Gender Differences in Weight Loss Extent Following Bariatric Surgery
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Measures and Statistics
2.3. Sample Size and Power Considerations
2.4. Ethics
3. Results
3.1. Baseline Characteristics of the Population
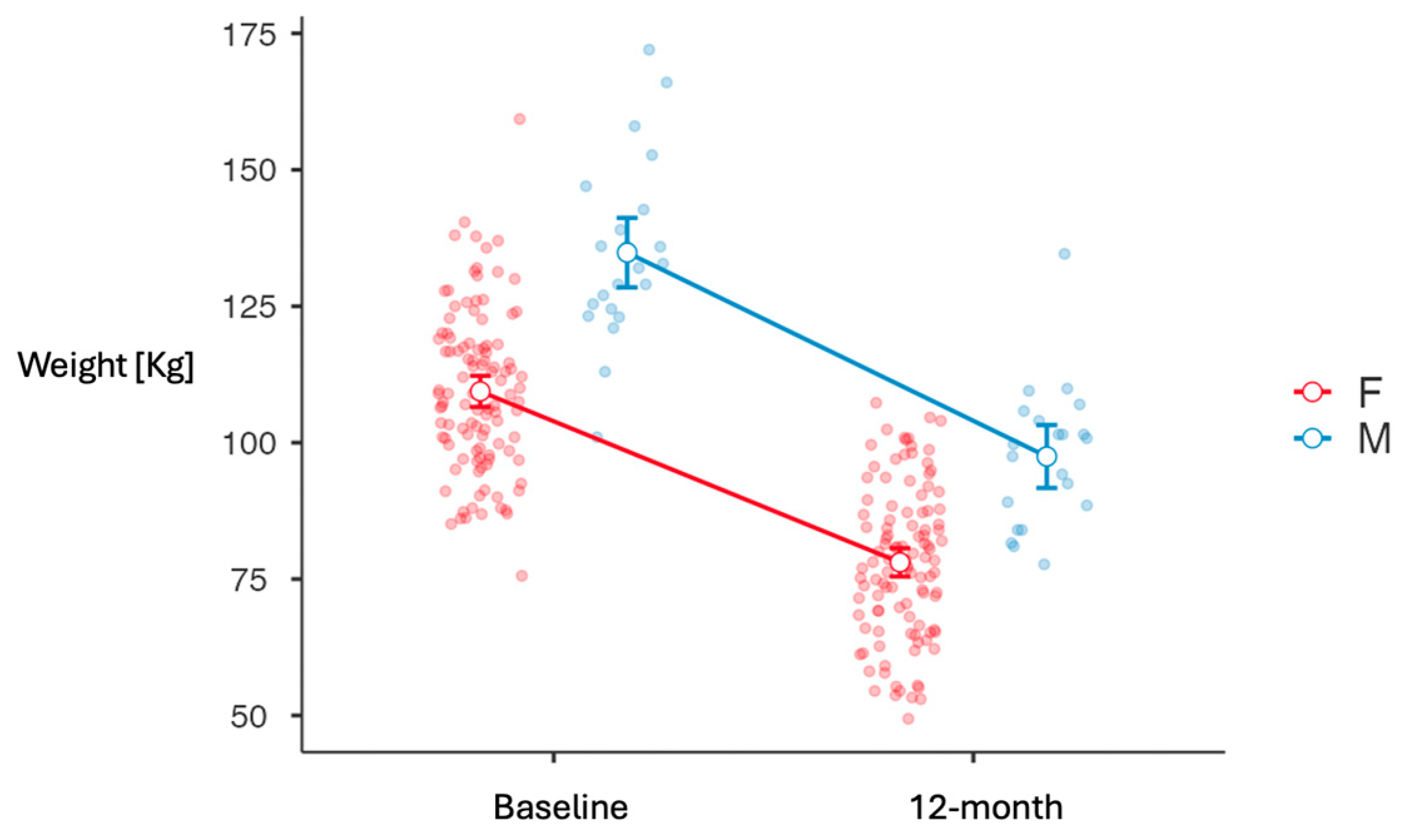
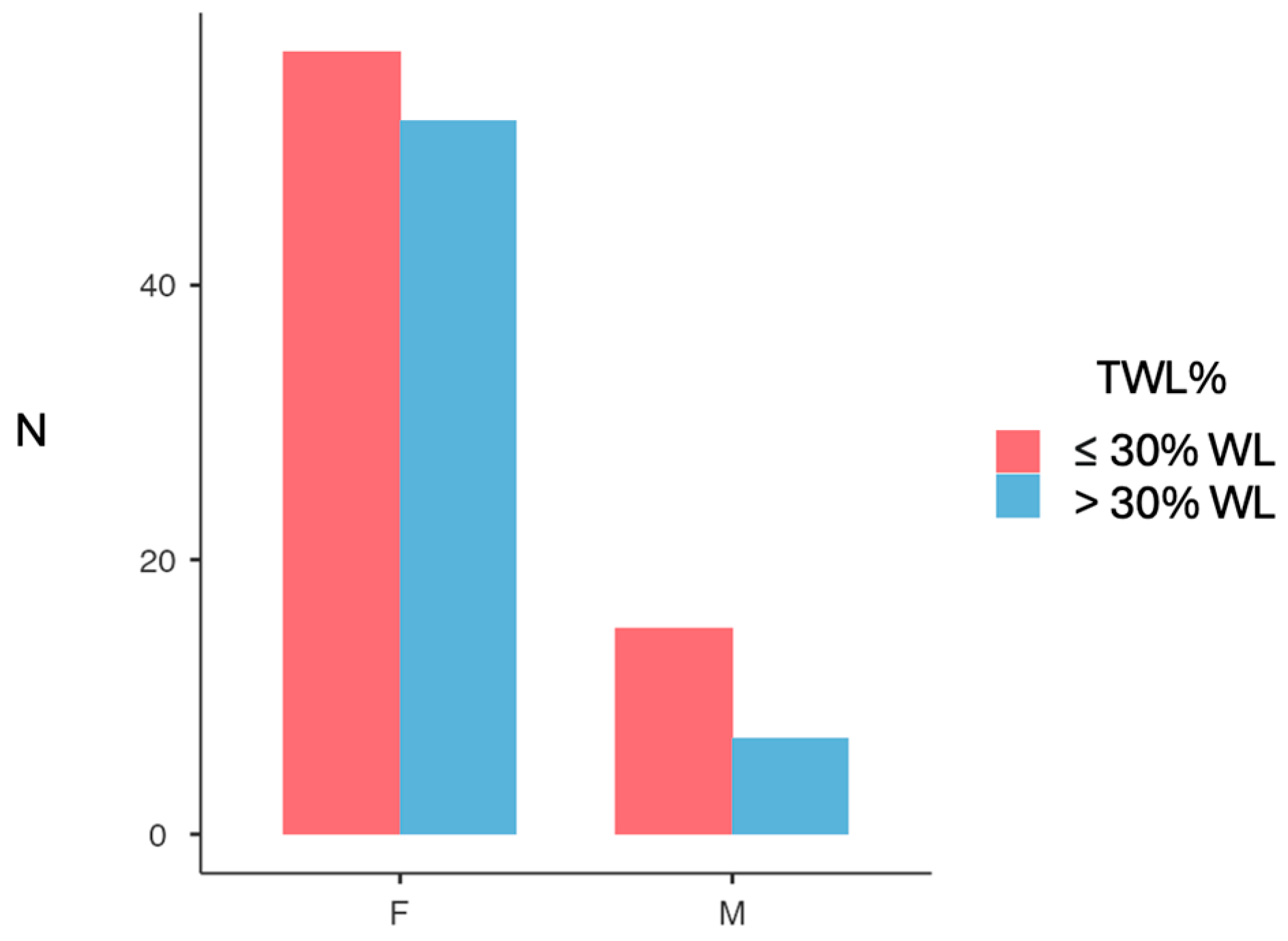
3.2. Weight Loss Outcomes
3.3. Linear Regression Analyses
3.4. Waist Circumference
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef]
- Pfefferkorn, U.; Hort, S.; Beluli, M.; La Vista, M.; Züger, T. Weight Loss After Bariatric Surgery in Different Age Groups. Obes. Surg. 2023, 33, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ananín, S.; Ballester, E.; Gonzalo, B.; Codina, C.; Miñambres, I.; Pérez, A.; Gich, I.J.; González, S.; Serrano, C.; Balagué, C. Is Sleeve Gastrectomy as Effective in Older Patients as in Younger Patients? A Comparative Analysis of Weight Loss, Related Comorbidities, and Medication Requirements. Obes. Surg. 2022, 32, 1909–1917. [Google Scholar] [CrossRef]
- Kochkodan, J.; Telem, D.A.; Ghaferi, A.A. Physiologic and psychological gender differences in bariatric surgery. Surg. Endosc. 2018, 32, 1382–1388. [Google Scholar] [CrossRef]
- Guerreiro, V.; Neves, J.S.; Salazar, D.; Ferreira, M.J.; Oliveira, S.C.; Souteiro, P.; Pedro, J.; Magalhães, D.; Varela, A.; Belo, S.; et al. Long-Term Weight Loss and Metabolic Syndrome Remission after Bariatric Surgery: The Effect of Sex, Age, Metabolic Parameters and Surgical Technique—A 4-Year Follow-Up Study. Obes. Facts 2019, 12, 639–652. [Google Scholar] [CrossRef]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Sjöholm, K.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.A.; Taube, M.; Carlsson, B.; Peltonen, M. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N. Engl. J. Med. 2020, 383, 1535–1543. [Google Scholar] [CrossRef]
- Golzarand, M.; Toolabi, K.; Farid, R. The bariatric surgery and weight losing: A meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg. Endosc. 2017, 31, 4331–4345. [Google Scholar] [CrossRef]
- Andersen, J.R.; Aadland, E.; Nilsen, R.M.; Våge, V. Predictors of weight loss are different in men and women after sleeve gastrectomy. Obes. Surg. 2014, 24, 594–598. [Google Scholar] [CrossRef]
- Perrone, F.; Bianciardi, E.; Benavoli, D.; Tognoni, V.; Niolu, C.; Siracusano, A.; Gaspari, A.L.; Gentileschi, P. Gender Influence on Long-Term Weight Loss and Comorbidities After Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: A Prospective Study With a 5-Year Follow-up. Obes. Surg. 2016, 26, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.; Coley, R.Y.; Clark, J.M.; McBride, C.L.; Cirelli, E.; McTigue, K.; Arterburn, D.; Coleman, K.J.; Wellman, R.; Anau, J.; et al. Interventions and Operations 5 Years After Bariatric Surgery in a Cohort From the US National Patient-Centered Clinical Research Network Bariatric Study. JAMA Surg. 2020, 155, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; Dos Reis Falcão, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef]
- De Luca, M.; Zese, M.; Bandini, G.; Zappa, M.A.; Bardi, U.; Carbonelli, M.G.; Carrano, F.M.; Casella, G.; Chianelli, M.; Chiappetta, S.; et al. SICOB Italian clinical practice guidelines for the surgical treatment of obesity and associated diseases using GRADE methodology on bariatric and metabolic surgery. Updat. Surg. 2024, 77, 1603–1625. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 28, O1–O58. [Google Scholar]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef]
- Burton, P.; Smit, H.J.; Lightowler, H.J. The influence of restrained and external eating patterns on overeating. Appetite 2007, 49, 191–197. [Google Scholar] [CrossRef]
- Bennett, J.; Greene, G.; Schwartz-Barcott, D. Perceptions of emotional eating behavior. A qualitative study of college students. Appetite 2013, 60, 187–192. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Marinari, G.; Foletto, M.; Nagliati, C.; Navarra, G.; Borrelli, V.; Bruni, V.; Fantola, G.; Moroni, R.; Tritapepe, L.; Monzani, R.; et al. Enhanced recovery after bariatric surgery: An Italian consensus statement. Surg. Endosc. 2022, 36, 7171–7486. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef] [PubMed]

| Female (SD) n = 109 | Male (SD) n = 22 | Total (SD) n = 131 | p Value | |
|---|---|---|---|---|
| Age [years] | 45.2 (10.8) | 47.1 (9.8) | 45.5 (10.6) | 0.435 1 |
| Weight [kg] | 109.4 (14.8) | 134.8 (16.7) | 113.7 (17.8) | <0.001 1 |
| BMI [kg/m2] | 41.8 (4.2) | 42.3 (4.0) | 41.9 (4.2) | 0.605 1 |
| Waist [cm] | 118.9 (11.0) | 134.3 (9.7) | 121.6 (12.2) | 0.001 1 |
| Glycaemia [mg/dL] | 100.5 (18.1) | 113.8 (56.7) | 102.6 (28.4) | 0.404 3 |
| T2D [%] | 12.1 | 5 | 11 | 0.321 2 |
| Hypertension [%] | 39 | 59 | 42 | 0.078 2 |
| Female (SD) n = 109 | Male (SD) n = 22 | p Value | |
|---|---|---|---|
| Weight Loss 12M [kg] | −31.2 (10.40) | −36.6 (10.33) | 0.028 1 |
| TWL% 12M | −28.6 (8.99) | −27.2 (6.24) | 0.48 2 |
| EWL% 12M | 48.4 (15.25) | 45.9 (12.05) | 0.46 2 |
| Waist Circumference (cm) | −28.0 (10.6) | −30.2 (7.81) | 0.45 1 |
| Predictor | Estimate (β) | SE | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender M-F | −5.89 | 2.197 | −10.233 | −1.537 | 0.008 |
| Age | 0.379 | 0.078 | 0.225 | 0.543 | <0.001 |
| BMI | −0.479 | 0.197 | −0.868 | −0.089 | 0.017 |
| Predictor | Estimate (β) | SE | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender M-F | 0.850 | 1.886 | −2.881 | 4.582 | 0.653 |
| Age | 0.293 | 0.067 | 0.162 | 0.425 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosimo, S.; Sileo, F.; Gambetti, A.; Frattini, F.; Bruno, A.; Mambrini, S.; Gilardini, L.; Barbera, F.; Gotti, A.; Vincenti, V.; et al. Gender Differences in Weight Loss Extent Following Bariatric Surgery. J. Clin. Med. 2025, 14, 6605. https://doi.org/10.3390/jcm14186605
Colosimo S, Sileo F, Gambetti A, Frattini F, Bruno A, Mambrini S, Gilardini L, Barbera F, Gotti A, Vincenti V, et al. Gender Differences in Weight Loss Extent Following Bariatric Surgery. Journal of Clinical Medicine. 2025; 14(18):6605. https://doi.org/10.3390/jcm14186605
Chicago/Turabian StyleColosimo, Santo, Federica Sileo, Andrea Gambetti, Francesco Frattini, Amalia Bruno, Sara Mambrini, Luisa Gilardini, Federica Barbera, Alice Gotti, Verdiana Vincenti, and et al. 2025. "Gender Differences in Weight Loss Extent Following Bariatric Surgery" Journal of Clinical Medicine 14, no. 18: 6605. https://doi.org/10.3390/jcm14186605
APA StyleColosimo, S., Sileo, F., Gambetti, A., Frattini, F., Bruno, A., Mambrini, S., Gilardini, L., Barbera, F., Gotti, A., Vincenti, V., Inì, L., Novelli, M., Cancello, R., Dionigi, G., & Bertoli, S. (2025). Gender Differences in Weight Loss Extent Following Bariatric Surgery. Journal of Clinical Medicine, 14(18), 6605. https://doi.org/10.3390/jcm14186605







