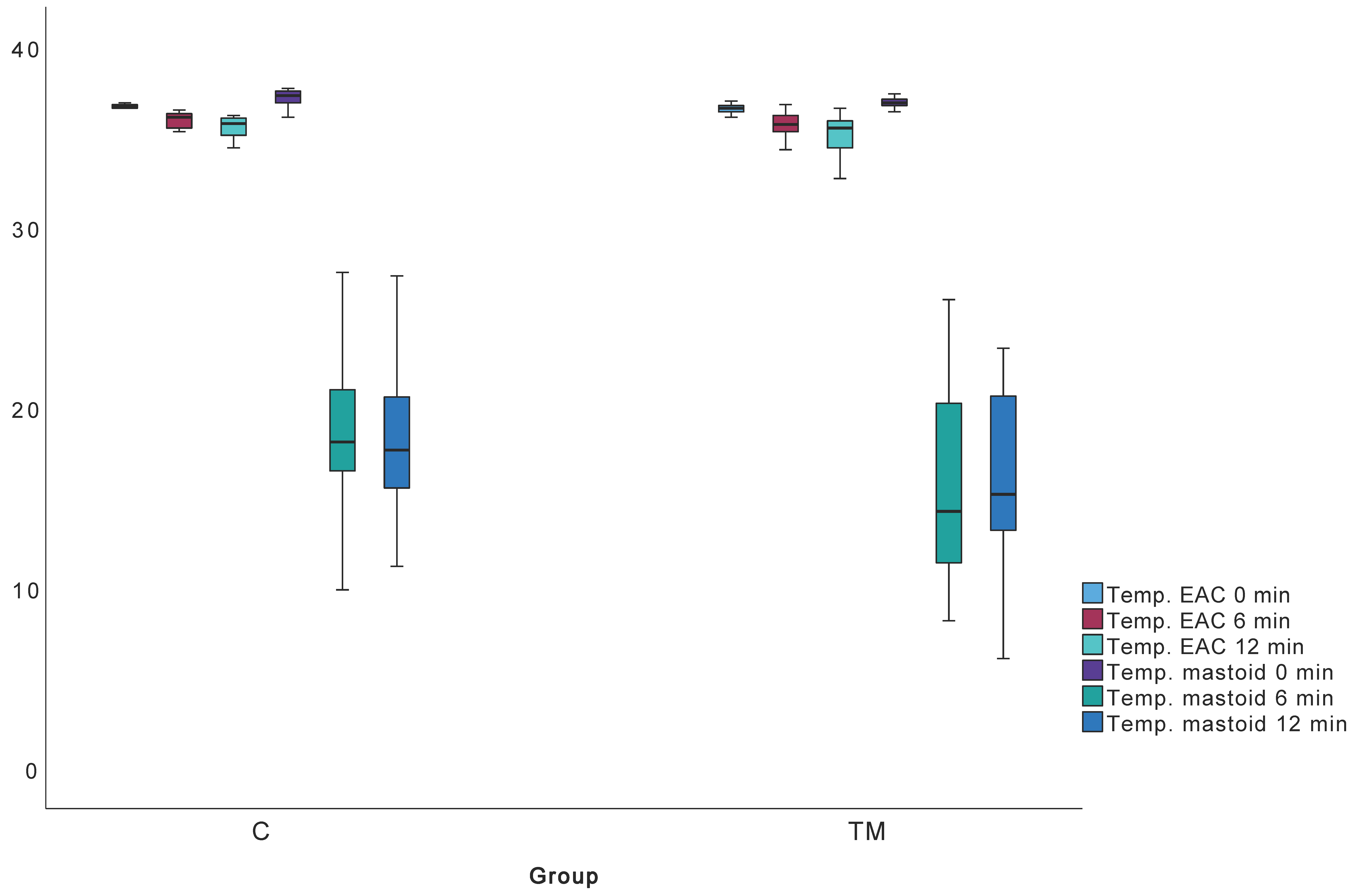
3.9. Mastoid Function Testing
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Acute mastoiditis |
| AOM | Acute otitis media |
| COMQ-12 | Chronic Otitis Media Questionnaire 12 |
| daPa | Deka pascal |
| DPOAE | Distortion-Product Otoacoustic Emissions |
| K | Kelvin |
| m | Meter |
| TPP | tympanometric peak pressure |
| VORg | vestibulo-ocular reflex gain |
| vHIT | video head impulse test |
| W | Watt |
References
- Luntz, M.; Brodsky, A.; Nusem, S.; Kronenberg, J.; Keren, G.; Migirov, L.; Cohen, D.; Zohar, S.; Shapira, A.; Ophir, D.; et al. Acute mastoiditis—The antibiotic era: A multicenter study. Int. J. Pediatr. Otorhinolaryngol. 2001, 57, 1–9. [Google Scholar] [CrossRef]
- Zanetti, D.; Nassif, N. Indications for surgery in acute mastoiditis and their complications in children. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1175–1182. [Google Scholar] [CrossRef]
- Kværner, K.J.; Bentdal, Y.; Karevold, G. Acute mastoiditis in Norway: No evidence for an increase. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1579–1583. [Google Scholar] [CrossRef]
- Psarommatis, I.M.; Voudouris, C.; Douros, K.; Giannakopoulos, P.; Bairamis, T.; Carabinos, C. Algorithmic management of pediatric acute mastoiditis. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.H.Y.; Barakate, M.S.; Havas, T.E. Mastoiditis in a paediatric population: A review of 11 years experience in management. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1520–1524. [Google Scholar] [CrossRef]
- Lin, H.W.; Shargorodsky, J.; Gopen, Q. Clinical Strategies for the Management of Acute Mastoiditis in the Pediatric Population. Clin. Pediatr. 2010, 49, 110–115. [Google Scholar] [CrossRef]
- Hullegie, S.; Venekamp, R.P.; Van Dongen, T.M.A.; Hay, A.D.; Moore, M.V.; Little, P.; Schilder, A.G.M.; Damoiseaux, R.A.M.J. Prevalence and Antimicrobial Resistance of Bacteria in Children with Acute Otitis Media and Ear Discharge: A Systematic Review. Pediatr. Infect. Dis. J. 2021, 40, 756–762. [Google Scholar] [CrossRef]
- Geva, A.; Oestreicher-Kedem, Y.; Fishman, G.; Landsberg, R.; DeRowe, A. Conservative management of acute mastoiditis in children. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Black, A.; Choo, D. What is the best practice for acute mastoiditis in children? Laryngoscope 2014, 124, 1057–1058. [Google Scholar] [CrossRef]
- Bakhos, D.; Trijolet, J.-P.; Morinière, S.; Pondaven, S.; Al zahrani, M.; Lescanne, E. Conservative Management of Acute Mastoiditis in Children. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 346. [Google Scholar] [CrossRef] [PubMed]
- Psarommatis, I.; Giannakopoulos, P.; Theodorou, E.; Voudouris, C.; Carabinos, C.; Tsakanikos, M. Mastoid subperiosteal abscess in children: Drainage or mastoidectomy? J. Laryngol. Otol. 2012, 126, 1204–1208. [Google Scholar] [CrossRef]
- Andersen, S.A.W.; Mikkelsen, P.T.; Konge, L.; Cayé-Thomasen, P.; Sørensen, M.S. Cognitive load in distributed and massed practice in virtual reality mastoidectomy simulation. Laryngoscope 2016, 126, E74–E79. [Google Scholar] [CrossRef]
- Attlmayr, B.; Zaman, S.; Scott, J.; Derbyshire, S.G.; Clarke, R.W.; De, S. Paediatric acute mastoiditis, then and now: Is it more of a problem now? J. Laryngol. Otol. 2015, 129, 955–959. [Google Scholar] [CrossRef]
- Nicastro, V.; Zagaria, A.; Abita, P.; Alberti, G.; Loteta, S.; Azieli, C. Association between obstructive sleep apnea and hearing loss: A literary review. Acta Medica Mediterr. 2019, 35, 3411–3416. [Google Scholar]
- Glynn, F.; Osman, L.; Colreavy, M.; Rowley, H.; Dwyer, T.P.O.; Blayney, A. Acute mastoiditis in children: Presentation and long term consequences. J. Laryngol. Otol. 2008, 122, 233–237. [Google Scholar] [CrossRef]
- Guillén-Lozada, E.; Bartolomé-Benito, M.; Moreno-Juara, Á. Surgical management of mastoiditis with intratemporal and intracranial complications in children. Outcome, complications, and predictive factors. Int. J. Pediatr. Otorhinolaryngol. 2023, 171, 111611. [Google Scholar] [CrossRef] [PubMed]
- Harley, E.H.; Sdralis, T.; Berkowitz, R.G. Acute Mastoiditis in Children: A 12-Year Retrospective Study. Otolaryngol.–Head Neck Surg. 1997, 116, 26–30. [Google Scholar] [CrossRef]
- Petersen, C.G.; Ovesen, T.; Pedersen, C.B. Acute mastoidectomy in a Danish county from 1977 to 1997-operative findings and long-term results. Acta Oto-Laryngol. Suppl. 2000, 543, 122–126. [Google Scholar] [CrossRef]
- Enoksson, F.; Groth, A.; Hultcrantz, M.; Stalfors, J.; Stenfeldt, K.; Hermansson, A. Subperiosteal abscesses in acute mastoiditis in 115 Swedish children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Tos, M.; Poulsen, G. Attic Retractions Following Secretory Otitis. Acta Oto-Laryngol. 1980, 89, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sade, J.; Berco, E. Atelectasis and Secretory Otitis Media. Ann. Otol. Rhinol. Laryngol. 1976, 85, 66–72. [Google Scholar] [CrossRef]
- Kang, H.S.; Ahn, S.K.; Jeon, S.Y.; Hur, D.G.; Kim, J.P.; Park, J.J.; Kim, D.W.; Woo, S.H. Sensation recovery of auricle following chronic ear surgery by retroauricular incision. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 101–106. [Google Scholar] [CrossRef]
- Phillips, J.S.; Haggard, M.; Yung, M. A new health-related quality of life measure for active chronic otitis media (COMQ-12): Development and initial validation. Otol. Neurotol. 2014, 35, 454–458. [Google Scholar] [CrossRef]
- Vozel, D.; Steiner, N.; Božanić Urbančič, N.; Mladenov, D.; Battelino, S. Slovenian Cross-Cultural Adaptation and Validation of Health-Related Quality of Life Measures for Chronic Otitis Media (COMQ-12), Vertigo (DHI, NVI) and TINNITUS (THI). Zdr. Varst. 2020, 59, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hearing, C.O.; Balkany, T.A.; Gates, G.A.; Goldenberg, R.A.; Meyerhoff, W.L.; House, J.W.; Surg, O.H.N. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngology-Head Neck Surg. 1995, 113, 186–187. [Google Scholar]
- Magnuson, B. Functions of the mastoid cell system: Auto-regulation of temperature and gas pressure. J. Laryngol. Otol. 2003, 117, 99–103. [Google Scholar] [CrossRef]
- Fooken Jensen, P.V.; Gaihede, M. Congestion of mastoid mucosa and influence on middle ear pressure—Effect of retroauricular injection of adrenaline. Hear. Res. 2016, 340, 121–126. [Google Scholar] [CrossRef]
- Cros, O.; Borga, M.; Pauwels, E.; Dirckx, J.J.J.; Gaihede, M. Micro-channels in the mastoid anatomy. Indications of a separate blood supply of the air cell system mucosa by micro-CT scanning. Hear. Res. 2013, 301, 60–65. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Kadir, A.; Hayashi, N.; Takamoto, M. Direct influence of temperature on the semicircular canal receptor. J. Vestib. Res. Equilib. Orientat. 1998, 8, 169–173. [Google Scholar] [CrossRef]
- Smolders, J.W.T.; Klinke, R. Effects of temperature on the properties of primary auditory fibres of the spectacled caiman, Caiman crocodilus (L.). J. Comp. Physiol. A 1984, 155, 19–30. [Google Scholar] [CrossRef]
- Whitehead, M.L.; Wilson, J.P.; Baker, R.J. The Effects of Temperature on Otoacoustic Emission Tuning Properties. In Auditory Frequency Selectivity; Springer: Boston, MA, USA, 1986; pp. 39–48. Available online: http://link.springer.com/10.1007/978-1-4613-2247-4_5 (accessed on 4 September 2025).
- Ferber-Viart, C.; Savourey, G.; Garcia, C.; Duclaux, R.; Bittel, J.; Collet, J. Influence of hyperthermia on cochlear micromechanical properties in humans. Hear. Res. 1995, 91, 202–207. [Google Scholar] [CrossRef]
- Zenner, H.P.; Zimmermann, U. Caloric evoked motile responses of mammalian vestibular sensory cells. Acta Oto-Laryngol. 1995, 115, 484–487. [Google Scholar] [CrossRef]
- Ohtani, M.; Yamashita, T.; Amano, H.; Kubo, N.; Kumazawa, T. Thermal influence on intracellular calcium concentration in vestibular hair cells isolated from the guinea pig. A preliminary report. Acta Oto-Laryngol. Suppl. 1993, 500, 46–49. [Google Scholar] [CrossRef]
- Hood, J.D. Evidence of direct thermal action upon the vestibular receptors in the caloric test. A re-interpretation of the data of Coats and Smith. Acta Otolaryngol. 1989, 107, 161–165. [Google Scholar] [CrossRef]
- Feldmann, A.; Wili, P.; Maquer, G.; Zysset, P. The thermal conductivity of cortical and cancellous bone. Eur. Cells Mater. 2018, 35, 25–33. [Google Scholar] [CrossRef]
- Xu, F.; Lu, T.J.; Seffen, K.A.; Ng, E.Y.K. Mathematical modeling of skin bioheat transfer. Appl. Mech. Rev. 2009, 62, 050801. [Google Scholar] [CrossRef]
- Rojas-Altamirano, G.; Vargas, R.O.; Escandón, J.P.; Mil-Martínez, R.; Rojas-Montero, A. Calculation of Effective Thermal Conductivity for Human Skin Using the Fractal Monte Carlo Method. Micromachines 2022, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Sha, Y.; Dai, P.; Li, H. Quantitative morphology of facial nerve based on three-dimensional reconstruction of temporal bone. Otolaryngol. Head Neck Surg. 2008, 138, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Merati, M.; Kazemi, M.A.; Dabiri, S.; Kouhi, A. Radiologic evaluation of the mastoid segment of the facial nerve tract in the intact temporal bone. Surg. Radiol. Anat. 2021, 43, 145–151. [Google Scholar] [CrossRef]
- Mahboubi, H.; Wu, E.C.; Jahanbakhshi, R.; Coale, K.; Rothholtz, V.S.; Zardouz, S.; Djalilian, H.R. A novel method to determine standardized anatomic dimensions of the osseous external auditory canal. Otol. Neurotol. 2012, 33, 715–720. [Google Scholar] [CrossRef]
- Palva, T.; Virtanen, H.; Mäkinen, J. Acute and latent mastoiditis in children. J. Laryngol. Otol. 1985, 99, 127–136. [Google Scholar] [CrossRef]
- Van Zuijlen, D.A.; Schilder, A.G.M.; Van Balen, F.A.M.; Hoes, A.W. National differences in incidence of acute mastoiditis: Relationship to prescribing patterns of antibiotics for acute otitis media? Pediatr. Infect. Dis. J. 2001, 20, 140–144. [Google Scholar] [CrossRef]
- Bento, R.F.; Fonseca ACde, O. A brief history of mastoidectomy. Int. Arch. Otorhinolaryngol. 2013, 17, 168–178. [Google Scholar]
- Bartov, N.; Lahav, Y.; Lahav, G.; Zloczower, E.; Katzenell, U.; Halperin, D.; Hilly, O.; Shoffel-Havakuk, H. Management of Acute Mastoiditis With Immediate Needle Aspiration for Subperiosteal Abscess. Otol. Neurotol. 2019, 40, e612–e618. [Google Scholar] [CrossRef]
- Spratley, J.; Silveira, H.; Alvarez, I.; Pais-Clemente, M. Acute mastoiditis in children: Review of the current status. Int. J. Pediatr. Otorhinolaryngol. 2000, 56, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kerem, R.; Uri, N.; Rennert, H.; Peled, N.; Greenberg, E.; Efrat, M. Acute mastoiditis in children: Is surgical treatment necessary? J. Laryngol. Otol. 1999, 113, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Palva, T.; Ramsay, H. Incudal Folds and Epitympanic Aeration. Otol. Neurotol. 1996, 17, 700–708. Available online: https://pubmed.ncbi.nlm.nih.gov/8892564/ (accessed on 4 September 2025).
- Groth, A.; Enoksson, F.; Hultcrantz, M.; Stalfors, J.; Stenfeldt, K.; Hermansson, A. Acute mastoiditis in children aged 0–16 years—A national study of 678 cases in Sweden comparing different age groups. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1494–1500. [Google Scholar] [CrossRef]
- Stalfors, J.; Enoksson, F.; Hermansson, A.; Hultcrantz, M.; Robinson, Å.; Stenfeldt, K.; Groth, A. National assessment of validity of coding of acute mastoiditis: A standardised reassessment of 1966 records. Clin. Otolaryngol. 2013, 38, 130–135. [Google Scholar] [CrossRef]
- Enoksson, F. Acute Mastoiditis in Children—A National Study in Sweden. Ph.D. Thesis, Lund University Faculty of Medicine Doctoral Dissertation Series. Department of Otorhinolaryngology, Lund University, Lund, Sweden, 2015. Available online: http://lup.lub.lu.se/record/8146392 (accessed on 4 September 2025).
- Lee, H.J.; Woo, J.H.; Cho, S.; Oh, H.W.; Joo, H.; Baik, H.J. Risk Factors for Perioperative Respiratory Adverse Events in Children with Recent Upper Respiratory Tract Infection: A Single-Center-Based Retrospective Study. Ther. Clin. Risk Manag. 2020, 16, 1227. [Google Scholar] [CrossRef]
- Groth, A.; Enoksson, F.; Stalfors, J.; Stenfeldt, K.; Hultcrantz, M.; Hermansson, A. Recurrent acute mastoiditis—A retrospective national study in Sweden. Acta Oto-Laryngol. 2012, 132, 1275–1281. [Google Scholar] [CrossRef]
- Schwam, Z.G.; Michaelides, E.; Kuo, P.; Hajek, M.A.; Judson, B.L.; Schutt, C. Thirty-day morbidity and mortality following otologic/neurotologic surgery: Analysis of the national surgical quality improvement program. Laryngoscope 2018, 128, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Prinsley, P. An audit of “dead ear” after ear surgery. J. Laryngol. Otol. 2013, 127, 1177–1183. [Google Scholar] [CrossRef]
- Tait, A.R.; Malviya, S.; Voepel-Lewis, T.; Munro, H.M.; Seiwert, M.; Pandit, U.A. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology 2001, 95, 299–306. [Google Scholar] [CrossRef]
- Michel, F.; Vacher, T.; Julien-Marsollier, F.; Dadure, C.; Aubineau, J.-V.; Lejus, C.; Sabourdin, N.; Woodey, E.; Orliaguet, G.; Brasher, C.; et al. Peri-operative respiratory adverse events in children with upper respiratory tract infections allowed to proceed with anaesthesia: A French national cohort study. Eur. J. Anaesthesiol. 2018, 35, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Strömberg, A.-K.; Duan, M. Evaluation of the noise generated by otological electrical drills and suction during cadaver surgery. Acta Otolaryngol. 2011, 131, 1132–1135. [Google Scholar] [CrossRef]
- Michaelides, E.M.; Kartush, J.M. Implications of sound levels generated by otologic devices. Otolaryngol. Head Neck Surg. 2001, 125, 361–363. [Google Scholar] [CrossRef]
- Maccà, I.; Scapellato, M.L.; Carrieri, M.; Maso, S.; Trevisan, A.; Bartolucci, G.B. High-frequency hearing thresholds: Effects of age, occupational ultrasound and noise exposure. Int. Arch. Occup. Environ. Health 2015, 88, 197–211. [Google Scholar] [CrossRef]
- Stenfelt, S.; Goode, R.L. Transmission properties of bone conducted sound: Measurements in cadaver heads. J. Acoust. Soc. Am. 2005, 118, 2373–2391. [Google Scholar] [CrossRef]
- Abtahi, S.H.; Fazel, A.; Rogha, M.; Nilforoush, M.; Solooki, R. Effect of drill-induced noise on hearing in non-operated ear. Adv. Biomed. Res. 2016, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Rockette, H.E.; Hoberman, A.; Kurs-Lasky, M.; Paradise, J.L. Determination of the Minimal Important Difference for the Acute Otitis Media Severity of Symptom Scale. Pediatr. Infect. Dis. J. 2015, 34, e41–e43. [Google Scholar] [CrossRef] [PubMed]
- Frampton, S.J.; Pringle, M. Cutaneous sensory deficit following post-auricular incision. J. Laryngol. Otol. 2011, 125, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, S.D.; Gupta, S.R. Sensation Loss of Auricle Following Ear Surgery by Post-auricular Incision: Our Experience. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 120. [Google Scholar] [CrossRef]
- Zloczower, E.; Tsur, N.; Hershkovich, S.; Fink, N.; Marom, T. Efficacy of Oral Steroids for Acute Acoustic Trauma. Audiol. Neurootol. 2022, 27, 312–320. [Google Scholar] [CrossRef]
- Cayé-Thomasen, P.; Hermansson, A.; Tos, M.; Prellner, K. Bone modeling dynamics in acute otitis media. Laryngoscope 1999, 109, 723–729. [Google Scholar] [CrossRef]
- Swarts, J.D.; Cullen Doyle, B.M.; Alper, C.M.; Doyle, W.J. Surface area-volume relationships for the mastoid air cell system and tympanum in adult humans: Implications for mastoid function. Acta Oto-Laryngol. 2010, 130, 1230–1236. [Google Scholar] [CrossRef][Green Version]
- Gaihede, M. Middle ear volume and pressure effects on tympanometric middle ear pressure determination: Model experiments with special reference to secretory otitis media. Auris Nasus Larynx 2000, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cros, O.; Knutsson, H.; Andersson, M.; Pawels, E.; Borga, M.; Gaihede, M. Determination of the mastoid surface area and volume based on micro-CT scanning of human temporal bones. Geometrical parameters depend on scanning resolutions. Hear. Res. 2016, 340, 127–134. [Google Scholar] [CrossRef]
- Lima, M.A.R.; Farage, L.; Cury, M.C.L.; Júnior, F.B. Mastoid surface area-to-volume ratios in adult brazilian individuals. Braz. J. Otorhinolaryngol. 2013, 79, 446–453. [Google Scholar] [CrossRef]
- Kwon, O.J.; Sung, J.M.; Jung, H.K.; Kim, C.W. Postoperative Mastoid Aeration Following Canal Wall Up Mastoidectomy according to Preoperative Middle Ear Disease: Analysis of Temporal Bone Computed Tomography Scans. J. Audiol. Otol. 2017, 21, 140–145. [Google Scholar] [CrossRef][Green Version]
- Kaneko, K.; Kanemaru, S.; Kanai, R.; Atsushi, Y. Regeneration of Mastoid Air Cells in Vivo Using Autologous Cortical Bone. Surg. Sci. 2012, 2012, 514–517. [Google Scholar] [CrossRef]




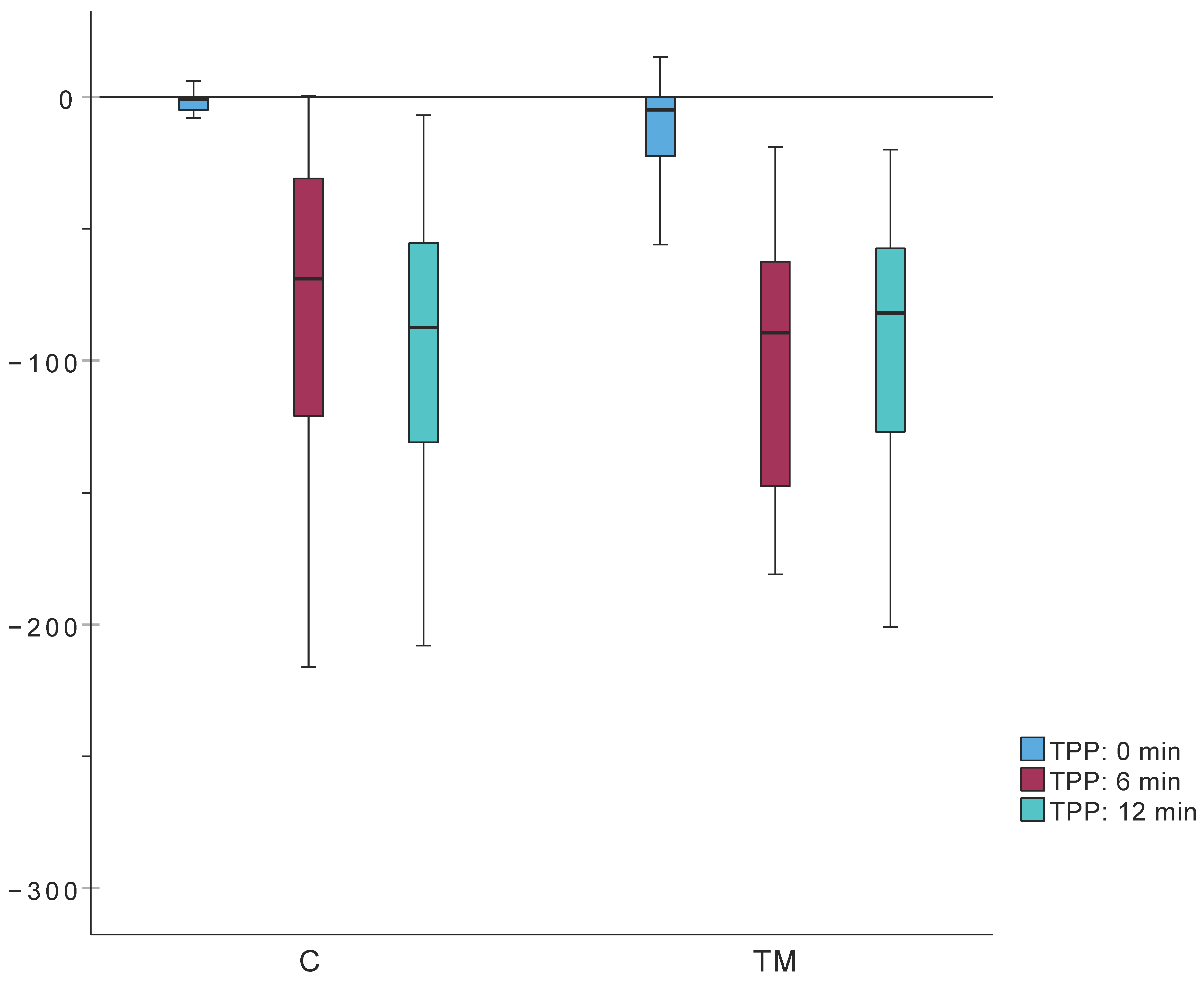

| Median (IQR) | Mean (St. Dev.) | Min.; Max. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q | TM | C | TM | C | TM | C | U | p | rrb |
| 1 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.2 (0.61) | 0.0 (0.0) | 0; 3 | 0; 0 | 467.0 | 0.34 | −0.269 |
| 2 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.07 (0.37) | 0.0 (0.0) | 0; 2 | 0; 0 | 464.0 | 0.3 | −0.131 |
| 3 | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.5 (1.01) | 0.0 (0.0) | 0; 4 | 0; 0 | 352.0 | 0.002 | −0.393 |
| 4 | 0.0 (Q1: 0.0; Q3: 2.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.93 (1.26) | 0.0 (0.0) | 0; 4 | 0; 0 | 288.0 | <0.001 | −0.499 |
| 5 | 0.0 (Q1: 0.0; Q3: 0.25) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.33 (0.71) | 0.0 (0.0) | 0; 3 | 0; 0 | 368.0 | 0.004 | −0.365 |
| 6 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.27 (0.69) | 0.0 (0.0) | 0; 3 | 0; 0 | 400.0 | 0.017 | −0.303 |
| 7 | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.47 (0.90) | 0.0 (0.0) | 0; 3 | 0; 0 | 352.0 | 0.002 | −0.393 |
| 8 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (0.0) | 0.0 (0.0) | 0; 0 | 0; 0 | 480.0 | 1 | / |
| 9 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.07 (0.37) | 0.0 (0.0) | 0; 2 | 0; 0 | 464.0 | 0.3 | −0.131 |
| 10 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.2 (0.76) | 0.0 (0.0) | 0; 3 | 0; 0 | 448.0 | 0.14 | −0.187 |
| 11 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.07 (0.36) | 0.0 (0.0) | 0; 2 | 0; 0 | 464.0 | 0.3 | −0.131 |
| 12 | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.2 (0.48) | 0.0 (0.0) | 0; 2 | 0; 0 | 450.0 | 0.17 | −0.303 |
| Median (IQR) | Mean (St. Dev.) | |||||||
|---|---|---|---|---|---|---|---|---|
| TM | T | C | TM | T | C | χ2 | p | |
| Atrophy | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.6 (0.68) | 0.4 (0.56) | 0.0 (0.0) | 19.06 | <0.001 |
| Thickening and scarring | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.27 (0.69) | 0.27 (0.69) | 0.0 (0.0) | 5.98 | 0.5 |
| Myringosclerosis | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.63 (0.89) | 0.63 (0.89) | 0.0 (0.0) | 17.32 | <0.001 |
| Myringitis | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.03 (0.18) | 0.03 (0.18) | 0.0 (0.0) | 1.09 | 0.58 |
| Retraction (Toš) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.43 (0.68) | 0.3 (0.54) | 0.0 (0.0) | 12.36 | 0.002 |
| Retraction (Sadé) | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 1.0) | 0.0 (Q1: 0.0; Q3: 0.0) | 0.1 (0.4) | 0.1 (0.4) | 0.0 (0.0) | 2.18 | 0.336 |
| C | T | TM | χ2 | p | V | ||
|---|---|---|---|---|---|---|---|
| LTA (dB) | Median (IQR) | 10.0 (Q1: 10.0; Q3: 11.66) | 11.66 (Q1: 10.0; Q3: 13.75) | 13.3 (Q1: 10.0; Q3: 18.33) | 8.06 | 0.018 | 0.30 |
| Min. | 10.0 | 10.0 | 10.0 | ||||
| Max. | 16.67 | 25.0 | 31.7 | ||||
| PTA (dB) | Median (IQR) | 10.5 (Q1: 10.0; Q3: 12.75) | 10 (Q1: 10.0; Q3: 12.25) | 11.0 (Q1: 10.0; Q3: 15.5) | 2.27 | 0.32 | 0.16 |
| Min. | 10.0 | 10.0 | 10.0 | ||||
| Max. | 19.0 | 21.8 | 35.0 | ||||
| HTA (dB) | Median (IQR) | 10 (Q1: 10.0; Q3: 10) | 10.0 (Q1: 10.0; Q3: 13.33) | 15.0 (Q1: 10.0; Q3: 19.17) | 21.7 | <0.001 | 0.48 |
| Min. | 10.0 | 10.0 | 10.0 | ||||
| Max. | 13.33 | 30.0 | 108.3 | ||||
| EHTA (dB) | Median (IQR) | 10.71 (Q1: 10.0; Q3: 14.29) | 12.5 (Q1: 10.0; Q3: 19.82) | 18.2 (Q1: 10.0; Q3: 25.71) | 9.3 | 0.01 | 0.32 |
| Min. | 7.11 | 9.3 | 8.6 | ||||
| Max. | 25.0 | 43.6 | 120 |
| C | T | TM | χ2 | p | ||
|---|---|---|---|---|---|---|
| LTA (dB) | Median (IQR) | 6.3 (Q1: 4.09; Q3: 9.76) | 4.38 (Q1: 2.8; Q3: 10.55) | 4.86 (Q1: −0.97; Q3: 8.35) | 0.76 | 0.68 |
| Min. | −3.05 | −4.45 | −6.05 | |||
| Max. | 17.15 | 17.35 | 16.10 | |||
| MTA (dB) | Median (IQR) | 19.93 (Q1: 10.38; Q3: 26.49) | 18.0 (Q1: 11.78; Q3: 23.61) | 16.51 (Q1: 9.83; Q3: 24.71) | 3.06 | 0.216 |
| Min. | 10.38 | −7.25 | −0.8 | |||
| Max. | 34.03 | 32.45 | 31.47 | |||
| HTA (dB) | Median (IQR) | 17.39 (Q1: 14.29; Q3: 20.58) | 10.08 (Q1: 5.48; Q3: 16.56) | 9.36 (Q1: 3.99; Q3: 12.24) | 20.23 | <0.001 |
| Min. | 1.8 | −1.1 | −2.62 | |||
| Max. | 30.02 | 23.95 | 12.24 |
| C | TM | U | p | ||
|---|---|---|---|---|---|
| Skin thickness (mm) | Median (IQR) | 3.73 (Q1: 3.6; Q3: 3.93) | 3.7 (Q1: 3.59; Q3: 3.89) | 477.0 | 0.929 |
| Min. | 3.0 | 3.3 | |||
| Max. | 4.7 | 4.3 | |||
| Cortical bone thickness (mm) | Median (IQR) | 5.71 (Q1: 5.2; Q3: 6.36) | 5.77 (Q1: 5.2; Q3: 6.42) | 444.0 | 0.976 |
| Min. | 4.13 | 1.93 | |||
| Max. | 7.97 | 7.97 | |||
| Min. | 1.8 | −2.62 | |||
| Max. | 30.02 | 12.24 |
| 0 Min | 6 Min | 12 Min | |||||
|---|---|---|---|---|---|---|---|
| C | TM | C | TM | C | TM | ||
| TC (°C) | Median (IQR) | 36.7 (Q1: 36.6; Q3: 36.8) | 36.6 (Q1: 36.4; Q3: 36.7) | 36.1 (Q1: 35.5; Q3: 36.3) | 35.8 (Q1: 35.3; Q3: 36.2) | 35.75 (Q1: 35.1; Q3: 36.1) | 35.5 (Q1: 34.4; Q3: 35.9) |
| Min. | 36.1 | 36.1 | 36.1 | 35.3 | 33.5 | 32.7 | |
| Max. | 36.9 | 37.3 | 36.9 | 36.2 | 36.2 | 36.6 | |
| TM (°C) | Median (IQR) | 37.3 (Q1: 36.9; Q3: 37.6) | 36.9 (Q1: 36.8; Q3: 37.1) | 18.1 (Q1: 16.5; Q3: 21.3) | 14.8 (Q1: 11.9; Q3: 20.3) | 17.65 (Q1: 15.4; Q3: 20.9) | 15.2 (Q1: 13.2; Q3: 20.7) |
| Min. | 33.2 | 33.7 | 9.9 | 8.2 | 11.2 | 6.1 | |
| Max. | 37.7 | 37.4 | 27.5 | 26.0 | 28.6 | 23.3 | |
| VORg | Median (IQR) | 0.89 (Q1: 0.80; Q3: 0.96) | 0.88 (Q1: 0.54; Q3: 1.24) | 0.93 (Q1: 0.86; Q3: 1.06) | 0.93 (Q1: 0.90; Q3: 1.06) | 1.00 (Q1: 0.95; Q3: 1.08) | 1.06 (Q1: 0.95; Q3: 1.13) |
| Min. | 0.66 | 0.81 | 0.76 | 0.80 | 0.81 | 0.91 | |
| Max. | 1.26 | 0.95 | 1.31 | 1.41 | 1.36 | 1.33 | |
| TPP (daPa) | Median (IQR) | −1.0 (Q1: −5; Q3: 0) | −5.0 (Q1: −23; Q3: 0) | −69.0 (Q1: −124; Q3: −31) | −89.5 (Q1: −65.3; Q3: −144.3) | −87.5 (Q1: −133; Q3: −53.8) | −82.0 (Q1: −130.5; Q3: −51.3) |
| Min. | −75 | −135 | −216 | −296 | −208 | −241 | |
| Max. | 11 | 15 | 0 | −19 | −7 | −20 | |
| C | TM | U | p | |
|---|---|---|---|---|
| TM 0 min (°C) | 37.3 (Q1: 36.9; Q3: 37.6) | 36.9 (Q1: 36.8; Q3: 37.1) | 260 | 0.002 |
| TM 6 min (°C) | 18.1 (Q1: 16.5; Q3: 21.3) | 14.8 (Q1: 11.9; Q3: 20.3) | 317.5 | 0.022 |
| TM 12 min (°C) | 17.65 (Q1: 15.4; Q3: 20.9) | 15.2 (Q1: 13.2; Q3: 20.7) | 302 | 0.030 |
| TC 0 min (°C) | 36.7 (Q1: 36.6; Q3: 36.8) | 36.6 (Q1: 36.4; Q3: 36.7) | 323 | 0.025 |
| TC 6 min (°C) | 36.1 (Q1: 35.5; Q3: 36.3) | 35.8 (Q1: 35.3; Q3: 36.2) | 395 | 0.230 |
| TC 12 min (°C) | 35.75 (Q1: 35.1; Q3: 36.1) | 35.5 (Q1: 34.4; Q3: 35.9) | 359.5 | 0.189 |
| ΔTM (0–6 min) (%) | −106.6 (Q1: −126; Q3: −76) | −146.2 (Q1: −212; Q3: −82) | 330 | 0.035 |
| ΔTM (6–12 min) (%) | 1.1 (Q1: −24.3; Q3: 10.4) | 2.9 (Q1: 14.2; Q3: −12.8) | 409.5 | 0.568 |
| ΔTM (0–12 min) (%) | −103.6 (Q1: −141.7; Q3: −79.1) | −141.6 (Q1: −176.4; Q3: −79.8) | 313 | 0.045 |
| ΔTC (0–6 min) (%) | −1.66 (Q1: −3.3; Q3: −1.37) | −1.8 (Q1: −3.6; Q3: −1.1) | 462 | 0.8 |
| ΔTC (6–12 min) (%) | −1.11 (Q1: −1.42; Q3: −0.83) | −1.13 (Q1: −0.13; Q3: −1.9) | 436 | 0.859 |
| ΔTC (0–12 min) (%) | −2.78 (Q1: −4.7; Q3: −2.01) | −2.7 (Q1: −5.5; Q3: −2.2) | 423 | 0.711 |
| VORg (0 min) | 0.89 (Q1: 0.80; Q3: 0.96) | 0.88 (Q1: 0.54; Q3: 1.24) | 450.5 | 0.677 |
| VORg (6 min) | 0.93 (Q1: 0.86; Q3: 1.06) | 0.93 (Q1: 0.90; Q3: 1.06) | 453 | 0.703 |
| VORg (12 min) | 1.00 (Q1: 0.95; Q3: 1.08) | 1.06 (Q1: 0.95; Q3: 1.13) | 377 | 0.292 |
| TPP (0 min) (daPa) | −1.0 (Q1: −5; Q3: 0) | −5.0 (Q1: −23; Q3: 0) | 342.5 | 0.051 |
| TPP (6 min) (daPa) | −69.0 (Q1: −124; Q3: −31) | −89.5 (Q1: −65.3; Q3: −144.3) | 357 | 0.083 |
| TPP (12 min) (daPa) | −87.5 (Q1: −133; Q3: −53.8) | −82.0 (Q1: −130.5; Q3: −51.3) | 442 | 0.929 |
| ΔVORg (0–6 min) | 0.06 (Q1: 0.032; Q3: 0.08) | 0.085 (Q1: 0.03; Q3: 0.12) | 378 | 0.152 |
| ΔVORg (6–12 min) | 0.045 (Q1: 0.023; Q3: 0.11) | 0.055 (Q1: 0.22; Q3: 0.11) | 386 | 0.357 |
| ΔVORg (0–12 min) | 0.10 (Q1: 0.09; Q3: 0.013) | 0.14 (Q1: 0.10; Q3: 0.18) | 304 | 0.033 |
| ΔTPP (0–6 min) (daPa) | −66.5 (Q1: −113.5; Q3: −27.8) | −72.5 (Q1: −127.8; Q3: −50) | 403.5 | 0.281 |
| ΔTPP (6–12 min) (daPa) | −21 (Q1: −33; Q3: −22.8) | −4 (Q1: −35; Q3: 45.3) | 339.5 | 0.108 |
| ΔTPP (0–12 min) (daPa) | −87 (Q1: −121; Q3: −51.5) | −71.5 (Q1: −101.8; Q3: −39.3) | 388.5 | 0.378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švagan, M. Evaluating the Sequelae of Mastoidectomy for Acute Mastoiditis: A Long-Term Follow-Up Study of Mastoid Function. J. Clin. Med. 2025, 14, 6689. https://doi.org/10.3390/jcm14196689
Švagan M. Evaluating the Sequelae of Mastoidectomy for Acute Mastoiditis: A Long-Term Follow-Up Study of Mastoid Function. Journal of Clinical Medicine. 2025; 14(19):6689. https://doi.org/10.3390/jcm14196689
Chicago/Turabian StyleŠvagan, Matija. 2025. "Evaluating the Sequelae of Mastoidectomy for Acute Mastoiditis: A Long-Term Follow-Up Study of Mastoid Function" Journal of Clinical Medicine 14, no. 19: 6689. https://doi.org/10.3390/jcm14196689
APA StyleŠvagan, M. (2025). Evaluating the Sequelae of Mastoidectomy for Acute Mastoiditis: A Long-Term Follow-Up Study of Mastoid Function. Journal of Clinical Medicine, 14(19), 6689. https://doi.org/10.3390/jcm14196689







