Impairment of Kidney Function in Patients with Chronic Coronary Syndromes
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Data Collection and Assays
2.3. Ethical Issues
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Estimated GFR in Patients with CCS
4.2. Prevalence of Albuminuria
4.3. The Impact of Albuminuria Measurement on CVD Risk Groups
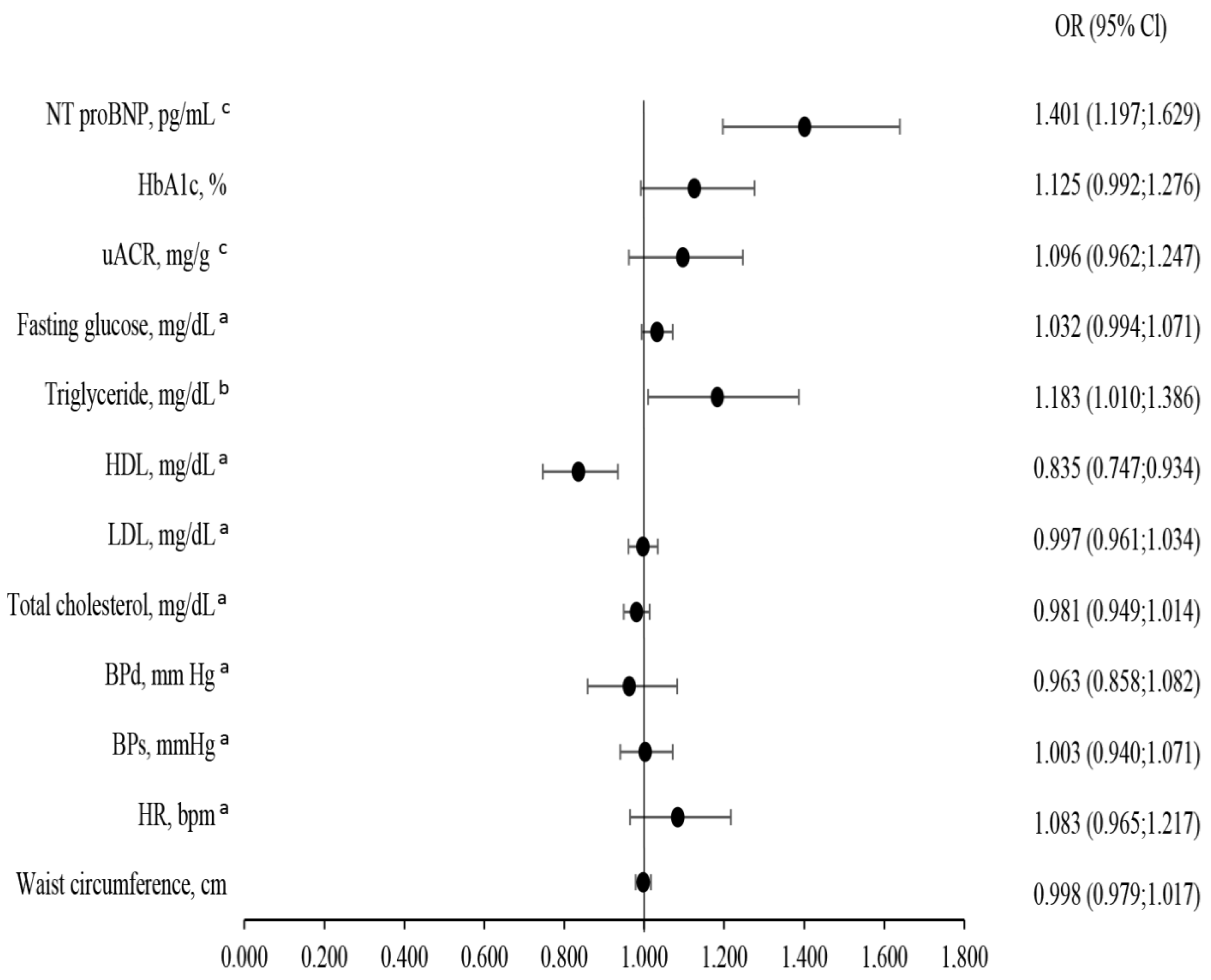
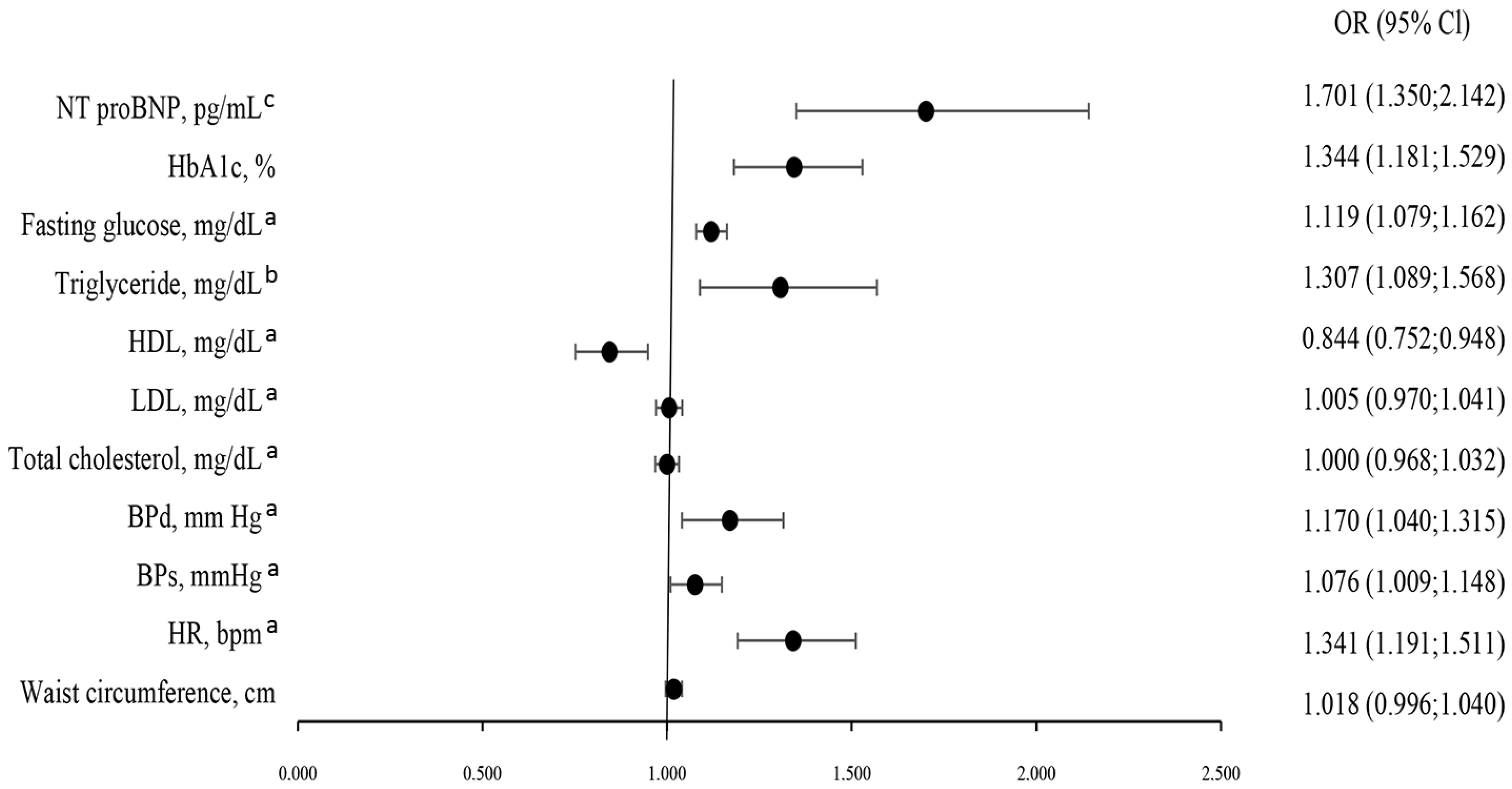
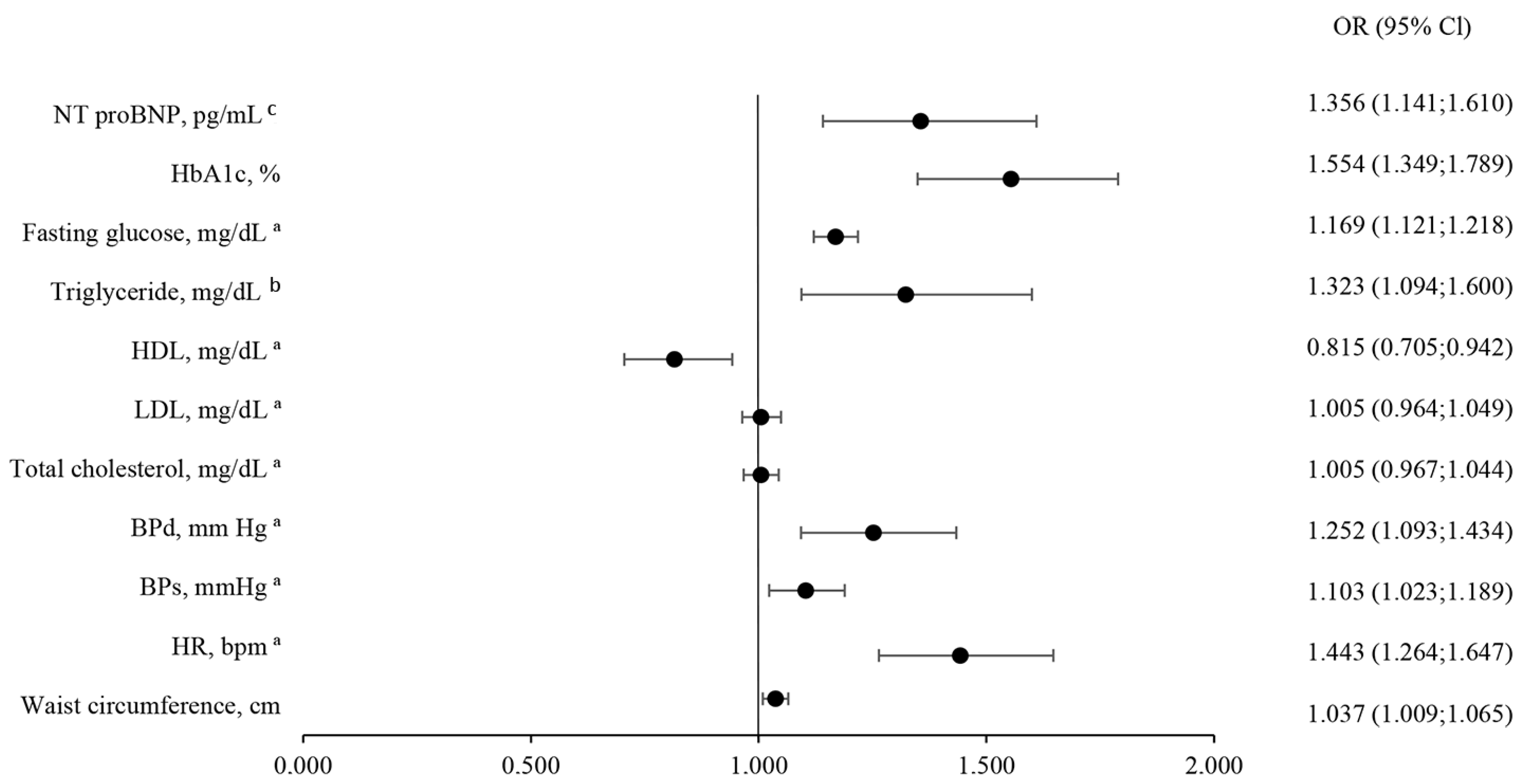
4.4. Predictors of CKD Severity
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, V.; Moroni, A.; Gall, E.; Benedetti, A.; Zivelonghi, C.; Picard, F. Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review. J Clin. Med. 2023, 12, 2833. [Google Scholar] [CrossRef]
- McCullough, A.P. Implications of CKD for Patients with CAD; Medscape: Northvile, MI, USA, 2009. [Google Scholar]
- Engelbertz, C.; Pinnschmidt, H.O.; Freisinger, E.; Reinecke, H.; Schmitz, B.; Fobker, M.; Schmieder, R.E.; Wegscheider, K.; Breithardt, G.; Pavenstädt, H.; et al. Sex-specific differences and long-term outcome of patients with coronary artery disease and chronic kidney disease: The Coronary Artery Disease and Renal Failure (CAD-REF) Registry. Clin. Res. Cardiol. 2021, 110, 1625–1636. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Lin, X.; Song, W.; Zhou, Y.; Gao, Y.; Wang, Y.; Wang, Y.; Liu, Y.; Deng, L.; Liao, Y.; Wu, B.; et al. Elevated urine albumin creatinine ratio increases cardiovascular mortality in coronary artery disease patients with or without type 2 diabetes mellitus: A multicenter retrospective study. Cardiovasc. Diabetol. 2023, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Wanner, C.; Gansevoort, R.; Council, E. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: A position statement by the Council of the European Renal Association. Eur. J. Prev. Cardiol. 2022, 29, 2211–2215. [Google Scholar] [CrossRef]
- Jankowski, P.; Kosior, D.A.; Sowa, P.; Szóstak-Janiak, K.; Kozieł, P.; Krzykwa, A.; Sawicka, E.; Haberka, M.; Setny, M.; Kamiński, K.; et al. Secondary prevention of coronary artery disease in Poland. Results from the POLASPIRE survey. Cardiol. J. 2020, 27, 533–540. [Google Scholar] [CrossRef]
- Jankowski, P.; Koczwarska-Maciejek, D.; Kamiński, K.; Gąsior, Z.; Kosior, D.A.; Styczkiewicz, M.; Kubica, A.; Rajzer, M.; Charkiewicz-Szeremeta, K.; Dąbek, J.; et al. Implementation of the cardiovascular prevention guidelines in clinical practice. Results from the POLASPIRE II survey. Kardiol. Pol. 2025, 83, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hawranek, M.; Gierlotka, M.; Gąsior, M.; Hudzik, B.; Desperak, P.; Ciślak, A.; Tajstra, M.; Osadnik, T.; Rozentryt, P.; Poloński, L. Renal function on admission affects both treatment strategy and long-term outcomes of patients with myocardial infarction (from the Polish Registry of Acute Coronary Syndromes). Kardiol. Pol. 2017, 75, 332–343. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Arranz-Martínez, E.; Iturmendi-Martínez, N.; Fernández-Vicente, T.; Rivera-Teijido, M.; García-Álvarez, J.C. Prevalence rates of chronic kidney disease and its association with cardiometabolic factors and cardiovascular diseases. SIMETAP-CKD study. Clin. Investig. Arter. 2023, 35, 64–74. [Google Scholar] [CrossRef]
- Cases Amenós, A.; González-Juanatey, J.R.; Conthe Gutiérrez, P.; Matalí Gilarranz, A.; Garrido Costa, C. Prevalence of chronic kidney disease in patients with or at a high risk of cardiovascular disease. Rev. Esp. Cardiol. 2010, 63, 225–228. [Google Scholar]
- Samaan, F.; Damiani, B.B.; Kirsztajn, G.M.; Sesso, R. A Cross-Sectional Study on the Prevalence and Risk Stratification of Chronic Kidney Disease in Cardiological Patients in São Paulo, Brazil. Diagnostics 2023, 13, 1146. [Google Scholar] [CrossRef]
- Malleshappa, P.; Shah, B.V. Prevalence of Chronic Kidney Disease and the Incidence of Acute Kidney Injury in Patients with Coronary Artery Disease in Mumbai, India. Heart Views 2015, 16, 47–52. [Google Scholar] [CrossRef]
- Xia, F.; Liu, G.; Shi, Y.; Zhang, Y. Impact of microalbuminuria on incident coronary heart disease, cardiovascular and all-cause mortality: A meta-analysis of prospective studies. Int. J. Clin. Exp. Med. 2015, 8, 1–9. [Google Scholar] [PubMed]
- Barzilay, J.I.; Farag, Y.M.K.; Durthaler, J. Albuminuria: An Underappreciated Risk Factor for Cardiovascular Disease. J. Am. Heart Assoc. 2024, 13, e030131. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Tripepi, G. Risk Factors of Chronic Kidney Disease Progression: Between Old and New Concepts. J. Clin. Med. 2024, 13, 678. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.S.; Kim, H.W.; Nam, K.H.; Young Lee, J.; Chang, T.I.; Park, J.T.; Yoo, T.-H.; Lee, J.; Kim, S.W.; Oh, Y.K.; et al. Association Between Longitudinal Blood Pressure Trajectory and the Progression of Chronic Kidney Disease: Results From the KNOW-CKD. Hypertension 2021, 78, 1355–1364. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Lee, Y.; Kang, M.W.; Park, S.; Park, S.; Han, K.; Paek, J.H.; Park, W.Y.; Jin, K.; et al. Predictive value of triglyceride/high-density lipoprotein cholesterol for major clinical outcomes in advanced chronic kidney disease: A nationwide population-based study. Clin. Kidney J. 2021, 14, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.; Kappes, J.; Rottmann, F.A.; Westermann, L.; Welte, T. HbA1c-dependent projection of long-term renal outcomes. J. Intern. Med. 2024, 295, 206–215. [Google Scholar] [CrossRef] [PubMed]

| Study Population (n = 1957) | ||||
|---|---|---|---|---|
| Variables | All Study Population | Subjects with <60 (n = 359) | Subjects with ≥60 (n = 1414) | p Values |
| Age, years | 67.00 (61.00–72.00) | 72.00 (67.00–76.00) | 65.00 (60.00–71.00) | <0.001 |
| Gender, male | 1394 (71.23) | 216 (60.17) | 1061 (75.04) | <0.001 |
| Weight, kg | 83.00 (73.00–94.00) | 80.20 (71.00–92.00) | 83.00 (74.00–94.00) | 0.012 |
| Waist circumference, cm | 101.00 (94.00–110.00) | 101.00 (93.00–110.00) | 101.00 (94.00–109.00) | 0.787 |
| HR, bpm | 68.00 (61.00–75.00) | 69.00 (62.00–76.00) | 67.00 (61.00–75.00) | 0.159 |
| BPs, mmHg | 135.00 (122.00–150.00) | 137.00 (120.25–150) | 135.00 (123.00–150.00) | 0.240 |
| BPd, mmHg | 80.00 (72.00–88.00) | 80.00 (70.00–86.00) | 80.00 (74.00–89.00) | 0.022 |
| eGFR, ml/min/1.73 m2 | 80.21 (63.23–92.27) | 48.40 (39.84–55.04) | 86.14 (74.73–94.76) | <0.001 |
| Total cholesterol, mg/dL | 158.55 (132.25–193.26) | 153.52 (127.00–189.37) | 158.55 (134.00–193.35) | 0.037 |
| LDL, mg/dL | 89.09 (65.74–119.88) | 86.76 (63.09–115.16) | 89.17 (67.00–120.00) | 0.179 |
| HDL, mg/dL | 44.66 (36.67–54.00) | 42.90 (35.00–52.07) | 45.00 (37.00–54.14) | 0.020 |
| Triglyceride, mg/dL | 111.00 (84.00–157.00) | 110.09 (86.00–168.50) | 111.00 (84.00–155.88) | 0.449 |
| Fasting glucose, mg/dL | 108.00 (95.40–124.20) | 109.98 (95.92–129.60) | 107.37 (95.40–123.06) | 0.033 |
| uACR, mg/g | 7.26 (3.61–19.90) | 13.36 (5.06–63.10) | 6.74 (3.36–15.93) | 0.235 |
| HbA1c, % | 5.90 (5.60–6.40) | 6.00 (5.60–6.70) | 5.90 (5.60–6.30) | 0.004 |
| NT proBNP, pg/mL | 182.80 (93.28–432.18) | 366.65 (164.85–1029.50) | 162.80 (88.00–365.78) | <0.001 |
| Diabetes, % (n = 1655) | 675 (40.79) | 173 (44.5) | 450 (30.4) | <0.001 |
| Hypertension, % (n = 1921) | 1613 (83.97) | 338 (86.9) | 1147 (77.5) | <0.001 |
| Variables | Albuminuria < 30 | Albuminuria 30–300 | Albuminuria > 300 | p Value | |
|---|---|---|---|---|---|
| n = 994 | n = 182 | n = 45 | |||
| General information | Age, years | 66.00 (60.00–72.00) a,b | 68.00 (62.75–74.00) a | 69.00 (67.00–75.00) b | <0.001 |
| Gander, male | 716 (72.03) | 134 (73.63) | 33 (73.33) | 0.905 | |
| Weight, kg | 83.00 (74.08–94.00) | 82.00 (71.80–93.60) | 84.80 (75.85–97.75) | 0.319 | |
| Waist circumference, cm | 102.00 (95.00–110.00) b | 101.00 (95.00–110.00) | 106.00 (98.00–116.00) b | 0.034 | |
| Heart rate, bpm | 66.00 (60.00–73.50) a,b | 70.00 (63.00–79.25) a | 73.00 (65.00–80.50) b | <0.001 | |
| BPs, mmHg | 133.00 (120.00–147.00) b | 137.00 (122.00–152.00) | 142.00 (130.00–159.50) b | 0.003 | |
| BPd, mmHg | 80.00 (72.00–87.00) | 80.00 (74.00–90.00) | 81.00 (72.00–90.00) | 0.067 | |
| Laboratory tests | Total cholesterol, mg/dL | 160.31 (135.00–193.35) | 151.29 (123.16–195.30) | 166.00 (126.45–199.39) | 0.828 |
| LDL, mg/dL | 89.06 (66.78–121.01) | 85.46 (63.00–111.67) | 89.50 (59.84–128.30) | 0.944 | |
| HDL, mg/dL | 44.00 (36.67–53.17) | 40.86 (34.03–48.68) | 37.04 (33.25–44.00) | 0.021 | |
| Triglyceride, mg/dL | 110.00 (80.33–157.57) | 110.71 (85.03–158.00) | 141.12 (93.00–201.05) | 0.039 | |
| Fasting glucose, mg/dL | 108.00 (95.40–122.01) a,b | 124.20 (106.20–149.40) a | 134.91 (105.80–164.34) b | <0.001 | |
| eGFR, mL/min/1.73 m2 | 82.86 (68.00–93.34) a,b | 74.73 (56.39–90.66) a,c | 59.64 (41.10–80.84) b,c | <0.001 | |
| uACR, mg/g | 5.46 (3.00–10.30) b | 70.59 (45.43–131.09) c | 678.52 (441.31–1087.78) b,c | <0.001 | |
| HbA1c, % | 5.90 (5.60–6.30) a,b | 6.10 (5.70–7.20) a | 6.40 (5.90–7.70) b | <0.001 | |
| NT proBNP, pg/mL | 163.20 (83.96–367.30) a,b | 164.40 (147.78–1021.75) a,c | 729.00 (225.20–2260.50) b,c | <0.001 | |
| Comorbidities | Diabetes, % | 328 (31.1) a,b | 82 (46.3) a | 19 (57.6) b | <0.001 |
| Hypertension, % | 836 (79.4) a | 156 (88.1) a | 28 (84.8) | 0.002 |
| Study Population (n = 1153) | |||
|---|---|---|---|
| Variables | Patients in Whom Albuminuria Did Not Change CKD Classification (n = 930) | Patients with the Change of CKD Class After Assessment of Albuminuria (n = 200) | p Values |
| Age, years | 66.00 (60.00–71.5) | 68.00 (63.00–74.00) | <0.001 |
| Gender, male | 667 (71.72) | 151 (75.50) | <0.001 |
| Weight, kg | 83.10 (74.00–94.00) | 83.00 (72.75–94.15) | 0.735 |
| Waist circumference, cm | 102.00 (95.00–110.00) | 103.00 (95.25–111.38) | 0.170 |
| HR, bpm | 66.00 (60.00–74.00) | 71.00 (64.00–80.00) | <0.001 |
| BPs, mmHg | 133.00 (121.00–147.00) | 139.00 (125.00–152.25) | 0.008 |
| BPd, mmHg | 72.50 (80.00–87.00) | 80.00 (74.00–90.00) | 0.038 |
| eGFR, mL/min/1.73 m2 | 83.13 (68.47–93.37) | 74.90 (57.38–90.63) | <0.001 |
| Total cholesterol, mg/dL | 159.31 (135.26–193.35) | 153.30 (121.5–193.88) | 0.100 |
| LDL, mg/dL | 89.17 (66.56–120.83) | 85.58 (60.83–112.14) | 0.192 |
| HDL, mg/dL | 44.00 (36.67–53.00) | 39.99 (34.03–48.31) | 0.001 |
| Triglyceride, mg/dL | 110.00 (80.07–157.75) | 112.48 (87.00–170.05) | 0.168 |
| Fasting glucose, mg/dL | 108.00 (95.87–122.01) | 124.54 (108.00–150.30) | <0.001 |
| uACR, mg/g | 5.43 (2.95–10.30) | 87.64 (48.07–200.94) | <0.001 |
| HbA1c, % | 5.90 (5.60–6.30) | 6.10 (5.70–7.48) | <0.001 |
| NT proBNP, pg/mL | 163.40 (84.46–367.10) | 362.30 (151.70–1007.50) | <0.001 |
| Diabetes, % | 282 (30.3) | 96 (48.0) | <0.023 |
| Hypertension, % | 741 (79.9) | 175 (87.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkiewicz-Szeremeta, K.; Sawicka-Śmiarowska, E.; Czarnecka, D.; Dubatówka, M.; Gąsior, Z.; Hryszko, T.; Jankowski, P.; Knapp, M.; Kosior, D.A.; Kubica, A.; et al. Impairment of Kidney Function in Patients with Chronic Coronary Syndromes. J. Clin. Med. 2025, 14, 6607. https://doi.org/10.3390/jcm14186607
Charkiewicz-Szeremeta K, Sawicka-Śmiarowska E, Czarnecka D, Dubatówka M, Gąsior Z, Hryszko T, Jankowski P, Knapp M, Kosior DA, Kubica A, et al. Impairment of Kidney Function in Patients with Chronic Coronary Syndromes. Journal of Clinical Medicine. 2025; 14(18):6607. https://doi.org/10.3390/jcm14186607
Chicago/Turabian StyleCharkiewicz-Szeremeta, Katarzyna, Emilia Sawicka-Śmiarowska, Danuta Czarnecka, Marlena Dubatówka, Zbigniew Gąsior, Tomasz Hryszko, Piotr Jankowski, Małgorzata Knapp, Dariusz A. Kosior, Aldona Kubica, and et al. 2025. "Impairment of Kidney Function in Patients with Chronic Coronary Syndromes" Journal of Clinical Medicine 14, no. 18: 6607. https://doi.org/10.3390/jcm14186607
APA StyleCharkiewicz-Szeremeta, K., Sawicka-Śmiarowska, E., Czarnecka, D., Dubatówka, M., Gąsior, Z., Hryszko, T., Jankowski, P., Knapp, M., Kosior, D. A., Kubica, A., Mickiewicz, K., Pająk, A., Rajzer, M., Styczkiewicz, M., Wolfshaut-Wolak, R., & Kamiński, K. A. (2025). Impairment of Kidney Function in Patients with Chronic Coronary Syndromes. Journal of Clinical Medicine, 14(18), 6607. https://doi.org/10.3390/jcm14186607









