Intracranial Metastases from Uterine Leiomyosarcoma: A Systematic Review and Case Illustration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Literature Search and Review Protocol
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Search and Extraction
2.6. Quality Assessments and Analysis
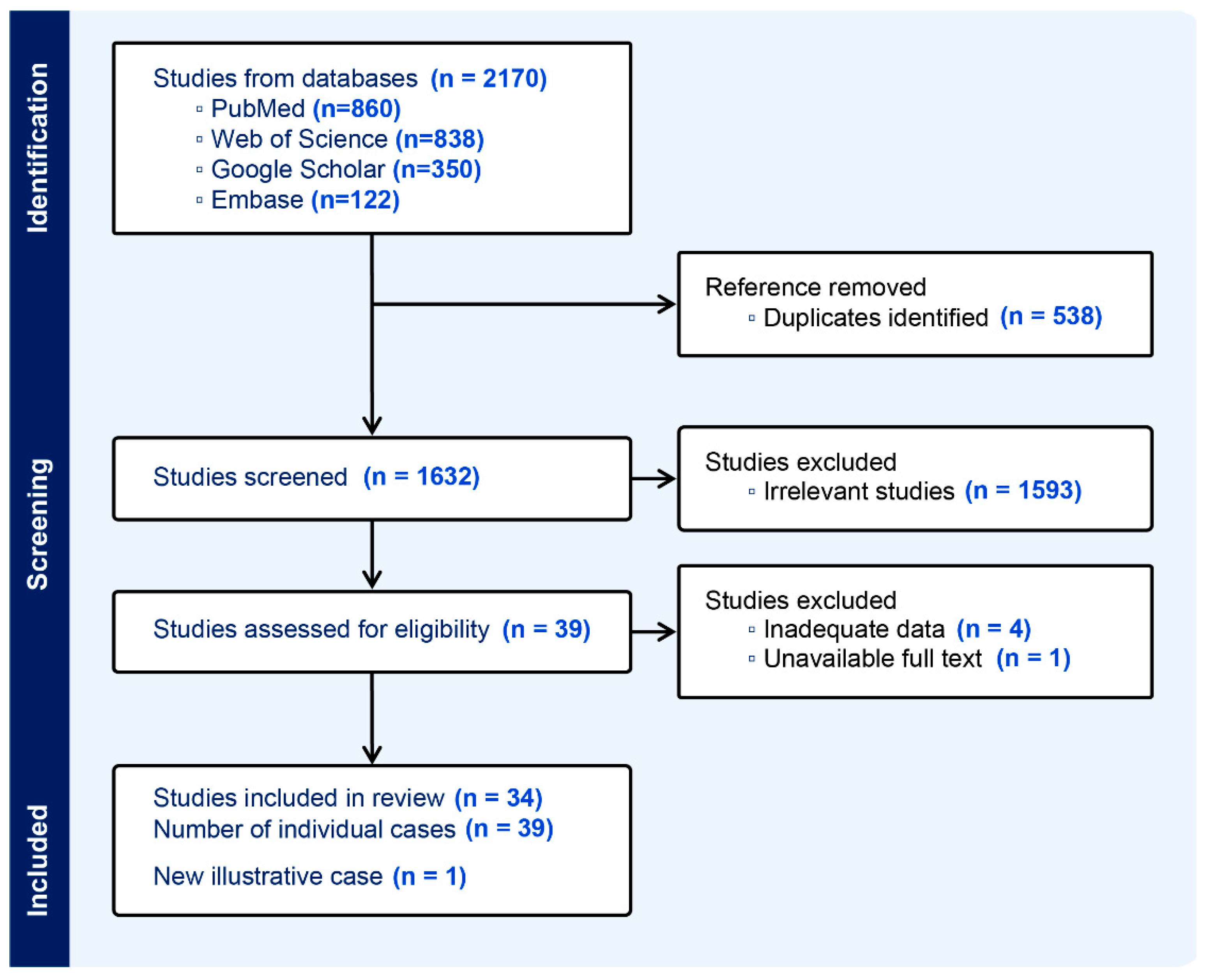
3. Results
3.1. Search Results and Study Characteristic
3.2. Patient Characteristics
3.3. Presentation Symptoms
3.4. Radiological Features and Diagnosis
3.5. Histopathology
3.6. Treatment Approaches and Outcomes
3.7. Methodological Quality and Risk of Bias Assessments
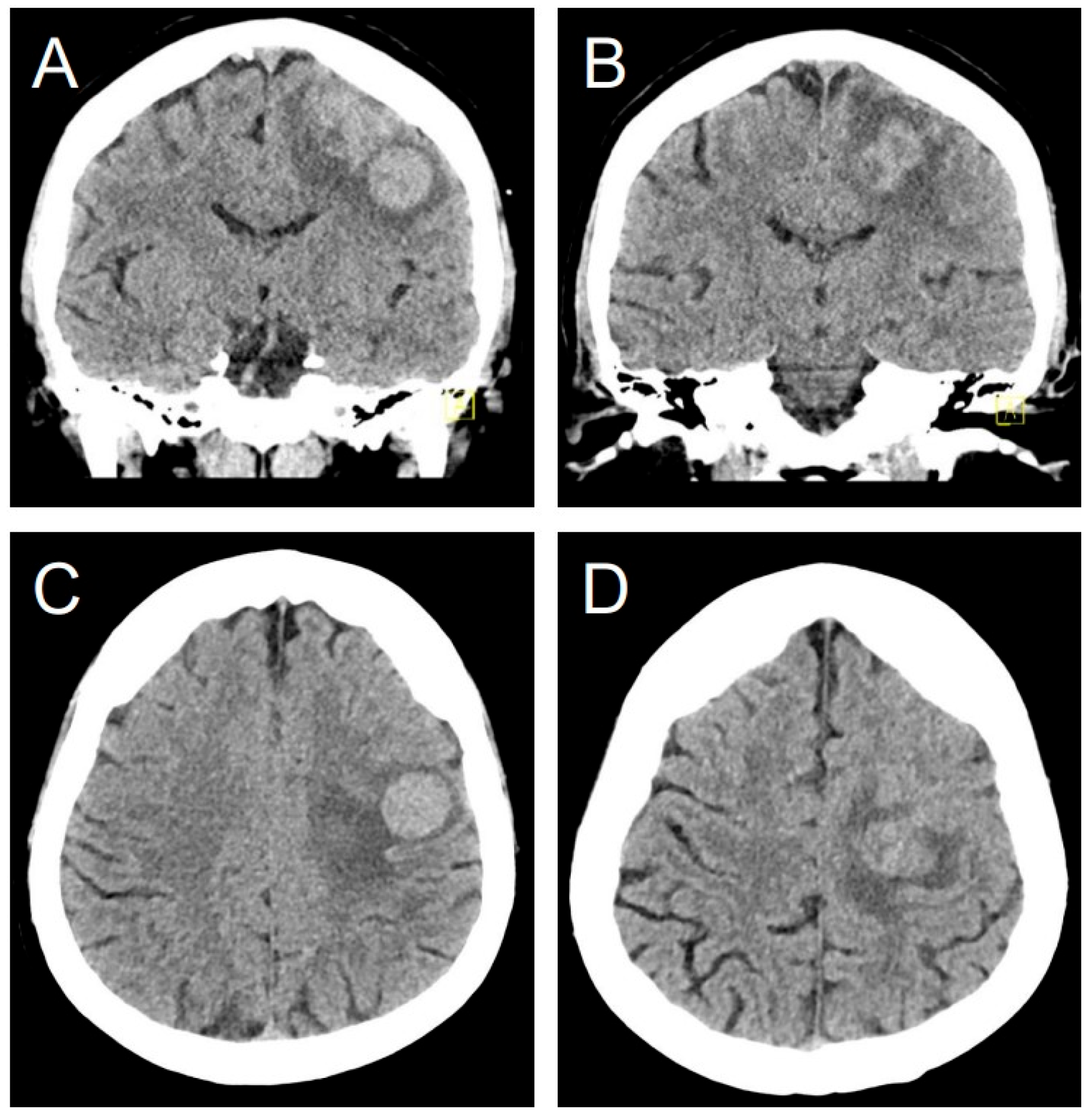
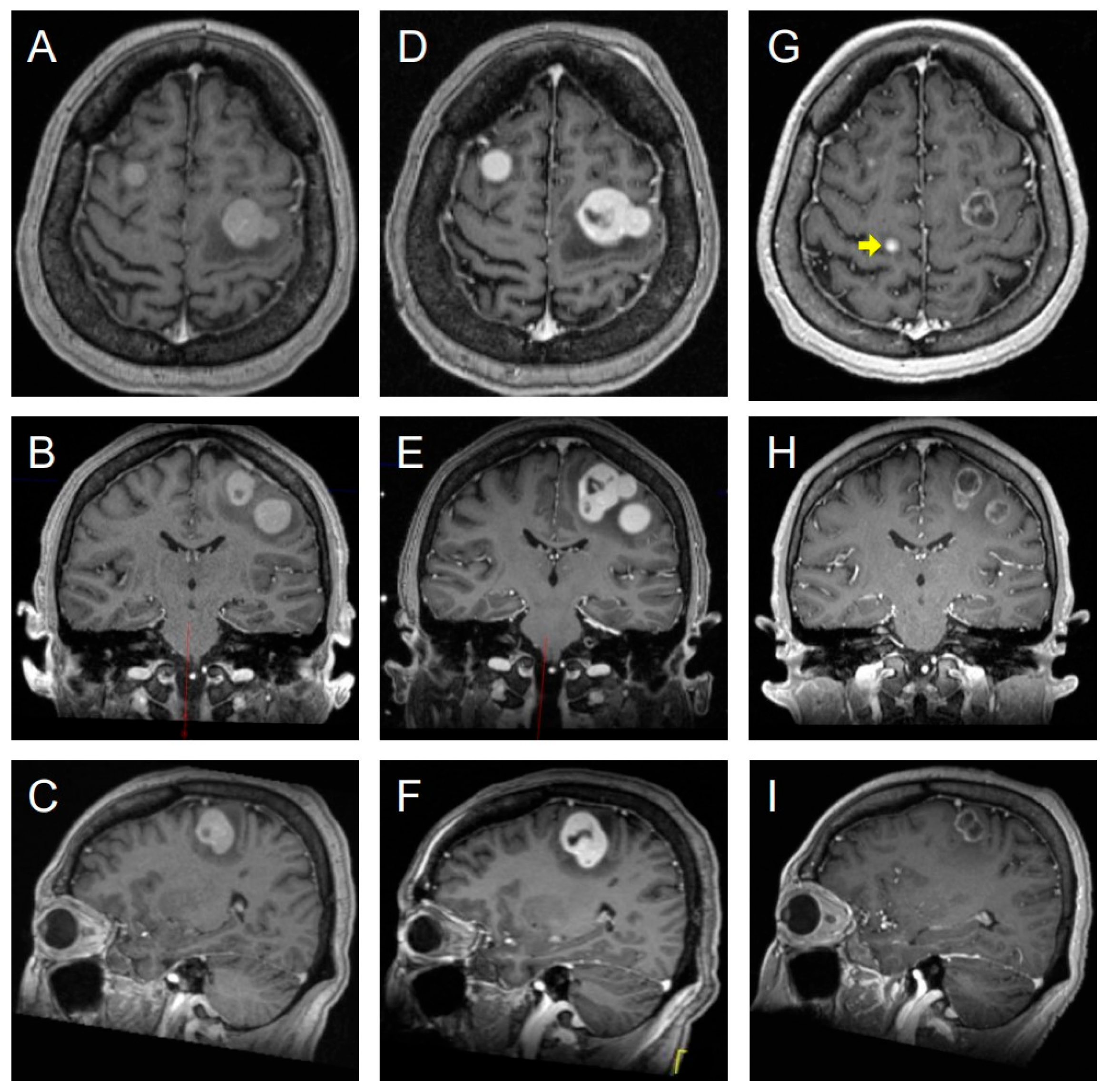
3.8. Illustrative Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMS | Leiomyosarcoma |
| ULMS | Uterine Leiomyosarcoma |
| BM | Brain Metastases |
| GKRS | Gamma-Knife Radiosurgery |
| WBRT | Whole Brain Radiotherapy |
| CT | Computer Tomography |
| MRI | Magnetic Resonance Imaging |
| ADC | Apparent Diffusion Coefficient |
References
- Hickman, A.; Siontis, B.L. Not All Leiomyosarcomas Are the Same: How to Best Classify LMS. Curr. Treat. Options Oncol. 2023, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lacuna, K.; Bose, S.; Ingham, M.; Schwartz, G. Therapeutic advances in leiomyosarcoma. Front. Oncol. 2023, 13, 1149106. [Google Scholar] [CrossRef]
- Mocellin, S. Leiomyosarcoma. In Soft Tissue Tumors: A Practical and Comprehensive Guide to Sarcomas and Benign Neoplasms; Springer: Cham, Switzerland, 2021; pp. 477–481. [Google Scholar]
- Salvati, M.; Cervoni, L.; Caruso, R.; Gagliardi, F.M.; Delfini, R. Sarcoma metastatic to the brain: A Series of 15 Cases. Surg. Neurol. 1998, 49, 441–444. [Google Scholar] [CrossRef]
- Sabhavath, M.; Annamaraju, S.S.; Amanchi, N.R.; Bhavanam, K.R.; Kancha, R.K. Soft tissue sarcoma. In Biomedical Aspects of Solid Cancers; Springer: Cham, Switzerland, 2024; pp. 279–288. [Google Scholar]
- Jędrys, W.; Leśniak, A.; Borkowska, A.; Rutkowski, P.; Sobczuk, P. Brain metastases of sarcoma: A rare phenomenon in rare tumours. J. Cancer Res. Clin. Oncol. 2023, 149, 18271–18281. [Google Scholar] [CrossRef]
- Carrillo-Uzeta, A.A.; Varela-Avalos, E.; Agustin-Godinez, E.; Uehara-Gonzalez, J.A.; Medina-Romero, J.R.; Velazquez-Zamarripa, A.I.; Quiñones-González, A.M.; Romero-García, P.A. A neurosurgical approach to skull metastasis from uterine leiomyosarcoma: Illustrative case. J. Neurosurg. Case Lessons 2025, 9, CASE24769. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Jordan, Z.; Pilla, B. From agenda to action: JBI Evidence Synthesis and the United Nations Sustainable Development Goals. JBI Evid. Synth. 2024, 22, 364–377. [Google Scholar] [CrossRef]
- Vaquero, J.; Martinez, R.; el Barkani, A.; Gomez-Angulo, J.C.; Escandon, J. Leiomyosarcoma metastatic to the brain with prolonged survival. J. Neurosurg. Sci. 1989, 33, 291–292. [Google Scholar] [PubMed]
- Prussia, P.R.; Clarke, H.A.; Mansoor, G.; Garriques, S.; Maheswaran, B. Uterine leiomyosarcoma with intracerebral metastasis: A case report. J. Natl. Med. Assoc. 1992, 84, 368–370. [Google Scholar] [PubMed]
- Wronski, M.; de Palma, P.; Arbit, E. Leiomyosarcoma of the uterus metastatic to brain: A case report and a review of the literature. Gynecol. Oncol. 1994, 54, 237–241. [Google Scholar] [CrossRef]
- Bindal, R.K.; Sawaya, R.E.; Leavens, M.E.; Taylor, S.H.; Guinee, V.F. Sarcoma metastatic to the brain: Results of surgical treatment. Neurosurgery 1994, 35, 185–190. [Google Scholar] [CrossRef]
- Uchino, M.; Endo, G.; Shibata, I.; Terao, H.; Kuramitsu, T.; Kushida, Y.; Nakamura, N. Uterine leiomyosarcoma metastasis to the skull—Case report. Neurol. Med. Chir. 1996, 36, 469–471. [Google Scholar] [CrossRef]
- Ziyal, I.M.; Musluman, M.; Bejjani, G.K.; Tanik, C.; Turkmen, C.S.; Aydin, Y. Cerebral metastasis of a uterine leiomyosarcoma—Case report. Neurol. Med. Chir. 1999, 39, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Mawrin, C.; Kirches, E.; Dietzmann, K.; Weis, S. Uterine leiomyosarcoma metastatic to the brain stem. Arch. Gynecol. Obstet. 2002, 266, 119–121. [Google Scholar] [CrossRef]
- Yip, C.M.; Yang, K.C.; Lo, Y.S.; Liao, W.C.; Chen, J.Y.; Hsu, S.S. Skull metastasis from uterine leiomyosarcoma: A case report. Acta Neurol. Taiwan. 2006, 15, 109–113. [Google Scholar] [PubMed]
- Munakata, A.; Asano, K.; Hatayama, T.; Itoh, K.; Suzuki, S.; Ohkuma, H. Leiomyosarcoma of the uterus metastatic to the brain. No Shinkei Geka 2006, 34, 409–413. [Google Scholar] [PubMed]
- Melone, G.A.; D’Elia, A.; Brogna, C.; Salvati, M. Uterine leiomyosarcoma metastatic to the brain: Case report. Tumori 2008, 94, 856–860. [Google Scholar] [CrossRef]
- Kaya, A.O.; Büyükberber, S.; Yildiz, R.; Öztürk, B.; Karagülle, K.; Yaman, E. Uterine Leiomyosarcoma Presenting with Neurological Symptoms: Differential Diagnosis. Turk. Klin. J. Med. Sci. 2009, 29, 281–284. [Google Scholar]
- Benizelos, J.; Anagnostou, E.; Papathomas, T.; Spandos, V.; Kampas, L.; Rasala, V.; Sioutopoulou, D.; Destouni, C.; Tatsiou, Z.; Tsantila, I. Abstracts of the 22nd European Congress of Pathology, September 4–9, 2009, Florence, Italy. Virchows Arch. 2009, 455 (Suppl. 1), S1–S448. [Google Scholar] [CrossRef]
- Pereira, F.O.C.; Pereira, D.C.; de Castro Aguiar, R.; Lombardi, I.A.S.; Zanini, M.A. Leiomyosarcoma Metastatic to the skull and spine: A case report. Rev. Bras. Cancerol. 2011, 57, 63–66. [Google Scholar] [CrossRef]
- Honeybul, S.; Ha, T. Leiomyosarcoma of the uterus metastatic to the brain: A case report. Arch. Gynecol. Obs. 2009, 279, 391–393. [Google Scholar] [CrossRef]
- Venizelos, I.; Anagnostou, E.; Papathomas, T.; Spandos, V.; Marinopoulos, D.; Tsitsopoulos, P.; Tsonidis, C. A 57-year-old female with a cerebellar mass. Brain Pathol. 2011, 21, 351–354. [Google Scholar] [CrossRef]
- Yamada, S.; Yamada, S.M.; Nakaguchi, H.; Murakami, M.; Hoya, K.; Matsuno, A. A case of multiple brain metastases of uterine leiomyosarcoma with a literature review. Surg. Oncol. 2011, 20, e127–e131. [Google Scholar] [CrossRef]
- Mariniello, G.; Vergara, P.; Del Basso De Caro, M.L.; Maiuri, F. Intracranial dural metastasis from uterine leiomyosarcoma with orbital extension. Neurol. Sci. 2012, 33, 1173–1177. [Google Scholar] [CrossRef]
- Chen, H.; Xu, C.; Zhou, Y. Complete remission of leiomyosarcoma with lung and brain metastasis by chemoradiotherapy after surgery. Chin.-Ger. J. Clin. Oncol. 2013, 12, 502–504. [Google Scholar] [CrossRef]
- Shepard, M.J.; Fezeu, F.; Lee, C.C.; Sheehan, J.P. Gamma knife radiosurgery for the treatment of gynecologic malignancies metastasizing to the brain: Clinical article. J. Neurooncol. 2014, 120, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Gurram, M.K.; Pulivarthi, S.; McGary, C.T.; Defillo, A. Brain and multiorgan metastases from uterine leiomyosarcoma. Tumori 2014, 100, e8–e13. [Google Scholar] [CrossRef]
- Abrahao, C.M.; Maluf, F.C. Uterine leiomyosarcoma with central nervous system metastases. Ecancermedicalscience 2015, 9, 515. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, I.K.; Min, G.E.; Jin, K.H.; Shin, J.H. A Case of Orbital Metastasis of Uterine Leiomyosarcoma With Intracranial Extension Presenting With Proptosis. Ophthalmic Plast. Reconstr. Surg. 2016, 32, e51–e52. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsubamoto, H.; Tomogane, Y.; Kamihigashi, M.; Shibahara, H. Pazopanib-mediated long-term disease stabilization after resection of a uterine leiomyosarcoma metastasis to the brain: A case report. Gynecol. Oncol. Rep. 2016, 17, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Agarwal, P.; Sardana, R.; Bhaskar, S. Extensively Metastasizing Leiomyosarcoma: A Diagnostic Challenge. J. Midlife Health 2017, 8, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Sosa, P.; Cuadra, G.; Hidalgo, R. Metástasis meníngea de leiomiosarcoma uterino. Rep. Caso Revis. Lit. Neurocir. 2018, 29, 103–106. [Google Scholar]
- Chahdi, H.; Oukabli, M. Brain metastases from uterine leiomyosarcoma. Pan Afr. Med. J. 2018, 30, 90. [Google Scholar] [CrossRef]
- Imoumby, F.N.; Dokponou, Y.C.H.; Kouakou, F.; El Agouri, H.; El Asri, A.C.; Gazzaz, M. Brain metastasis of uterine leiomyosarcoma: A case report and review of the literature. Open J. Mod. Neurosurg. 2021, 11, 107. [Google Scholar] [CrossRef]
- Miki, K.; Samura, K.; Takahashi, K.; Kawashima, M. Treatment of skull metastasis from uterine leiomyosarcoma: A single-center experience with literature review. Interdiscip. Neurosurg. 2021, 23, 101004. [Google Scholar] [CrossRef]
- Soo, W.T.; Teo, E.G.; Mohamad, N.; Wong, A.S.H. Chronic Subdural Hematoma Caused by Calvarial and Dural Metastasis from Uterine Leiomyosarcoma. J. Neurosci. Rural Pr. 2022, 13, 351–353. [Google Scholar] [CrossRef]
- Rodríguez Delgado, J.E.; Morales Cruz, M.; López Hernández, C.J.; Rodríguez Morales, M.; Viscarra León, J.; Torres Álvarez, M.; Díaz Quiroz, G.; Aceves Chimal, J.L. Uterine Leiomyosarcoma: Infrequent Cardiac and Brain Metastasis A Case Report. J. Cardio-Thorac. Med. 2022, 10, 1099. [Google Scholar]
- Eatz, T.; Levy, A.; Merenzon, M.; Bystrom, L.; Berry, K.; Morell, A.; Bhatia, S.; Daggubati, L.; Higgins, D.; Schlumbrecht, M.; et al. Surgically Treated Brain Metastases from Uterine Origin: A Case Series and Systematic Review. World Neurosurg. 2023, 173, e91–e108. [Google Scholar] [CrossRef]
- Richards, H.; Alsalek, S.; Laiwalla, A.; Attiah, M.; Harary, M.; Kim, W.J.; Hirt, D.; Rahman, S.U. Brain and lung metastasis of uterine leiomyosarcoma: Illustrative case. J. Neurosurg. Case Lessons 2023, 5, CASE22557. [Google Scholar] [CrossRef]
- Seyal, A.R.; Parekh, K.; Velichko, Y.S.; Salem, R.; Yaghmai, V. Tumor growth kinetics versus RECIST to assess response to locoregional therapy in breast cancer liver metastases. Acad. Radiol. 2014, 21, 950–957. [Google Scholar] [CrossRef]
- Eminovic, S.; Orth, T.; Dell’Orco, A.; Baumgartner, L.; Morotti, A.; Wasilewski, D.; Guelen, M.S.; Scheel, M.; Penzkofer, T.; Nawabi, J. Clinical and imaging manifestations of intracerebral hemorrhage in brain tumors and metastatic lesions: A comprehensive overview. J. Neuro-Oncol. 2024, 170, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.P.; Paleologos, N.A.; Mikkelsen, T.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; et al. The role of chemotherapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2010, 96, 71–83. [Google Scholar] [CrossRef] [PubMed]


| Author | Age | Lung Mets | Time to BM | EOR | Adjuvant for BM | Positive IHC Markers | Cause of Death | Survival After BM, Month |
|---|---|---|---|---|---|---|---|---|
| Vaquero et al. [10] | 26 | Yes | NS | T | Chemo | NS | CNS | 50 |
| Prussia et al. [11] | 36 | NS | 72 | T | No | NS | NS | NS |
| Wronski et al. [12] | 60 | Yes | 78 | T | WBRT | NS | Systemic | 30 |
| Bindal et al. [13] | 55 | Yes | 116 | T | WBRT | NS | Systemic | 14 |
| Uchino et al. [14] | 54 | No | 48 | T | Chemo | NS | Lung | 24 |
| Salvati et al. [4] | 58 | Yes | 3 | T | WBRT | NS | NS | 3 |
| 43 | Yes | 48 | T | WBRT | NS | NS | 5 | |
| 28 | Yes | 26 | T | WBRT | NS | NS | 5 | |
| Ziyal et al. [15] | 38 | Yes | 72 | T | WBRT | NS | CNS | 4 |
| Mawrin et al. [16] | 59 | Yes | 36 | Bx | WBRT | Laminin, Vimentin | CNS | 2 |
| Yip et al. [17] | 63 | NS | 15 | T | No | Actin, Desmin | Systemic | 4 |
| Munakata et al. [18] | 52 | No | 36 | Bx | GKRS | NS | NS | NS |
| Melone at al. [19] | 57 | Yes | 12 | T | WBRT + Chemo | NS | ___ | At least 5 |
| Kaya et al. [20] | 60 | Yes | 0 | ___ | WBRT + Chemo | Actin, Vimentin, Desmin | NS | NS |
| Benizelos et al. [21] | 51 | Yes | 6 | ___ | WBRT + Chemo | Actin, Desmin | Systemic | 3 |
| Pereira et al. [22] | 55 | No | 60 | T | No | Actin, Desmin | Systemic | 5 |
| Honeybul et al. [23] | 42 | Yes | 10 | T | No | NS | Systemic | 2 |
| Venizelos et al. [24] | 57 | Yes | 8 | STR | WBRT | Actin, Vimentin, Desmin | Systemic | 1.5 |
| Yamada et al. [25] | 50 | Yes | 28 | STR | GKRS | NS | Systemic | 12 |
| Mariniello et al. [26] | 57 | No | 8 | STR | Chemo | Actin, Vimentin | CNS | 4 |
| Chen et al. [27] | 54 | Yes | 48 | ___ | GKRS + Chemo | NS | ___ | At least 29 |
| Shepard et al. [28] | 54 | NS | 24 | ___ | GKRS | NS | Systemic | 1 |
| Gurram et al. [29] | 59 | Yes | 84 | STR | SRS | NS | Systemic | 2 |
| Abrahao et al. [30] | 45 | Yes | 31 | T | WBRT + Chemo | NS | NS | 27 |
| Abrahao et al. [30] | 51 | Yes | 22 | T | WBRT + Chemo | NS | Systemic | 16 |
| Kim et al. [31] | 57 | Yes | 36 | ___ | ____ | ___ | Systemic | 1 |
| Inoue et al. [32] | 48 | Yes | 77 | T | Chemo | Actin, EMA, Vimentin, Desmin | ___ | At least 18 |
| Ahuja et al. [33] | 60 | Yes | 6 | ___ | Chemo | Actin, Vimentin, pan-CK | ___ | At least 12 |
| Sosa et al. [34] | 43 | Yes | 0 | T | No | Actin | Systemic | 1 |
| Chahdi et al. [35] | 46 | NS | 84 | T | WBRT | Actin, h-Caldesmon | NS | NS |
| Imoumby et al. [36] | 46 | Yes | 60 | T | WBRT | Actin, Vimentin, h-Caldesmon | Systemic | 5 |
| Miki et al. [37] | 35 | NS | 36 | T | WBRT | NS | ___ | At least 17 |
| 70 | Yes | 96 | T | No | NS | ___ | At least 15 | |
| 51 | Yes | 48 | T | WBRT | NS | ___ | At least 7 | |
| Soo et al. [38] | 60 | No | 0 | T | WBRT + Chemo | NS | ___ | At least 6 |
| Delgado et al. [39] | 33 | Yes | 10 | T | WBRT + Chemo | NS | NS | NS |
| Eatz et al. [40] | 55 | Yes | 5 | Bx | SRS | NS | Systemic | 2 |
| Richards et al. [41] | 51 | Yes | 44 | T | Chemo + SRS | Myosin | ___ | At least 2 |
| Carrilo-Uzeta et al. [7] | 46 | No | 0 | T | WBRT | SMA, h-Caldesmon | ___ | At least 12 |
| Our Case | 49 | Yes | 2 | ___ | GKRS + Chemo | Actin, Vimentin | Systemic | 20 |
| Treatment | Number of Patients | Mean Survival Time | Median Survival Time |
|---|---|---|---|
| Surgery + Chemotherapy | 4 | 24 ± 19.2 | 18 (24–18) |
| Surgery | 5 | 5.4 ± 5.6 | 4 (5–2) |
| Surgery + RT | 13 | 9.3 ± 8 | 5 (12–4) |
| Surgery + RT + Chemotherapy | 5 | 11.2 ± 10.3 | 6 (16–5) |
| RT | 4 | 6.2 ± 9.2 | 2 (2–2) |
| With Chemotherapy | 12 | 15.1 ± 13.9 | 12 (24–5) |
| Without Chemotherapy | 23 | 7.7 ± 7.4 | 5 (12–2) |
| Case | Total Score (out of 8) | Case (Continuation) | Total Score (out of 8) |
|---|---|---|---|
| Vaquero et al., 1989 [10] | 6 | Honeybul et al., 2009 [23] | 8 |
| Prussia et al., 1992 [11] | 4.5 | Venizelos et al., 2011 [24] | 8 |
| Wronski et al., 1994 [12] | 7 | Yamada et al., 2011 [25] | 8 |
| Bindal et al., 1994 [13] | 7 | Mariniello et al., 2012 [26] | 8 |
| Uchino et al., 1996 [14] | 7.5 | Sosa et al., 2018 [34] | 8 |
| Salvati et al., 1998 [4] | 7 | Chahdi et al., 2018 [35] | 5 |
| Ziyal et al., 1999 [15] | 7.5 | Kim et al., 2016 [31] | 5.5 |
| Mawrin et al., 2002 [16] | 8 | Eatz et al., 2023 [40] | 7 |
| Yip et al., 2006 [17] | 7.5 | Soo et al., 2022 [38] | 7.5 |
| Chen et al., 2013 [27] | 7 | Carrillo-Uzeta et al., 2025 [7] | 8 |
| Shepard et al., 2014 [28] | 6 | Abrahao et al., 2015 [30] | 7 |
| Gurram et al., 2014 [29] | 8 | Delgado et al., 2022 [39] | 6 |
| Munakata et al., 2006 [18] | 5 | Inoue et al., 2016 [32] | 8 |
| Melone et al., 2008 [19] | 8 | Ahuja et al., 2017 [33] | 8 |
| Kaya et al., 2009 [20] | 6 | Miki et al., 2021 [37] | 7.5 |
| Benizelos et al., 2009 [21] | 7 | Richards et al., 2023 [41] | 8 |
| Pereira et al., 2011 [22] | 8 | Imoumby et al., 2021 [36] | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pour-Rashidi, A.; Zandpazandi, S.; Perronne, L.; Hill, V.B.; Krumpelman, C.; Subedi, K.; Kelahan, L.; Borhani, A.A.; Savas, H.; Avery, R.; et al. Intracranial Metastases from Uterine Leiomyosarcoma: A Systematic Review and Case Illustration. J. Clin. Med. 2025, 14, 6631. https://doi.org/10.3390/jcm14186631
Pour-Rashidi A, Zandpazandi S, Perronne L, Hill VB, Krumpelman C, Subedi K, Kelahan L, Borhani AA, Savas H, Avery R, et al. Intracranial Metastases from Uterine Leiomyosarcoma: A Systematic Review and Case Illustration. Journal of Clinical Medicine. 2025; 14(18):6631. https://doi.org/10.3390/jcm14186631
Chicago/Turabian StylePour-Rashidi, Ahmad, Sara Zandpazandi, Laetitia Perronne, Virginia B. Hill, Chase Krumpelman, Kamal Subedi, Linda Kelahan, Amir A. Borhani, Hatice Savas, Ryan Avery, and et al. 2025. "Intracranial Metastases from Uterine Leiomyosarcoma: A Systematic Review and Case Illustration" Journal of Clinical Medicine 14, no. 18: 6631. https://doi.org/10.3390/jcm14186631
APA StylePour-Rashidi, A., Zandpazandi, S., Perronne, L., Hill, V. B., Krumpelman, C., Subedi, K., Kelahan, L., Borhani, A. A., Savas, H., Avery, R., Agirlar Trabzonlu, T., Bagci, U., Sachdev, S., Dixit, K., Lukas, R. V., Kumthekar, P., & Velichko, Y. S. (2025). Intracranial Metastases from Uterine Leiomyosarcoma: A Systematic Review and Case Illustration. Journal of Clinical Medicine, 14(18), 6631. https://doi.org/10.3390/jcm14186631





