Hypertension, Diabetes and Depression as Modifiable Risk Factors for Dementia: A Common Data Model Approach in a Population-Based Cohort, with Study Protocol and Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Setting
2.3. Exposure and Outcome
2.4. Data Sources and Common Data Model Approach
- Hospital Discharge—containing primary and secondary diagnoses coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9CM).
- Pharmaceutical Prescription—including both community and direct hospital pharmacy dispensing of all medications reimbursed by the INHS coded using Anatomic Therapeutic Chemical (ATC) codes for drug classification; the ATC system is the drug classification system adopted by the World Health Organization [27].
- Co-Payment Exemption—listing individuals certified by an INHS specialist as having a disease qualifying for medical co-payment exemption.
- Demographic Population Registry—identifying all residents under the INHS, including death and out-migration information.
2.5. Statistical Analysis
2.6. Meta-Analysis
3. Results
3.1. Common Data Model
3.2. Algorithm Definition
3.2.1. Dementia
3.2.2. Depression
3.2.3. Diabetes
3.2.4. Hypertension
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| INHS | Italian National Health System |
| CDM | Common Data Model |
| HUDs | Healthcare Utilization Databases |
| PAF | Population Attributable Fraction |
| PIF | Potential Impact Fraction |
| LHT | Local Health Trust |
| ICD9CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| ATC | Anatomic Therapeutic Drug |
| SHR | Sub Hazard Ratio |
| ISTAT | National Institute of Statistics |
References
- Rao, A.; Suliman, A.; Vuik, S.; Aylin, P.; Darzi, A. Outcomes of dementia: Systematic review and meta-analysis of hospital administrative database studies. Arch. Gerontol. Geriatr. 2016, 66, 198–204. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Durand, D.; Olivera, E.; Sáez, A.; Carniglia, L.; Caruso, C.; Lasaga, M. Alzheimer’s disease risk after COVID-19: A view from the perspective of the infectious hypothesis of neurodegeneration. Neural Regen. Res. 2023, 18, 1404–1410. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446, Erratum in Lancet 2023, 402, 1132. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.L.; Butler, M.; Fink, H.A.; Brasure, M.; Davila, H.; Desai, P.; Jutkowitz, E.; McCreedy, E.; Nelson, V.A.; McCarten, J.R.; et al. Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia; Comparative Effectiveness Review No. 188; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017.
- Asta, F.; Marcon, G.; Cella, S.; Ricciardi, W.; Boccia, S. Preventing dementia in Italy: Estimations of modifiable risk factors and public health implications. J. Prev. Alzheimer’s Dis. 2025, 12, 100055. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Wolters, F.J.; Chibnik, L.B.; Waziry, R.; Anderson, R.; Berr, C.; Beiser, A.; Bis, J.C.; Blacker, D.; Bos, D.; Brayne, C.; et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020, 95, e519–e531. [Google Scholar] [CrossRef]
- Skoog, I.; Börjesson-Hanson, A.; Kern, S.; Johansson, L.; Falk, H.; Sigström, R.; Östling, S. Decreasing prevalence of dementia in 85-year-olds examined 22 years apart: The influence of education and stroke. Sci. Rep. 2017, 7, 6136. [Google Scholar] [CrossRef]
- Ahmadi-Abhari, S.; Guzman-Castillo, M.; Bandosz, P.; Shipley, M.J.; Muniz-Terrera, G.; Singh-Manoux, A.; Kivimäki, M.; Steptoe, A.; Capewell, S.; O’fLaherty, M.; et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: Modelling study. BMJ 2017, 358, j2856. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, K.; Han, E.; Kim, G.; Cho, H.; Kim, K.J.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Brayne, C.; et al. Risk of Incident Dementia According to Metabolic Health and Obesity Status in Late Life: A Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 2942–2952. [Google Scholar] [CrossRef]
- Rolandi, E.; Zaccaria, D.; Vaccaro, R.; Abbondanza, S.; Pettinato, L.; Davin, A.; Guaita, A. Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: A population-based study. Alzheimer’s Res. Ther. 2020, 12, 94. [Google Scholar] [CrossRef]
- Lespinasse, J.; Chêne, G.; Mangin, J.F.; Dubois, B.; Blanc, F.; Paquet, C.; Hanon, O.; Planche, V.; Gabelle, A.; Ceccaldi, M.; et al. MEMENTO study group. Associations among hypertension, dementia biomarkers, and cognition: The MEMENTO cohort. Alzheimer’s Dement. 2022, 19, 2332–2342. [Google Scholar] [CrossRef]
- He, P.; Zhou, C.; Ye, Z.; Liu, M.; Zhang, Y.; Wu, Q.; Zhang, Y.; Yang, S.; Xiaoqin, G.; Qin, X. Walking pace, handgrip strength, age, APOE genotypes, and new-onset dementia: The UK Biobank prospective cohort study. Alzheimer’s Res. Ther. 2023, 15, 9. [Google Scholar] [CrossRef]
- Feigin, V.L. The Evolution of Neuroepidemiology: Marking the 40-Year Anniversary of Publishing Studies on Epidemiology of Neurological Disorders. Neuroepidemiology 2022, 56, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, A.M.; Nonino, F.; Vanacore, N. Chronic Neurological Diseases between epidemiology and public health. Are we doing enough? The contribution of the Italian network NeuroEpiNet. Epidemiol. Prev. 2022, 46, 281–283. [Google Scholar]
- Schneeweiss, S.; Brown, J.; Bate, A.; Trifirò, G.; Bartels, D.B. Choosing among Common Data Models for Real-World Data Analyses Fit for Making Decisions About the Effectiveness of Medical Products. Clin. Pharmacol. Ther. 2019, 107, 827–833. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. The REporting of studies Conducted using Observational Routinely-collected Health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef] [PubMed]
- Zenesini, C.; Baldin, E.; Vignatelli, L.; Poluzzi, E.; Antonazzo, I.; Calandra-Buonaura, G.; Guarino, M.; De Carolis, P.; Cortelli, P.; D’ALessandro, R.; et al. Use of antidepressants and the risk of Parkinson’s disease in the Local Health Trust of Bologna: A historical cohort study. J. Neurol. Sci. 2019, 405, 116421. [Google Scholar] [CrossRef]
- Gnavi, R.; Karaghiosoff, L.; Balzi, D.; Barchielli, A.; Canova, C.; Demaria, M.; Pellizzari, M.; Rigon, S.; Tessari, R.; Simonato, L. Stima della prevalenza di diabete basata su dati sanitari correnti mediante un algoritmo comune in differenti aree italiane. Epidemiol. Prev. 2008, 32 (Suppl. S1), 15–21. [Google Scholar] [PubMed]
- Gini, R.; Schuemie, M.J.; Mazzaglia, G.; Lapi, F.; Francesconi, P.; Pasqua, A.; Bianchini, E.; Montalbano, C.; Roberto, G.; Barletta, V.; et al. Automatic identification of type 2 diabetes, hypertension, ischaemic heart disease, heart failure and their levels of severity from Italian General Practitioners’ electronic medical records: A validation study. BMJ Open 2016, 6, e012413. [Google Scholar] [CrossRef]
- Bacigalupo, I.; Lombardo, F.L.; Bargagli, A.M.; Cascini, S.; Agabiti, N.; Davoli, M.; Scalmana, S.; Di Palma, A.; Greco, A.; Rinaldi, M.; et al. Identification of dementia and MCI cases in health information systems: An Italian validation study. Alzheimer’s Dement. 2022, 8, e12327. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2019; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2018. [Google Scholar]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W.; Putter, H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: Cumulative total failure probability may exceed 1. Stat. Med. 2021, 40, 4200–4212. [Google Scholar] [CrossRef]
- Von Korff, M.; Wagner, E.H.; Saunders, K. A chronic disease score from automated pharmacy data. J. Clin. Epidemiol. 1992, 45, 197–203. [Google Scholar] [CrossRef]
- Kusoro, O.; Roche, M.; Del-Pino-Casado, R.; Leung, P.; Orgeta, V. Time to diagnosis in dementia: A systematic review with meta-analysis. Int. J. Geriatr. Psychiatry. 2025, 40, e70129. [Google Scholar] [CrossRef]
- Fiest, K.M.; Jette, N.; Quan, H.; St Germaine-Smith, C.; Metcalfe, A.; Patten, S.B.; Beck, C.A. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry 2014, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Di Domenicantonio, R.; Cappai, G.; Cascini, S.; Narduzzi, S.; Porta, D.; Bauleo, L.; Lallo, A.; Renzi, M.; Cesaroni, G.; Agabiti, N.; et al. Validazione degli algoritmi per l’identificazione di persone con patologia cronica attraverso i dati dei sistemi informativi. Epidemiol. Prev. 2018, 42, 316–325. [Google Scholar]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Belleudi, V.; Fortinguerra, F.; Poggi, F.R.; Perna, S.; Bortolus, R.; Donati, S.; Clavenna, A.; Locatelli, A.; Davoli, M.; Addis, A.; et al. The Italian Network for Monitoring Medication Use During Pregnancy (MoM-Net): Experience and Perspectives. Front. Pharmacol. 2021, 12, 699062. [Google Scholar] [CrossRef] [PubMed]
- Trifirò, G.; Isgrò, V.; Ingrasciotta, Y.; Ientile, V.; L’Abbate, L.; Foti, S.S.; Belleudi, V.; Poggi, F.; Fontana, A.; Moretti, U.; et al. Large-Scale Postmarketing Surveillance of Biological Drugs for Immune-Mediated Inflammatory Diseases Through an Italian Distributed Multi-Database Healthcare Network: The VALORE Project. BioDrugs 2021, 35, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Dalla Zuanna, T.; Pitter, G.; Canova, C.; Simonato, L.; Gnavi, R. A Systematic Review of Case-Identification Algorithms Based on Italian Healthcare Administrative Databases for Two Relevant Diseases of the Endocrine System: Diabetes Mellitus and Thyroid Disorders. Epidemiol. Prev. 2019, 43 (Suppl. S2), 17–36. [Google Scholar]
- Lorenzoni, G.; Baldi, I.; Soattin, M.; Gregori, D.; Buja, A. A Systematic Review of Case-Identification Algorithms Based on Italian Healthcare Administrative Databases for Three Relevant Diseases of the Cardiovascular System: Hypertension, Heart Failure, and Congenital Heart Diseases. Epidemiol. Prev. 2019, 43 (Suppl. S2), 51–61. [Google Scholar]
- Gini, R.; Sturkenboom, M.C.J.; Sultana, J.; Cave, A.; Landi, A.; Pacurariu, A.; Roberto, G.; Schink, T.; Candore, G.; Slattery, J.; et al. Different strategies to execute multi-database studies for medicines surveillance in real-world setting: A reflection on the European model. Clin. Pharmacol. Ther. 2020, 108, 228–235. [Google Scholar] [CrossRef]
- Lo Scalzo, A.; Donatini, A.; Orzella, L.; Cicchetti, A.; Profili, S.; Maresso, A. Italy: Health system review. Health Syst. Transit. 2009, 11, 1–216. [Google Scholar]



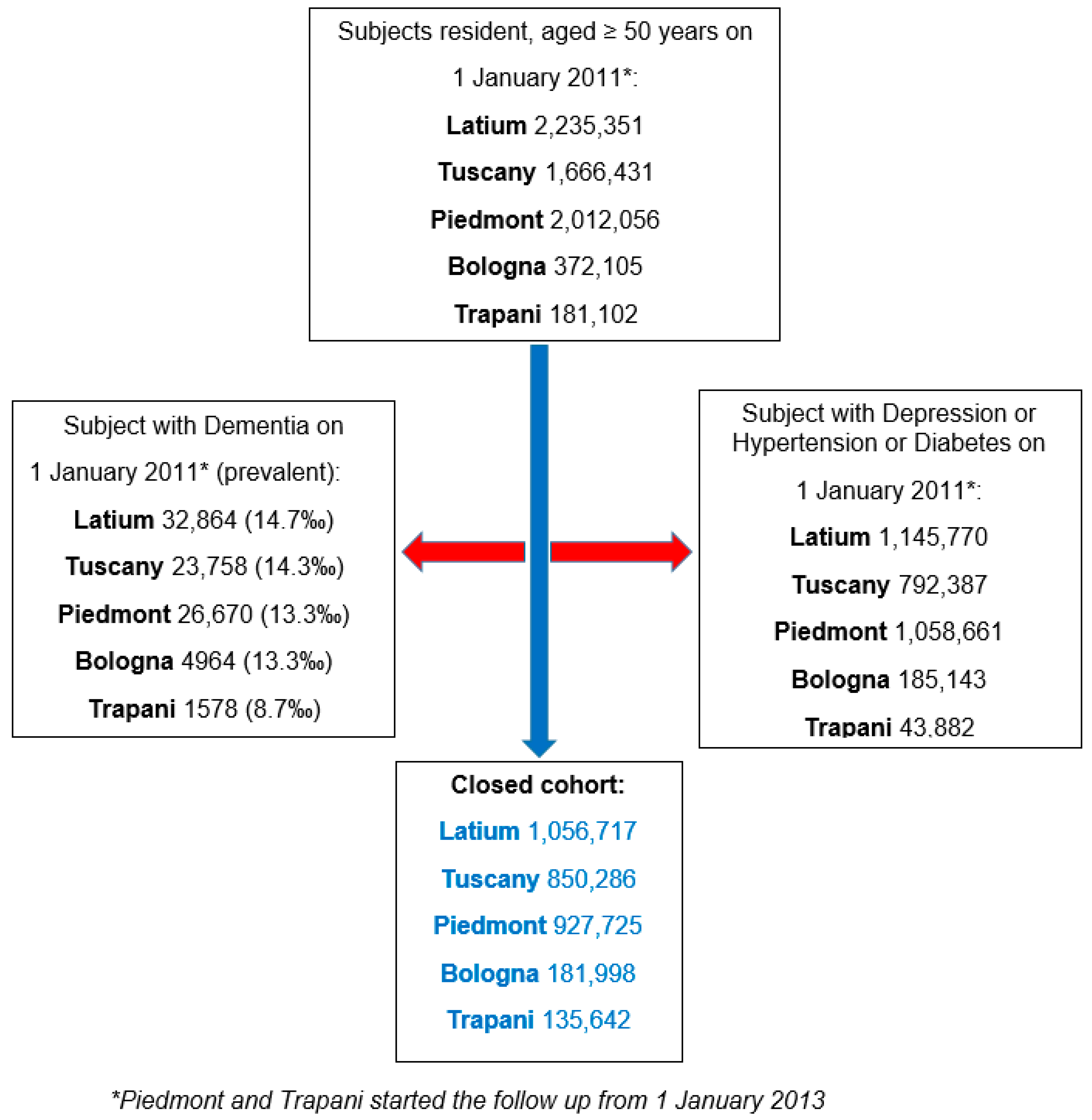
| Regions | Cohorts Population-N | Age-Years Mean (SD) | Sex: Female n (%) |
|---|---|---|---|
| Piedmont | 927,725 | 63.6 (11.4) | 483,182 (52.1) |
| Latium | 1,056,717 | 63.1 (10.9) | 572,024 (54.1) |
| Tuscany | 850,286 | 64.8 (11.4) | 456,465 (53.7) |
| Bologna (Emilia-Romagna) | 181,998 | 63.1 (10.4) | 99,786 (54.8) |
| Trapani (Sicily) | 135,642 | 65.2 (11.2) | 72,990 (53.8) |
| Linkage Key for All Health Administrative Databases | |||
|---|---|---|---|
| Variable Name | Definition | Variable Type (Length) | Encoding |
| codice | Patient identification code-linkage key | Character | Alphanumeric |
| Hospital Discharge (Schede di Dimissioni Ospedaliera) | |||
| diag | Principal diagnosis | Character (5) | ICD-9-CM |
| diagsec1-5 | From 1st to 5th secondary diagnosis | Character (5) | ICD-9-CM |
| interv | Main surgical intervention | Character (4) | ICD-9-CM |
| interv1-5 | From 1st to 5th secondary operation/procedure | Character (4) | ICD-9-CM |
| data_amm | Date of admission | Date (10) | dd/mm/yyyy |
| data_dim | Date of discharge | Date (10) | dd/mm/yyyy |
| tip_dim | 0: discharge | ||
| Type of discharge | Character (1) | 1: transferred | |
| 2: deceased | |||
| 3: other | |||
| regric | 1: ordinary | ||
| 2: day-hospital | |||
| Admission regime | Character (1) | 3: home treatment | |
| 4: day-surgery with overnight stay | |||
| Drug prescriptions (Assistenza Farmaceutica Territoriale and Farmaci a Erogazione Diretta) | |||
| data_erog | Drug dispensing date | Date (10) | dd/mm/yyyy |
| atc | Anatomical Therapeutic Chemical classification | Character (7) | Alphanumeric |
| aic | Drug code (Autorizzazione all’Immissione in Commercio) | Character (9) | Alphanumeric |
| days | Prescription coverage days | Numeric | >0 |
| pezzi | Number of packages | Numeric | >0 |
| Exemption for Pathology (Esenzioni per Patologia) | |||
| cod_esen | Exemption code | Character (8) | Alphanumeric |
| data_inizio | Start date | Date (10) | dd/mm/yyyy |
| data_fine | End date | Date (10) | dd/mm/yyyy |
| Demographic population registry (Anagrafe Sanitaria Assistiti) and mortality registry | |||
| sesso | Sex | Character (1) | 1: male, 2: female |
| datanas | Date of birth | Date (10) | dd/mm/yyyy |
| dinizio_resid | Residence start date | Date (10) | dd/mm/yyyy |
| dfine_resid | Residence end date | Date (10) | dd/mm/yyyy |
| reg_res | Region of residence | Character (3) | ISTAT code |
| com_res | Municipality of residence | Character (6) | ISTAT code |
| data_dec | Date of death | Date (10) | dd/mm/yyyy |
| Pathologies | Drug Prescription (ATC Code) | Hospital Discharge in Primary or Secondary Diagnosis (ICD-9 Code) | Exemption for Pathology |
|---|---|---|---|
| Dementia | At least two prescriptions in one year: N06DA04; N06DA03; N06DA02; N06DX0 | 290 *; 291.2; 294.0–294.21; 292.82; 331.0–331.2; 331.5, 331.7; 331.8; 331.82–331.9; 046.1 | 011; 029 |
| Depression | At least 180 days of antidepressants prescriptions in one year: N06AA; N06AB; N06AX | 296.20–296.25; 296.30–296.35; 300.4; 311; 296.6; 296.82; 296.90; 309.0; 309.1; 309.28 | |
| Diabetes | At least two prescriptions in one year of all antidiabetic drugs: A10A; A10B | 250 * | 013 |
| Hypertension | At least two prescriptions in one year: C02; C07; C08C; C09 | 401 *; 402 *; 403 *; 404 *; 405 *, 36211 Exclusion criteria (at least one episode of heart failure): 428 *, 36211; 39891; 40201; 40211; 40291; 40401; 40403; 40411; 40413; 40491; 40493 | 031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenesini, C.; Cascini, S.; Picariello, R.; Profili, F.; Belotti, L.M.B.; Maniscalco, L.; Acampora, A.; Gnavi, R.; Francesconi, P.; Vignatelli, L.; et al. Hypertension, Diabetes and Depression as Modifiable Risk Factors for Dementia: A Common Data Model Approach in a Population-Based Cohort, with Study Protocol and Preliminary Results. J. Clin. Med. 2025, 14, 6622. https://doi.org/10.3390/jcm14186622
Zenesini C, Cascini S, Picariello R, Profili F, Belotti LMB, Maniscalco L, Acampora A, Gnavi R, Francesconi P, Vignatelli L, et al. Hypertension, Diabetes and Depression as Modifiable Risk Factors for Dementia: A Common Data Model Approach in a Population-Based Cohort, with Study Protocol and Preliminary Results. Journal of Clinical Medicine. 2025; 14(18):6622. https://doi.org/10.3390/jcm14186622
Chicago/Turabian StyleZenesini, Corrado, Silvia Cascini, Roberta Picariello, Francesco Profili, Laura Maria Beatrice Belotti, Laura Maniscalco, Anna Acampora, Roberto Gnavi, Paolo Francesconi, Luca Vignatelli, and et al. 2025. "Hypertension, Diabetes and Depression as Modifiable Risk Factors for Dementia: A Common Data Model Approach in a Population-Based Cohort, with Study Protocol and Preliminary Results" Journal of Clinical Medicine 14, no. 18: 6622. https://doi.org/10.3390/jcm14186622
APA StyleZenesini, C., Cascini, S., Picariello, R., Profili, F., Belotti, L. M. B., Maniscalco, L., Acampora, A., Gnavi, R., Francesconi, P., Vignatelli, L., Nonino, F., Bargagli, A., Tarantino, D., Salemi, G., Vanacore, N., & Matranga, D. (2025). Hypertension, Diabetes and Depression as Modifiable Risk Factors for Dementia: A Common Data Model Approach in a Population-Based Cohort, with Study Protocol and Preliminary Results. Journal of Clinical Medicine, 14(18), 6622. https://doi.org/10.3390/jcm14186622







