Risk-Stratifying Pituitary Adenoma Treatment: A Cohort Analysis and Risk Prediction of Hypopituitarism
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Population
2.2. Patient Selection Criteria
2.3. Clinical and Hormonal Assessment
2.4. Imaging Assessment
2.5. Treatment Protocols
2.6. Outcome Measures and Definitions
2.7. Statistical Analysis
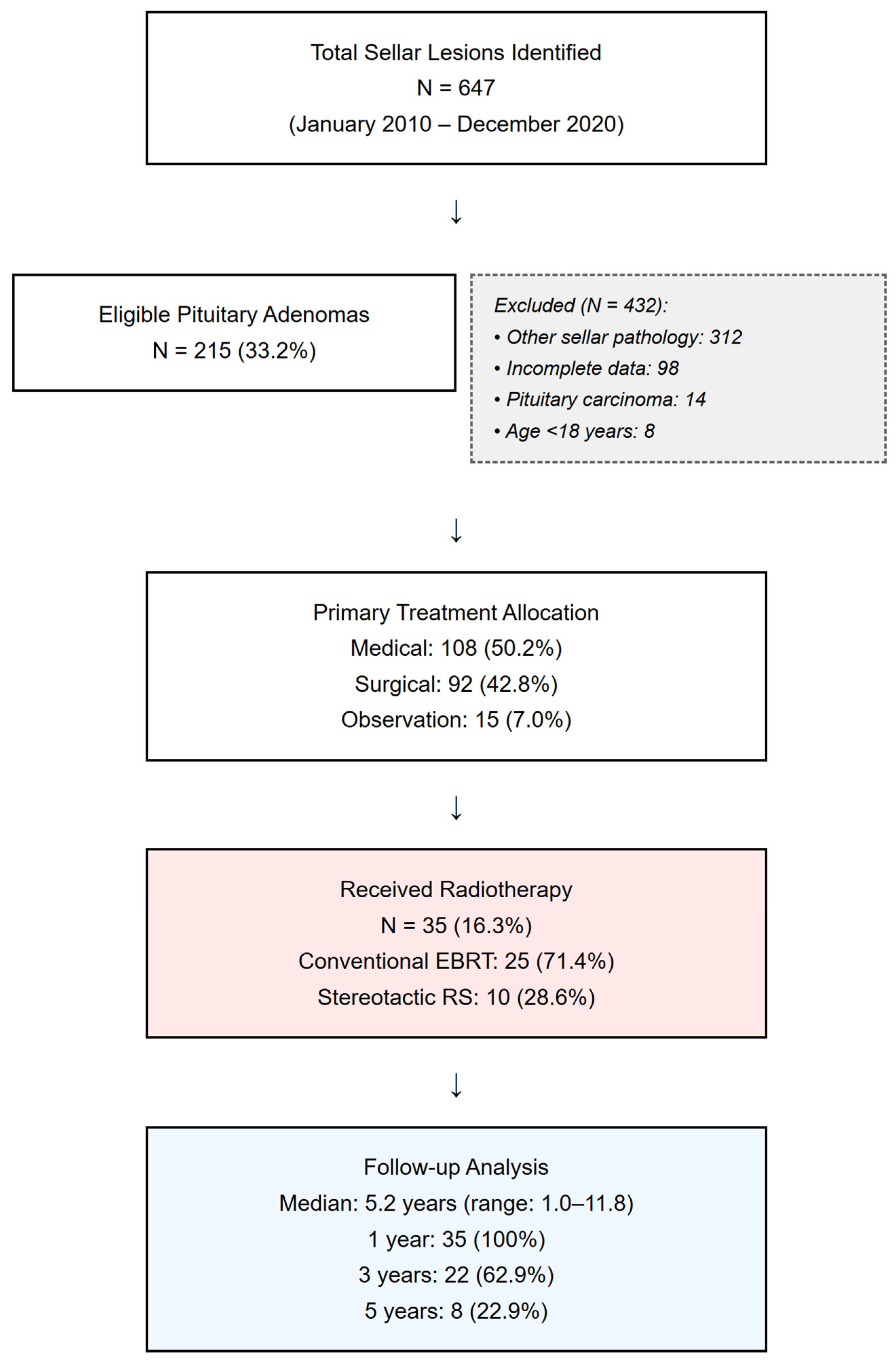
3. Results
3.1. Patient Demographics and Baseline Characteristics
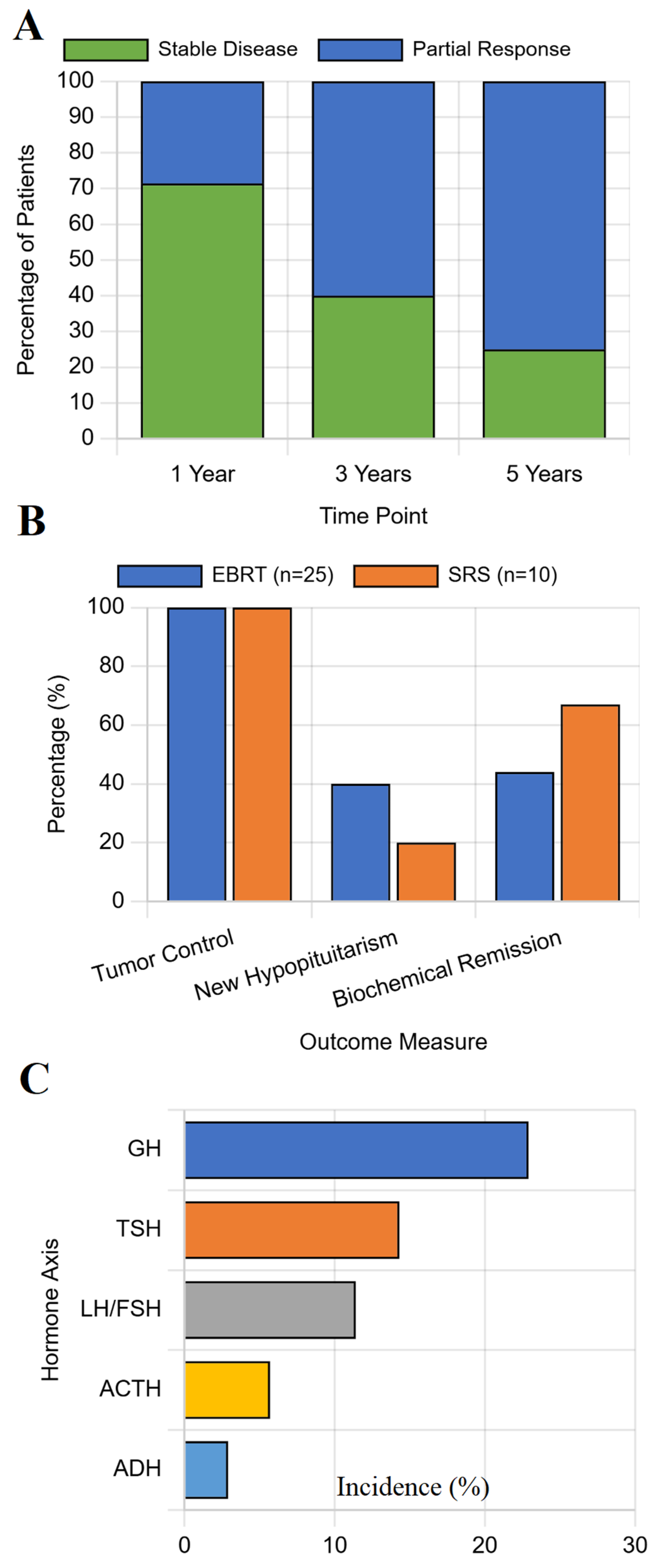
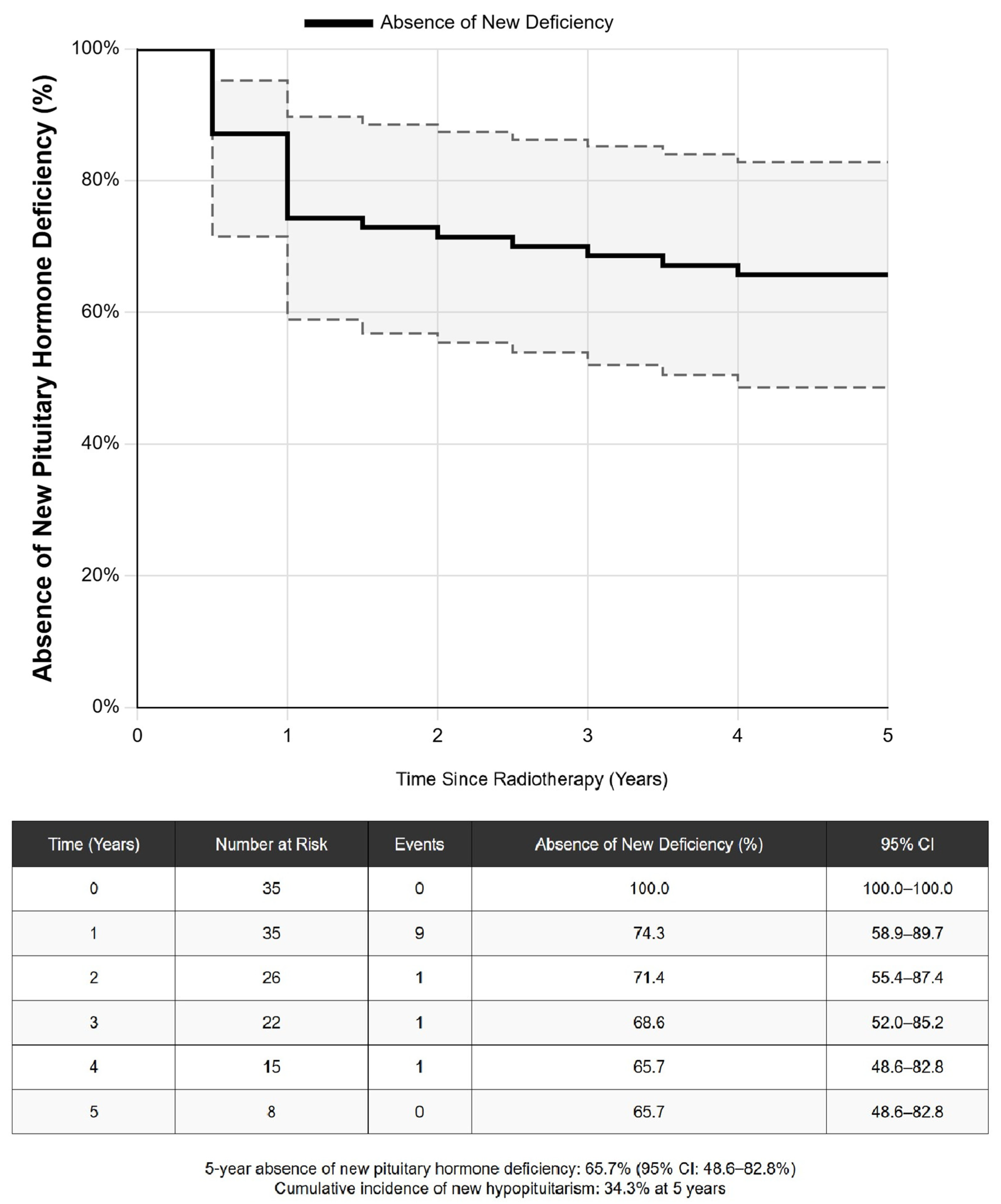
3.2. Long-Term Outcomes Across All Treatment Modalities
3.3. Predictors of Treatment-Related Hypopituitarism
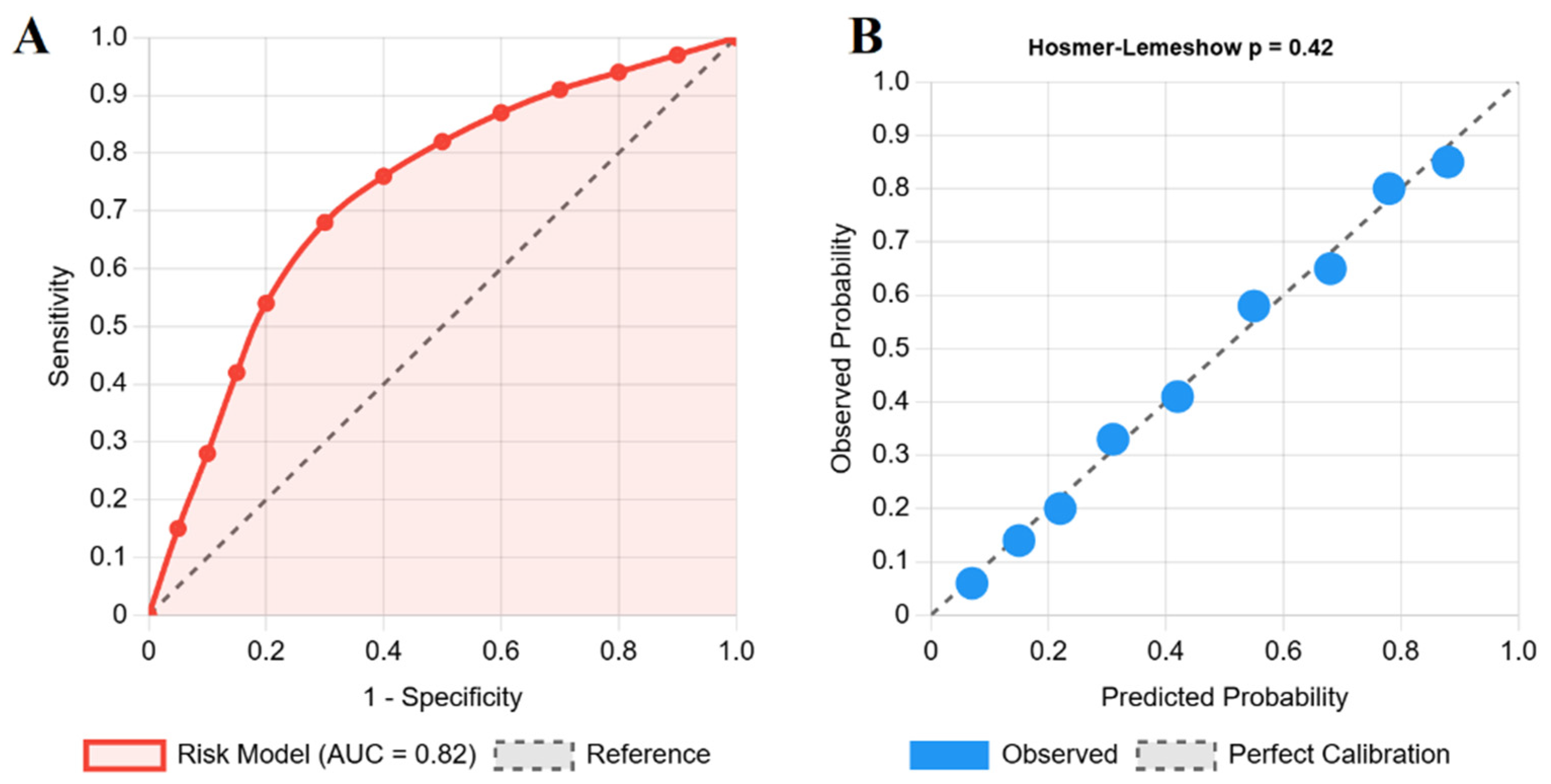
3.4. Development of Model for Risk Prediction of Hypopituitarism
4. Discussion
4.1. Comparative Treatment Outcomes in a Real-World Setting
4.2. A Pragmatic Tool for Individualized Risk Prediction
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of pituitary adenomas: A systematic review. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S. Pituitary-adenoma endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal replacement in hypopituitarism in adults: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
- Molitch, M.E. Diagnosis and treatment of pituitary adenomas: A review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef]
- Hall, W.A.; Luciano, M.G.; Doppman, J.L.; Patronas, N.J.; Oldfield, E.H. Pituitary magnetic resonance imaging in normal human volunteers: Occult adenomas in the general population. Ann. Intern. Med. 1994, 120, 817–820. [Google Scholar] [CrossRef]
- Petersenn, S.; Fleseriu, M.; Casanueva, F.F.; Giustina, A.; Biermasz, N.; Biller, B.M.K.; Bronstein, M.; Chanson, P.; Fukuoka, H.; Gadelha, M.; et al. Diagnosis and management of prolactin-secreting pituitary adenomas: A Pituitary Society international consensus statement. Nat. Rev. Endocrinol. 2023, 19, 722–740. [Google Scholar] [CrossRef]
- Zaidi, H.A.; Awad, A.W.; Bohl, M.A.; Chapple, K.; Knecht, L.; Jahnke, H.; White, W.L.; Little, A.S. Comparison of outcomes between a less experienced surgeon using a fully endoscopic technique and a very experienced surgeon using a microscopic transsphenoidal technique for pituitary adenoma. J. Neurosurg. 2016, 124, 596–604. [Google Scholar] [CrossRef]
- Minniti, G.; Flickinger, J.; Tolu, B.; Paolini, S. Radiotherapy and radiosurgery for pituitary adenomas. Pituitary 2023, 26, 45–58. [Google Scholar]
- Sheehan, J.P.; Pouratian, N.; Steiner, L.; Laws, E.R.; Vance, M.L. Gamma Knife surgery for pituitary adenomas: Factors related to radiological and endocrine outcomes. J. Neurosurg. 2011, 114, 303–309. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Holden, N.; Hills, R.K.; Wheatley, K.; Clayton, R.N.; Bates, A.; Sheppard, M.; Stewart, P. Association between premature mortality and hypopituitarism. Lancet 2001, 357, 425–431. [Google Scholar] [CrossRef]
- Higham, C.E.; Johannsson, G.; Shalet, S.M. Hypopituitarism. Lancet 2016, 388, 2403–2415. [Google Scholar] [CrossRef]
- Xu, Z.; Vance, M.L.; Schlesinger, D.; Sheehan, J.P. Hypopituitarism after stereotactic radiosurgery for pituitary adenomas. Neurosurgery 2013, 72, 630–637. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Malgo, F.; Neelis, K.J.; Coremans, I.; Biermasz, N.R.; Pereira, A.M. Pituitary dysfunction in adult patients after cranial irradiation for head and nasopharyngeal tumours. Radiother. Oncol. 2014, 113, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, D.; Xu, Z.; Mehta, G.U.; Ding, D.; Vance, M.L.; Kano, H.; Sisterson, N.; Yang, H.C.; Kondziolka, D.; Lunsford, L.D.; et al. Hypopituitarism after Gamma Knife radiosurgery for pituitary adenomas: A multicenter, international study. J. Neurosurg. 2018, 131, 1188–1196. [Google Scholar] [CrossRef]
- Mohammadzadeh, I.; Hajikarimloo, B.; Niroomand, B.; Eini, P.; Habibi, M.A.; Mortezaei, A.; Bagheri, M.H.; Günkan, A.; Aaronson, D.M.; Himic, V.; et al. Using machine learning to predict remission after surgery for pituitary adenoma: A systematic review and meta-analysis. Endocrine 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Gurnell, M.; McCormack, A.; Fukuoka, H.; Glezer, A.; Langlois, F.; Schwartz, T.H.; Greenman, Y.; Agrawal, N.; Akirov, A.; et al. for The Pituitary Society International Incidentaloma Consensus Group. Pituitary incidentaloma: A Pituitary Society international consensus guideline statement. Nat. Rev. Endocrinol. 2025, 21, 638–655. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A.H. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Van den Bergh, A.C.M.; Van den Berg, G.A.; Schoorl, M.A.; Sluiter, W.J.; van der Vliet, A.M.; Hoving, E.W.; Szabó, B.G.; Langendijk, J.A.; Wolffenbuttel, B.H.; Dullaart, R.P. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: Beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 67, 863–869. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.F.; Zhang, F.X.; Li, J.X.; Cai, L.; Zhuge, Q.C.; Wu, Z.B. Gamma knife surgery for patients with volumetric classification of nonfunctioning pituitary adenomas: A systematic review and meta-analysis. Eur. J. Endocrinol. 2013, 169, 487–495. [Google Scholar] [CrossRef]
- Agha, A. Pituitary Adenoma Hypopituitarism Risk Calculator. Available online: https://hypopituitarism-risk.netlify.app/ (accessed on 13 September 2025).
- Dekkers, O.M.; Hammer, S.; de Keizer, R.J.; Roelfsema, F.; Schutte, P.J.; Smit, J.W.A.; Romijn, J.A.; Pereira, A.M. The natural course of non-functioning pituitary macroadenomas. Eur. J. Endocrinol. 2007, 156, 217–224. [Google Scholar] [CrossRef]
- Dos Santos Nunes, V.; El Dib, R.; Boguszewski, C.L.; Nogueira, C.R. Cabergoline versus bromocriptine in the treatment of hyperprolactinemia: A systematic review of randomized controlled trials and meta-analysis. Pituitary 2011, 14, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Management of medically refractory prolactinoma. J. Neurooncol. 2014, 117, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, M.; Wei, L.; Ciric, I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 843–849. [Google Scholar] [CrossRef]
- Tabaee, A.; Anand, V.K.; Barrón, Y.; Hiltzik, D.H.; Brown, S.M.; Kacker, A.; Mazumdar, M.; Schwartz, T.H. Endoscopic pituitary surgery: A systematic review and meta-analysis. J. Neurosurg. 2009, 111, 545–554. [Google Scholar] [CrossRef]
- Pollock, B.E.; Cochran, J.; Natt, N.; Brown, P.D.; Erickson, D.; Link, M.J.; Garces, Y.I.; Foote, R.L.; Stafford, S.L.; Schomberg, P.J. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: Results from a 15-year experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1325–1329. [Google Scholar] [CrossRef]
- McLaren, D.S.; Devi, A.; Kyriakakis, N.; Kwok-Williams, M.; Murray, R.D. The impact of radiotherapy on the hypothalamo-pituitary axis: Old vs new radiotherapy techniques. Endocr. Connect. 2023, 12, e220490. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.M. Ethics and governance of trustworthy medical artificial intelligence. BMC Med. Inform. Decis. Mak. 2023, 23, 7. [Google Scholar] [CrossRef]
- Kyriakakis, N.; Lynch, J.; Orme, S.M.; Gerrard, G.; Hatfield, P.; Short, S.C.; Loughrey, C.; Murray, R.D. Hypothalamic-pituitary axis irradiation dose thresholds for the development of hypopituitarism in adult-onset gliomas. Clin. Endocrinol. 2019, 91, 131–140. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Cohort (N = 215) | Radiotherapy Group (N = 35) | No Radiotherapy (N = 180) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis, mean ± SD | 43.2 ± 14.1 | 48.1 ± 12.3 | 42.2 ± 14.3 | 0.018 |
| Female, n (%) | 127 (59.1) | 18 (51.4) | 109 (60.6) | 0.312 |
| BMI, mean ± SD | 27.8 ± 5.2 | 28.4 ± 4.9 | 27.7 ± 5.3 | 0.462 |
| Adenoma type, n (%) | ||||
| Prolactinoma | 107 (49.8) | 5 (14.3) | 102 (56.7) | <0.001 |
| Non-functioning | 77 (35.8) | 20 (57.1) | 57 (31.7) | |
| GH-secreting | 18 (8.4) | 7 (20.0) | 11 (6.1) | |
| ACTH-secreting | 8 (3.7) | 2 (5.7) | 6 (3.3) | |
| Other/Multiple | 5 (2.3) | 1 (2.9) | 4 (2.2) | |
| Adenoma size characteristics | ||||
| Macroadenoma, n (%) | 117 (54.4) | 29 (82.9) | 88 (48.9) | <0.001 |
| Maximum diameter, mm | 18.2 ± 11.4 | 26.8 ± 12.1 | 16.5 ± 10.3 | <0.001 |
| Cavernous sinus invasion, n (%) | 45 (20.9) | 15 (42.9) | 30 (16.7) | <0.001 |
| Suprasellar extension, n (%) | 89 (41.4) | 24 (68.6) | 65 (36.1) | <0.001 |
| Clinical presentation | ||||
| Headache, n (%) | 102 (47.4) | 22 (62.9) | 80 (44.4) | 0.044 |
| Visual field defect, n (%) | 48 (22.3) | 15 (42.9) | 33 (18.3) | 0.002 |
| Incidental finding, n (%) | 28 (13.0) | 2 (5.7) | 26 (14.4) | 0.159 |
| Baseline hormonal status | ||||
| Any hypopituitarism, n (%) | 71 (33.0) | 19 (54.3) | 52 (28.9) | 0.004 |
| Number of deficient axes | 0.6 ± 1.0 | 1.1 ± 1.3 | 0.5 ± 0.9 | 0.001 |
| GH deficiency, n (%) | 32 (14.9) | 10 (28.6) | 22 (12.2) | 0.014 |
| TSH deficiency, n (%) | 28 (13.0) | 8 (22.9) | 20 (11.1) | 0.058 |
| ACTH deficiency, n (%) | 21 (9.8) | 5 (14.3) | 16 (8.9) | 0.323 |
| LH/FSH deficiency, n (%) | 43 (20.0) | 12 (34.3) | 31 (17.2) | 0.023 |
| Panhypopituitarism, n (%) | 11 (5.1) | 4 (11.4) | 7 (3.9) | 0.071 |
| Variable | Univariable Analysis OR (95% CI) | p-Value | Multivariable Analysis OR (95% CI) | p-Value |
|---|---|---|---|---|
| Treatment Modality | ||||
| Medical therapy | Reference | Reference | ||
| Surgery | 2.52 (0.94–6.75) | 0.066 | 2.18 (0.78–6.09) | 0.138 |
| Radiotherapy | 9.21 (3.35–25.32) | <0.001 | 8.45 (3.82–18.71) | <0.001 |
| Observation | 0.89 (0.18–4.42) | 0.887 | 0.92 (0.17–4.98) | 0.923 |
| Baseline Characteristics | ||||
| Age > 50 years | 2.31 (1.21–4.41) | 0.011 | 1.92 (0.96–3.84) | 0.065 |
| Adenoma Characteristics | ||||
| Macroadenoma | 3.45 (1.52–7.83) | 0.003 | 2.01 (0.82–4.93) | 0.127 |
| Cavernous sinus invasion | 2.78 (1.44–5.37) | 0.002 | 2.15 (1.03–4.49) | 0.042 |
| Suprasellar extension | 1.92 (1.01–3.65) | 0.047 | 1.45 (0.71–2.96) | 0.308 |
| Baseline Hormonal Status | ||||
| Pre-existing hypopituitarism | 4.12 (2.13–7.97) | <0.001 | 3.21 (1.58–6.52) | 0.001 |
| Radiotherapy-Specific (n = 35) | ||||
| EBRT vs. SRS | 3.14 (1.12–8.81) | 0.030 | 2.78 (1.15–6.72) | 0.023 |
| Dose > 50 Gy | 2.89 (1.23–6.79) | 0.015 | 2.45 (0.98–6.13) | 0.055 |
| Previous surgery before RT | 1.67 (0.48–5.81) | 0.421 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agha, A.; Gunasekaran, S.D.; Noor, E.M.; Al Dahmani, K.M.A. Risk-Stratifying Pituitary Adenoma Treatment: A Cohort Analysis and Risk Prediction of Hypopituitarism. J. Clin. Med. 2025, 14, 6656. https://doi.org/10.3390/jcm14186656
Agha A, Gunasekaran SD, Noor EM, Al Dahmani KMA. Risk-Stratifying Pituitary Adenoma Treatment: A Cohort Analysis and Risk Prediction of Hypopituitarism. Journal of Clinical Medicine. 2025; 14(18):6656. https://doi.org/10.3390/jcm14186656
Chicago/Turabian StyleAgha, Adnan, Shriram Dorairaj Gunasekaran, Entessor Mohammed Noor, and Khaled Mohammed Asad Al Dahmani. 2025. "Risk-Stratifying Pituitary Adenoma Treatment: A Cohort Analysis and Risk Prediction of Hypopituitarism" Journal of Clinical Medicine 14, no. 18: 6656. https://doi.org/10.3390/jcm14186656
APA StyleAgha, A., Gunasekaran, S. D., Noor, E. M., & Al Dahmani, K. M. A. (2025). Risk-Stratifying Pituitary Adenoma Treatment: A Cohort Analysis and Risk Prediction of Hypopituitarism. Journal of Clinical Medicine, 14(18), 6656. https://doi.org/10.3390/jcm14186656







