Dinutuximab Beta for the Treatment of High-Risk Neuroblastoma: Data from the Hungarian Pediatric Oncology Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Assessments and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients
3.2. Treatment
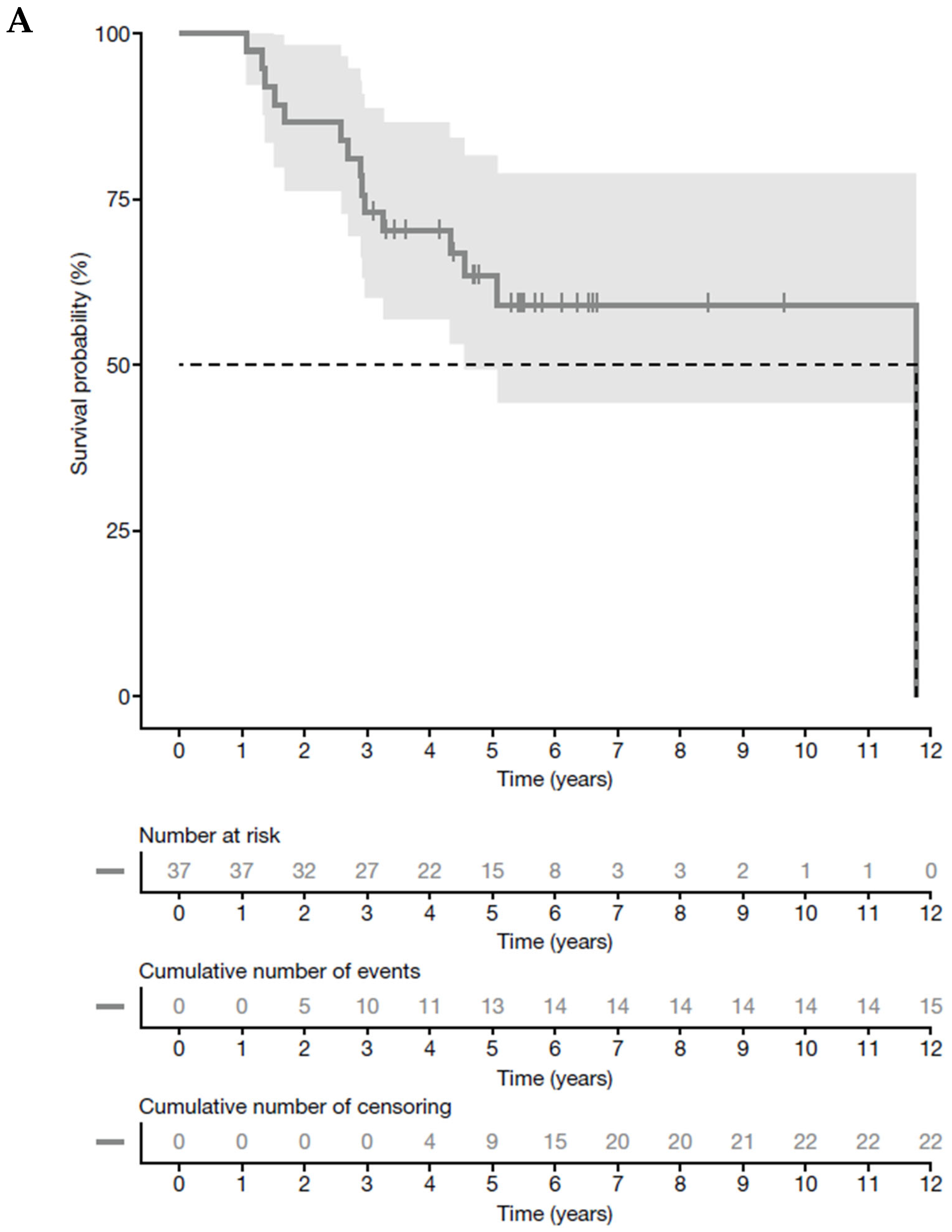
3.3. Tumor Response and Survival Analysis
3.4. Toxicities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponzoni, M.; Bachetti, T.; Corrias, M.V.; Brignole, C.; Pastorino, F.; Calarco, E.; Bensa, V.; Giusto, E.; Ceccherini, I.; Perri, P. Recent advances in the developmental origin of neuroblastoma: An overview. J. Exp. Clin. Cancer Res. 2022, 41, 92. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Dessypris, N.; Baka, M.; Moschovi, M.; Papadakis, V.; Polychronopoulou, S.; Kourti, M.; Hatzipantelis, E.; Stiakaki, E.; Dana, H.; et al. Neuroblastoma among children in Southern and Eastern European cancer registries: Variations in incidence and temporal trends compared to US. Int. J. Cancer 2018, 142, 1977–1985. [Google Scholar] [CrossRef]
- Swift, C.C.; Eklund, M.J.; Kraveka, J.M.; Alazraki, A.L. Updates in Diagnosis, Management, and Treatment of Neuroblastoma. Radiographics 2018, 38, 566–580. [Google Scholar] [CrossRef]
- London, W.B.; Castleberry, R.P.; Matthay, K.K.; Look, A.T.; Seeger, R.C.; Shimada, H.; Thorner, P.; Brodeur, G.; Maris, J.M.; Reynolds, C.P.; et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J. Clin. Oncol. 2005, 23, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Bender, H.G.; Irwin, M.S.; Hogarty, M.D.; Castleberry, R.; Maris, J.M.; Kao, P.C.; Zhang, F.F.; Naranjo, A.; Cohn, S.L.; London, W.B. Survival of Patients With Neuroblastoma After Assignment to Reduced Therapy Because of the 12- to 18-Month Change in Age Cutoff in Children's Oncology Group Risk Stratification. J. Clin. Oncol. 2023, 41, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A.; Maris, J.M. New strategies in refractory and recurrent neuroblastoma: Translational opportunities to impact patient outcome. Clin. Cancer Res. 2012, 18, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef]
- Ploessl, C.; Pan, A.; Maples, K.T.; Lowe, D.K. Dinutuximab: An Anti-GD2 Monoclonal Antibody for High-Risk Neuroblastoma. Ann. Pharmacother. 2016, 50, 416–422. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef]
- Chung, C.; Boterberg, T.; Lucas, J.; Panoff, J.; Valteau-Couanet, D.; Hero, B.; Bagatell, R.; Hill-Kayser, C.E. Neuroblastoma. Pediatr. Blood Cancer 2021, 68 (Suppl. 2), e28473. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.L.; Poetschger, U.; Valteau-Couanet, D.; Gray, J.; Luksch, R.; Balwierz, W.; Castel, V.; Ash, S.; Beck Popovic, M.; Laureys, G.; et al. Randomization of dose-reduced subcutaneous interleukin-2 (scIL2) in maintenance immunotherapy (IT) with anti-GD2 antibody dinutuximab beta (DB) long-term infusion (LTI) in front–line high-risk neuroblastoma patients: Early results from the HR-NBL1/SIOPEN trial. J. Clin. Oncol. 2019, 37, 10013. [Google Scholar] [CrossRef]
- European Medicines Agency. Qarziba (Dinutuximab Beta) Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/qarziba-epar-product-information_en.pdf (accessed on 5 September 2025).
- Lode, H.N.; Valteau-Couanet, D.; Gray, J.; Luksch, R.; Wieczorek, A.; Castel, V.; Ash, S.; Laureys, G.; Papadakis, V.; Owens, C.; et al. Randomized use of anti-GD2 antibody dinutuximab beta (DB) long-term infusion with and without subcutaneous interleukin-2 (scIL-2) in high-risk neuroblastoma patients with relapsed and refractory disease: Results from the SIOPEN LTI-trial. J. Clin. Oncol. 2019, 37, 10014. [Google Scholar] [CrossRef]
- SIOPEN Research Network. High Risk Neuroblastoma Study 1 (HR-NBL1). Available online: https://www.siopen.org/siopen-clinical-trials-1/hr1 (accessed on 3 September 2025).
- Nyári, T.A.; Kajtár, P.; Parker, L. Neuroblastoma in hungary. Eur. J. Cancer 2006, 42, 2350–2354. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F.; et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Liang, W.H.; Federico, S.M.; London, W.B.; Naranjo, A.; Irwin, M.S.; Volchenboum, S.L.; Cohn, S.L. Tailoring Therapy for Children With Neuroblastoma on the Basis of Risk Group Classification: Past, Present, and Future. JCO Clin. Cancer Inform. 2020, 4, 895–905. [Google Scholar] [CrossRef]
- Ladenstein, R.; Valteau-Couanet, D.; Brock, P.; Yaniv, I.; Castel, V.; Laureys, G.; Malis, J.; Papadakis, V.; Lacerda, A.; Ruud, E.; et al. Randomized Trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J. Clin. Oncol. 2010, 28, 3516–3524. [Google Scholar] [CrossRef]
- Amoroso, L.; Erminio, G.; Makin, G.; Pearson, A.D.J.; Brock, P.; Valteau-Couanet, D.; Castel, V.; Pasquet, M.; Laureys, G.; Thomas, C.; et al. Topotecan-Vincristine-Doxorubicin in Stage 4 High-Risk Neuroblastoma Patients Failing to Achieve a Complete Metastatic Response to Rapid COJEC: A SIOPEN Study. Cancer Res. Treat. 2018, 50, 148–155. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef]
- Barone, G.; Barry, A.; Bautista, F.; Brichard, B.; Defachelles, A.S.; Herd, F.; Manzitti, C.; Reinhardt, D.; Rubio, P.M.; Wieczorek, A.; et al. Managing Adverse Events Associated with Dinutuximab Beta Treatment in Patients with High-Risk Neuroblastoma: Practical Guidance. Paediatr. Drugs 2021, 23, 537–548. [Google Scholar] [CrossRef]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 16 December 2024).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Therneau, T.M.; Lumley, T.; Atkinson, E.; Crowson, C. A Package for Survival Analysis in R. Available online: https://CRAN.R-project.org/package=survival (accessed on 16 December 2024).
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using ‘ggplot2’. Available online: https://CRAN.R-project.org/package=survminer (accessed on 16 December 2024).
- Corbacioglu, S.; Lode, H.; Ellinger, S.; Zeman, F.; Suttorp, M.; Escherich, G.; Bochennek, K.; Gruhn, B.; Lang, P.; Rohde, M.; et al. Irinotecan and temozolomide in combination with dasatinib and rapamycin versus irinotecan and temozolomide for patients with relapsed or refractory neuroblastoma (RIST-rNB-2011): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2024, 25, 922–932. [Google Scholar] [CrossRef]
- Aitken, J.F.; Youlden, D.R.; Moore, A.S.; Baade, P.D.; Ward, L.J.; Thursfield, V.J.; Valery, P.C.; Green, A.C.; Gupta, S.; Frazier, A.L. Assessing the feasibility and validity of the Toronto Childhood Cancer Stage Guidelines: A population-based registry study. Lancet Child. Adolesc. Health 2018, 2, 173–179. [Google Scholar] [CrossRef]
- Waetzig, R.; Matthes, M.; Leister, J.; Penkivech, G.; Heise, T.; Corbacioglu, S.; Sommer, G. Comparing mTOR inhibitor Rapamycin with Torin-2 within the RIST molecular-targeted regimen in neuroblastoma cells. Int. J. Med. Sci. 2021, 18, 137–149. [Google Scholar] [CrossRef]
- Nonnenmacher, L.; Westhoff, M.A.; Fulda, S.; Karpel-Massler, G.; Halatsch, M.E.; Engelke, J.; Simmet, T.; Corbacioglu, S.; Debatin, K.M. RIST: A potent new combination therapy for glioblastoma. Int. J. Cancer 2015, 136, E173–E187. [Google Scholar] [CrossRef]
- Kieran, M.W.; Turner, C.D.; Rubin, J.B.; Chi, S.N.; Zimmerman, M.A.; Chordas, C.; Klement, G.; Laforme, A.; Gordon, A.; Thomas, A.; et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J. Pediatr. Hematol. Oncol. 2005, 27, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Robison, N.J.; Campigotto, F.; Chi, S.N.; Manley, P.E.; Turner, C.D.; Zimmerman, M.A.; Chordas, C.A.; Werger, A.M.; Allen, J.C.; Goldman, S.; et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr. Blood Cancer 2014, 61, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Żebrowska, U.; Ussowicz, M.; Sokół, A.; Stypińska, M.; Dembowska-Bagińska, B.; Pawińska-Wąsikowska, K.; Balwierz, W. Dinutuximab Beta Maintenance Therapy in Patients with High-Risk Neuroblastoma in First-Line and Refractory/Relapsed Settings-Real-World Data. J. Clin. Med. 2023, 12, 5252. [Google Scholar] [CrossRef] [PubMed]
- Giljević, J.S.; Rajačić, N.; Mikulić, D.; Batoš, A.T. Dinutuximab Beta in Children with High-Risk Neuroblastoma: Experience from a Single Center in Croatia. Children 2022, 9, 943. [Google Scholar] [CrossRef]
- Achbergerová, M.; Hederová, S.; Hrašková, A.; Kolenová, A. Dinutuximab beta in the treatment of high-risk neuroblastoma: A follow-up of a case series in Bratislava. Medicine 2022, 101, e28716. [Google Scholar] [CrossRef]
- Papp, M.; Kőrösi, L.; Sándor, J.; Nagy, C.; Juhász, A.; Ádány, R. Workforce crisis in primary healthcare worldwide: Hungarian example in a longitudinal follow-up study. BMJ Open 2019, 9, e024957. [Google Scholar] [CrossRef] [PubMed]
- Kovács, N.; Pálinkás, A.; Sipos, V.; Nagy, A.; Harsha, N.; Kőrösi, L.; Papp, M.; Ádány, R.; Varga, O.; Sándor, J. Factors Associated with Practice-Level Performance Indicators in Primary Health Care in Hungary: A Nationwide Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 3153. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Nyári, T.A.; Lantos, T. Patterns of Childhood Cancer Mortality in Hungary Since the Turn of the Millennium, Including the Two Years of the COVID-19 Pandemic. Cancers 2024, 16, 3961. [Google Scholar] [CrossRef] [PubMed]

| Number of Patients | N = 37 |
|---|---|
| Patients with HR-NB who received dinutuximab beta, n (%) a | 37 (100) |
| First line | 31 (83.8) |
| Relapsed disease | 6 (16.2) |
| Distant relapse | 4 (10.8) |
| Male, n (%) | 26 (70.3) |
| Age, median (range) months | 39.2 (0.7–150.4) |
| INSS stage at diagnosis, n (%) | |
| 2 | 1 (2.7) |
| 3 | 2 (5.4) |
| 4 | 27 (73.0) |
| 4S | 1 (2.7) |
| rec b | 6 (16.2) |
| MYCN amplified, c n (%) | 15 (40.5) |
| INSS stage 2 | 1 (2.7) |
| INSS stage 3 c | 1 (2.7) |
| INSS stage 4 | 11 (29.7) |
| INSS stage 4S | 1 (2.7) |
| rec b | 1 (2.7) |
| Unfavorable histopathology, d n (%) | 30 (81.1) |
| Primary tumor major location, n (%) | |
| Adrenal glands | 34 (91.9) |
| Abdomen | 2 (5.4) |
| Lymph nodes | 1 (2.7) |
| Number of metastatic compartments at diagnosis, n (%) | |
| 0 | 4 (10.8) |
| 1 | 11 (29.7) |
| 2 | 7 (18.9) |
| 3 | 9 (24.3) |
| ≥4 | 6 (16.2) |
| Number of Patients | N = 37 |
|---|---|
| Number of dinutuximab beta cycles received, n (%) | |
| 1 | 4 (10.8) |
| 2 | 2 (5.4) |
| 3 | 4 (10.8) |
| 4 | 2 (5.4) |
| 5 | 23 (62.2) |
| 6 | 1 (2.7) |
| 9 | 1 (2.7) |
| Subsequent treatment after initial dinutuximab beta course, n (%) | 11 (29.7) |
| First-line dinutuximab beta | 9 (24.3) |
| RIST | 6 (16.2) |
| MIBG + HSCT | 1 (2.7) |
| Radiotherapy, RIST | 1 (2.7) |
| KIERAN | 1 (2.7) |
| Second-line dinutuximab beta (relapse after 5 first-line cycles) | 2 (5.4) |
| RIST, 1 × N5, 1 × N6, MIBG + haplo-HSCT | 1 (2.7) |
| 4 × N5, 4 × N6, MIBG + haplo-HSCT | 1 (2.7) |
| No further treatment after initial dinutuximab beta course, n (%) | 26 (70.3) |
| Number of Patients | N = 37 (100%) |
|---|---|
| Objective response rate, n (%) | 19 (51.4) |
| Complete response (CR) | 19 (51.4) |
| Partial response (PR) | 0 (0.0) |
| Disease control rate, n (%) | 20 (54.1) |
| Stable disease (SD) | 1 (2.7) |
| Progressive disease, n (%) | 2 (5.4) |
| Death, n (%) | 15 (40.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernádfői, M.; Szabados, M.; Brückner, E.; Varga, Á.; Hauser, P.; Ottóffy, G.; Vojcek, Á.; Csanádi, K.; Kertész, G.; Jakab, Z.; et al. Dinutuximab Beta for the Treatment of High-Risk Neuroblastoma: Data from the Hungarian Pediatric Oncology Network. J. Clin. Med. 2025, 14, 6641. https://doi.org/10.3390/jcm14186641
Hernádfői M, Szabados M, Brückner E, Varga Á, Hauser P, Ottóffy G, Vojcek Á, Csanádi K, Kertész G, Jakab Z, et al. Dinutuximab Beta for the Treatment of High-Risk Neuroblastoma: Data from the Hungarian Pediatric Oncology Network. Journal of Clinical Medicine. 2025; 14(18):6641. https://doi.org/10.3390/jcm14186641
Chicago/Turabian StyleHernádfői, Márk, Márton Szabados, Edit Brückner, Ágnes Varga, Péter Hauser, Gábor Ottóffy, Ágnes Vojcek, Krisztina Csanádi, Gabriella Kertész, Zsuzsanna Jakab, and et al. 2025. "Dinutuximab Beta for the Treatment of High-Risk Neuroblastoma: Data from the Hungarian Pediatric Oncology Network" Journal of Clinical Medicine 14, no. 18: 6641. https://doi.org/10.3390/jcm14186641
APA StyleHernádfői, M., Szabados, M., Brückner, E., Varga, Á., Hauser, P., Ottóffy, G., Vojcek, Á., Csanádi, K., Kertész, G., Jakab, Z., Agócs, G., & Garami, M. (2025). Dinutuximab Beta for the Treatment of High-Risk Neuroblastoma: Data from the Hungarian Pediatric Oncology Network. Journal of Clinical Medicine, 14(18), 6641. https://doi.org/10.3390/jcm14186641









