Evaluation of the Effectiveness of TECAR and Vibration Therapy as Methods Supporting Muscle Recovery After Strenuous Eccentric Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
- Group 1 (n = 20)–VT recovery
- Group 2 (n = 21)–TECAR recovery
2.2. Study Design
2.3. Procedures
2.3.1. EMG Measurements
2.3.2. TMG Measurements
2.3.3. Fatiguing Eccentric Protocol
2.3.4. VT Intervention
2.3.5. TECAR Intervention
2.4. Statistical Analysis
3. Results
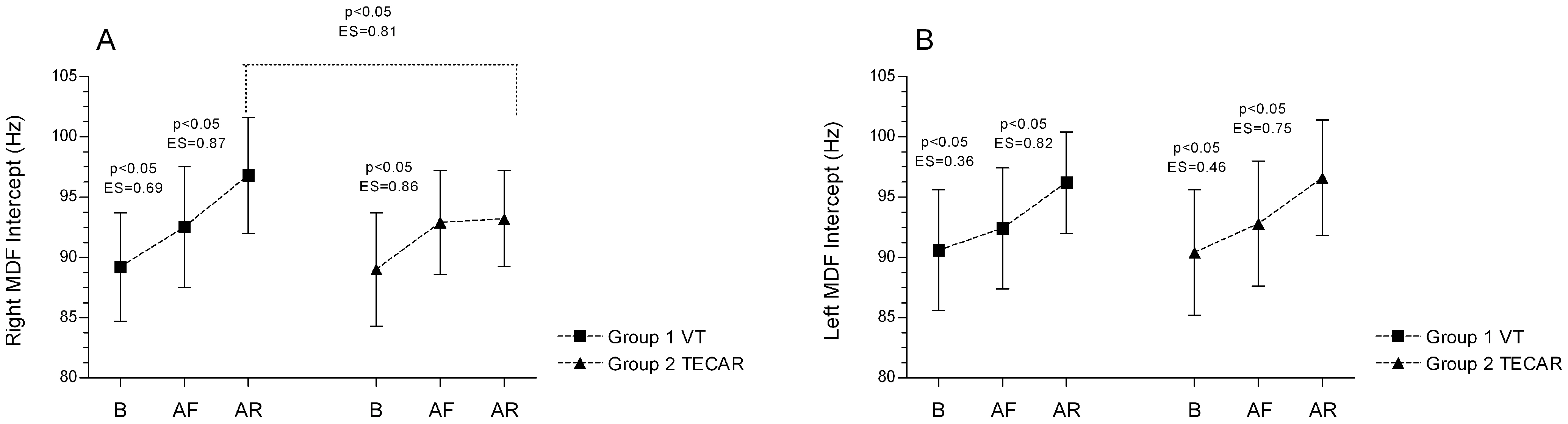
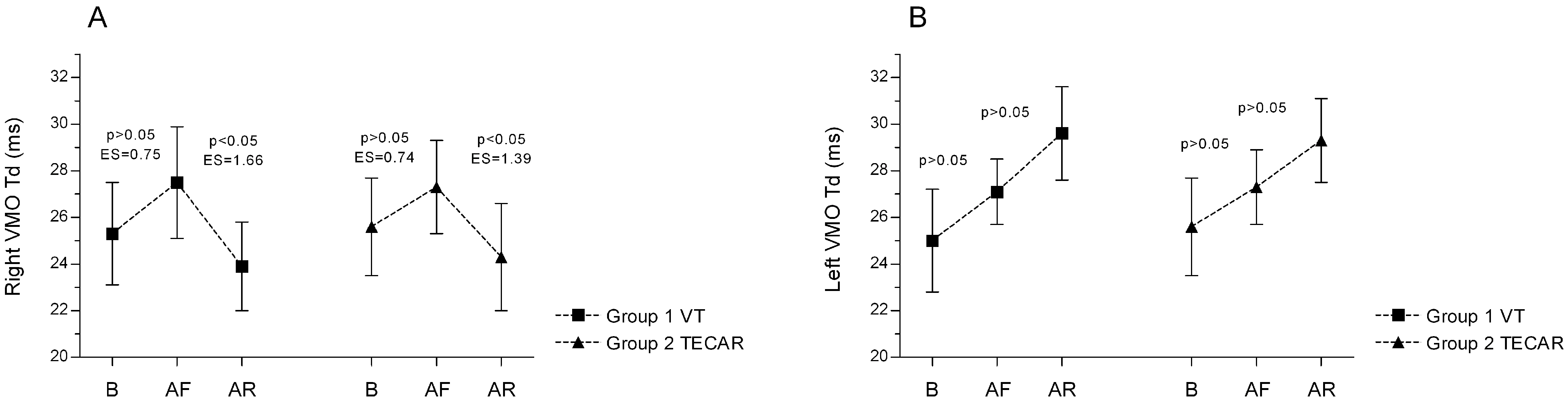
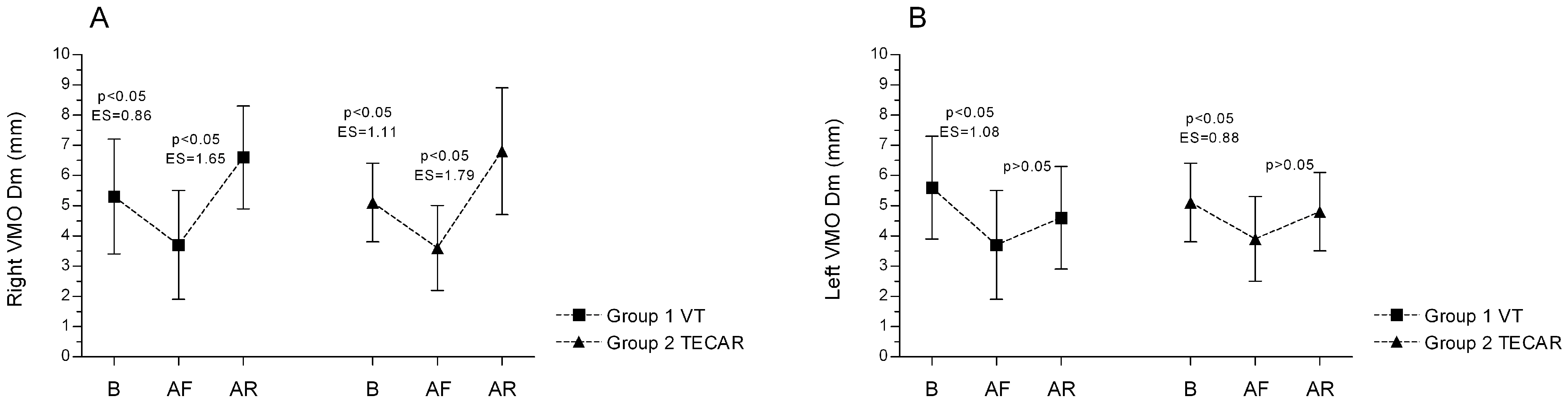
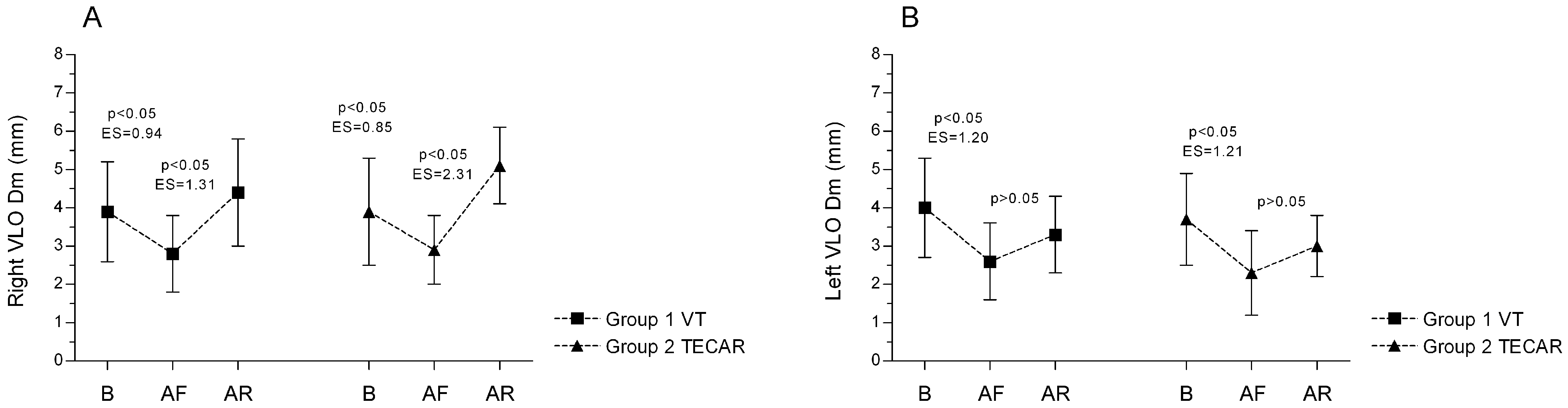
3.1. Electromyography (EMG)
3.2. Tensiomyography (TMG)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iodice, P.; Ripari, P.; Pezzulo, G. Local high-frequency vibration therapy following eccentric exercises reduces muscle soreness perception and posture alterations in elite athletes. Eur. J. Appl. Physiol. 2019, 119, 539–549. [Google Scholar] [CrossRef]
- Percival, S.; Sims, D.T.; Stebbings, G.K. Local Vibration Therapy, Oxygen Resaturation Rate, and Muscle Strength After Exercise-Induced Muscle Damage. J. Athl. Train. 2022, 57, 502–509. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Ferrauti, A.; Chupel, M.U.; Hacker, S.; Weyh, C.; Valenzuela, P.L.; Lucia, A.; Krüger, K.; Reichel, T. Acute Inflammatory Response to Eccentric Exercise in Young and Master Resistance-trained Athletes. Int. J. Sports Med. 2024, 45, 897–907. [Google Scholar] [CrossRef]
- Marklund, P.; Mattsson, C.M.; Wåhlin-Larsson, B.; Ponsot, E.; Lindvall, B.; Lindvall, L.; Ekblom, B.; Kadi, F. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J. Appl. Physiol. 2013, 114, 66–72. [Google Scholar] [CrossRef]
- Duñabeitia, I.; Arrieta, H.; Torres-Unda, J.; Gil, J.; Santos-Concejero, J.; Gil, S.M.; Irazusta, J.; Bidaurrazaga-Letona, I. Effects of a capacitive-resistive electric transfer therapy on physiological and biomechanical parameters in recreational runners: A randomized controlled crossover trial. Phys. Ther. Sport 2018, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.L.; Casazza, G.A.; Davis, B.A. Passive Recovery Strategies after Exercise: A Narrative Literature Review of the Current Evidence. Curr. Sports Med. Rep. 2021, 20, 351–358. [Google Scholar] [CrossRef]
- Wilcock, I.M.; Cronin, J.B.; Hing, W.A. Physiological response to water immersion: A method for sport recovery? Sports Med. 2006, 36, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cogburn, J.; Marcussen, B.; Slayman, T. Optimizing Athletes’ Recovery and Performance: A Review of Vibration Therapy, Compression Garments, and Massage. Curr. Sports Med. Rep. 2025, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, G.J.; Coutts, A.J.; Reaburn, P.; Hill-Haas, S. Effect of post-match cold-water immersion on subsequent match running performance in junior soccer players during tournament play. J. Sports Sci. 2011, 29, 1–6. [Google Scholar] [CrossRef]
- Tiidus, P.M. Alternative treatments for muscle injury: Massage, cryotherapy, and hyperbaric oxygen. Curr. Rev. Musculoskelet. Med. 2015, 8, 162–167. [Google Scholar] [CrossRef]
- Tashiro, Y.; Hasegawa, S.; Yokota, Y.; Nishiguchi, S.; Fukutani, N.; Shirooka, H.; Tasaka, S.; Matsushita, T.; Matsubara, K.; Nakayama, Y.; et al. Effect of Capacitive and Resistive electric transfer on haemoglobin saturation and tissue temperature. Int. J. Hyperth. 2017, 33, 696–702. [Google Scholar] [CrossRef]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the Application of Tecar Therapy Affect Temperature and Perfusion of Skin and Muscle Microcirculation? A Pilot Feasibility Study on Healthy Subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar] [CrossRef]
- Szabo, D.A.; Neagu, N.; Teodorescu, S.; Predescu, C.; Sopa, I.S.; Panait, L. TECAR Therapy Associated with High-Intensity Laser Therapy (Hilt) and Manual Therapy in the Treatment of Muscle Disorders: A Literature Review on the Theorised Effects Supporting Their Use. J. Clin. Med. 2022, 11, 6149. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Lu, J.; You, Y.; Zhang, L.; Zhu, D.; Yao, F. Does vibration benefit delayed-onset muscle soreness? A meta-analysis and systematic review. J. Int. Med. Res. 2019, 47, 3–18. [Google Scholar] [CrossRef] [PubMed]
- de Benito, A.M.; Valldecabres, R.; Ceca, D.; Richards, J.; Barrachina Igual, J.; Pablos, A. Effect of vibration vs non-vibration foam rolling techniques on flexibility, dynamic balance and perceived joint stability after fatigue. PeerJ 2019, 7, e8000. [Google Scholar] [CrossRef]
- Cochrane, D.J. Effectiveness of using wearable vibration therapy to alleviate muscle soreness. Eur. J. Appl. Physiol. 2017, 117, 501–509. [Google Scholar] [CrossRef]
- Fattorini, L.; Rodio, A.; Pettorossi, V.E.; Filippi, G.M. Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review. J. Funct. Morphol. Kinesiol. 2021, 6, 39. [Google Scholar] [CrossRef]
- Merletti, R.; Parker, P. Electromyography: Physiology, Engineering, and Non-Invasive Applications; Wiley-IEEE Press: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Nogales, R.; Guilcapi, J.; Benalcazar, F.; Vargas, J. A Brief Literature Review of Mathematical Models of EMG Signals through Hierarchical Analytical Processing. In Advances and Applications in Computer Science, Electronics, and Industrial Engineering; Garcia, M.V., Fernández-Peña, F., Gordón-Gallegos, C., Eds.; Springer: Cham, Switzerland, 2022; Volume 433, pp. 233–248. [Google Scholar]
- Casolo, A.; Del Vecchio, A.; Balshaw, T.G.; Maeo, S.; Lanza, M.B.; Felici, F.; Folland, J.P.; Farina, D. Behavior of motor units during submaximal isometric contractions in chronically strength-trained individuals. J. Appl. Physiol. 2021, 131, 1584–1598. [Google Scholar] [CrossRef]
- Zhu, X.; Pang, Y.; Li, L.; Sun, W.; Ding, L.; Song, Q.; Shen, P. Standard isometric contraction has higher reliability than maximum voluntary isometric contraction for normalizing electromyography during level walking among older adults with knee osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1276793. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Karduna, A. Submaximal contractions can serve as a reliable technique for shoulder electromyography normalization. J. Biomech. 2022, 134, 111014. [Google Scholar] [CrossRef]
- Bigliassi, M.; Scalassara, P.R.; Kanthack, T.F.D. Fourier and Wavelet Spectral Analysis of EMG Signals in 1-km Cycling Time-Trial. Appl. Math. 2014, 5, 1878–1886. [Google Scholar] [CrossRef]
- Camata, T.V.; Dantas, J.L.; Abrão, T. Fourier and Wavelet Spectral Analysis of EMG signals in Supramaximal Constant Load Dynamic Exercise. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 13–16. [Google Scholar]
- Rainoldi, A.; Bullock-Saxton, J.E.; Cavarretta, F.; Hogan, N. Repeatability of maximal voluntary force and of surface EMG variables during voluntary isometric contraction of quadriceps muscles in healthy subjects. J. Electromyogr. Kinesiol. 2001, 11, 425–438. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.J.; Callaghan, M.J.; Oldham, J.A. The reliability of isometric strength and fatigue measures in patients with knee osteoarthritis. Man. Ther. 2008, 13, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, Ł.; Mika, A.; Sopa, M.; Stolarczyk, A.; Adamska, O.; Szczudło, M.; Kielnar, R.; Hagner-Derengowska, M.; Buryta, R.; Nowak, M.J.; et al. Evaluation of Lateral and Medial Parts of the Hamstring Muscle Fatigue Symmetry in Professional Footballers Cleared to Play After ACL Reconstruction. J. Clin. Med. 2024, 13, 6521. [Google Scholar] [CrossRef]
- Lohr, C.; Braumann, K.M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of tensiomyography and myotonometry in detecting mechanical and contractile characteristics of the lumbar erector spinae in healthy volunteers. Eur. J. Appl. Physiol. 2018, 118, 1349–1359. [Google Scholar] [CrossRef]
- Gorianovas, G.; Skurvydas, A.; Streckis, V.; Brazaitis, M.; Kamandulis, S.; McHugh, M.P. Repeated bout effect was more expressed in young adult males than in elderly males and boys. Biomed. Res. Int. 2013, 2013, 218970. [Google Scholar] [CrossRef] [PubMed]
- Chwała, W.; Pogwizd, P.; Rydzik, Ł.; Ambroży, T. Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise. Int. J. Environ. Res. Public Health 2021, 18, 11680. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kang, S.R.; Kim, H.S.; Won, Y.H.; Park, S.H.; Seo, J.H.; Ko, M.H.; Kim, G.W. Effects of Low-Frequency Whole-Body Vibration on Muscle Activation, Fatigue, and Oxygen Consumption in Healthy Young Adults: A Single-Group Repeated-Measures Controlled Trial. J. Sport Rehabil. 2022, 31, 984–992. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, K.; He, B.; Yan, Y. Effect of Vertical Vibration Stimulation at Different Frequencies on Delayed Muscle Soreness in Athletes: A Randomized Trial. Front. Public Health 2022, 10, 980454. [Google Scholar] [CrossRef]
- Vahdatpour, B.; Haghighat, S.; Sadri, L.; Taghian, M.; Sadri, S. Effects of Transfer Energy Capacitive and Resistive on Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Galen Med. J. 2022, 11, e2407. [Google Scholar]
- Beltrame, R.; Ronconi, G.; Ferrara, P.E.; Salgovic, L.; Vercelli, S.; Solaro, C.; Ferriero, G. Capacitive and Resistive Electric Transfer Therapy in Rehabilitation: A Systematic Review. Int. J. Rehabil. Res. 2020, 43, 291–298. [Google Scholar] [CrossRef]
- Yeste-Fabregat, M.; Baraja-Vegas, L.; Vicente-Mampel, J.; Pérez-Bermejo, M.; Bautista González, I.J.; Barrios, C. Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 8756. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G. Effect Size Guidelines for Individual and Group Differences in Physiotherapy. Arch. Phys. Med. Rehabil. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895. [Google Scholar] [CrossRef]
- Choobsaz, H.; Ghotbi, N.; Mohamadi, P. Comparison Between the Effects of Transfer Energy Capacitive and Resistive Therapy and Therapeutic Ultrasound on Hamstring Muscle Shortness in Male Athletes: A Single-Blind Randomized Controlled Trial. Galen Med. J. 2023, 12, e2981. [Google Scholar] [CrossRef]
- Ribeiro, S.; Henriques, B.; Cardoso, R. The Effectiveness of Tecar Therapy in Musculoskeletal Disorders. Int. J. Public Health Health Syst. 2018, 3, 77–83. [Google Scholar]
- Ida, A.M.; Neves, E.B.; Stadnik, A.M.W. Effects of Tecartherapy on Body Tissue: A Systematic Review. J. Biomed. Sci. Eng. 2023, 16, 445–460. [Google Scholar] [CrossRef]
- Yokota, Y.; Sonoda, T.; Tashiro, Y.; Suzuki, Y.; Kajiwara, Y.; Zeidan, H.; Nakayama, Y.; Kawagoe, M.; Shimoura, K.; Tatsumi, M.; et al. Effect of Capacitive and Resistive Electric Transfer on Changes in Muscle Flexibility and Lumbopelvic Alignment after Fatiguing Exercise. J. Phys. Ther. Sci. 2018, 30, 719–725. [Google Scholar] [CrossRef]
- Manimmanakorn, N.; Ross, J.J.; Manimmanakorn, A.; Lucas, S.J.; Hamlin, M.J. Effect of whole-body vibration therapy on performance recovery. Int. J. Sports Physiol. Perform. 2015, 10, 388–395. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Boccia, G.; Cavazzuti, L.; Magnani, E.; Mariani, E.; Rainoldi, A.; Casale, R. Localized muscle vibration reverses quadriceps muscle hypotrophy and improves physical function: A clinical and electrophysiological study. Int. J. Rehabil. Res. 2017, 40, 339–346. [Google Scholar] [CrossRef]
- Olmos, A.A.; Sontag, S.A.; Sterczala, A.J.; Parra, M.E.; Dimmick, H.L.; Miller, J.D.; Deckert, J.A.; Herda, T.J.; Trevino, M.A. High-Intensity Cycling Training Necessitates Increased Neuromuscular Demand of the Vastus Lateralis During a Fatiguing Contraction. Res. Q. Exerc. Sport 2024, 95, 313–324. [Google Scholar] [CrossRef]
- Muceli, S.; Merletti, R. Tutorial. Frequency analysis of the surface EMG signal: Best practices. J. Electromyogr. Kinesiol. 2024, 79, 102937. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, Q.; Chu, M.; Geng, B.; Jia, H.; Li, X.; Lv, T.; Jiang, S. Long-Term Effect of Vibration Therapy for Training-Induced Muscle Fatigue in Elite Athletes. Int. J. Environ. Res. Public Health 2022, 19, 7531. [Google Scholar] [CrossRef]
- Muñoz-López, A.; De Hoyo, M.; Nuñez, F.J.; Sañudo, B. Using Tensiomyography to Assess Changes in Knee Muscle Contraction Properties After Concentric and Eccentric Fatiguing Muscle Actions. J. Strength Cond. Res. 2022, 36, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Kalc, M.; Puš, K.; Paravlic, A.; Urbanc, J.; Šimunič, B. Diagnostic accuracy of Tensiomyography parameters for monitoring peripheral neuromuscular fatigue. J. Electromyogr. Kinesiol. 2023, 70, 102775. [Google Scholar] [CrossRef] [PubMed]
| Group 1 | Group 2 | |
|---|---|---|
| Number of subjects (n) | 20 | 21 |
| Male/Female | 3/17 | 4/17 |
| Height (cm) | 168 ± 10 | 167 ± 7 |
| Weight (kg) | 63 ± 14 | 64 ± 12 |
| Age | 19 ± 2 | 19 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksy, Ł.; Mika, A.; Daszkiewicz, M.; Sopa, M.; Szczudło, M.; Kuchciak, M.; Stolarczyk, A.; Adamska, O.; Reichert, P.; Dzięcioł-Anikiej, Z.; et al. Evaluation of the Effectiveness of TECAR and Vibration Therapy as Methods Supporting Muscle Recovery After Strenuous Eccentric Exercise. J. Clin. Med. 2025, 14, 6648. https://doi.org/10.3390/jcm14186648
Oleksy Ł, Mika A, Daszkiewicz M, Sopa M, Szczudło M, Kuchciak M, Stolarczyk A, Adamska O, Reichert P, Dzięcioł-Anikiej Z, et al. Evaluation of the Effectiveness of TECAR and Vibration Therapy as Methods Supporting Muscle Recovery After Strenuous Eccentric Exercise. Journal of Clinical Medicine. 2025; 14(18):6648. https://doi.org/10.3390/jcm14186648
Chicago/Turabian StyleOleksy, Łukasz, Anna Mika, Maciej Daszkiewicz, Martyna Sopa, Miłosz Szczudło, Maciej Kuchciak, Artur Stolarczyk, Olga Adamska, Paweł Reichert, Zofia Dzięcioł-Anikiej, and et al. 2025. "Evaluation of the Effectiveness of TECAR and Vibration Therapy as Methods Supporting Muscle Recovery After Strenuous Eccentric Exercise" Journal of Clinical Medicine 14, no. 18: 6648. https://doi.org/10.3390/jcm14186648
APA StyleOleksy, Ł., Mika, A., Daszkiewicz, M., Sopa, M., Szczudło, M., Kuchciak, M., Stolarczyk, A., Adamska, O., Reichert, P., Dzięcioł-Anikiej, Z., & Kielnar, R. (2025). Evaluation of the Effectiveness of TECAR and Vibration Therapy as Methods Supporting Muscle Recovery After Strenuous Eccentric Exercise. Journal of Clinical Medicine, 14(18), 6648. https://doi.org/10.3390/jcm14186648










