Management of Ventricular Arrhythmias in Patients with Left Ventricular Assist Devices: Pathophysiology, Risk Stratification, and Ablation Strategies
Abstract
1. Introduction
2. Search Strategy and Study Selection
3. LVAD—General Structure and Functional Mechanisms
4. Etiology of Ventricular Arrhythmias in Patients with LVAD
5. LVAD and Cardiac Implantable Electronic Devices
6. Antiarrhythmic Drug Therapy in Patients with LVAD and Ventricular Arrhythmias
7. Catheter Ablation of Ventricular Arrhythmias
7.1. Ablation of VAs Before LVAD Implantation
7.2. Ablation of VAs During LVAD Implantation
7.3. Ablation of VAs After LVAD Implantation
7.4. Pre-Procedure Planning and Technical Considerations
7.5. Intraprocedural Considerations
7.6. Procedure-Related Complications and Recurrences
8. Conclusions
9. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| VAs | Ventricular Arrhythmias |
| LVADs | Left Ventricular Assist Devices |
| ICDs | Implantable Cardioverter Defibrillators |
| DT | Destination Therapy |
| AADs | Antiarrhythmic Drugs |
| BT | Bridge to cardiac Transplantation |
| HF | Heart Failure |
| VF | Ventricular Fibrillation |
| CRT-Ds | Cardiac Resynchronization Therapy with Defibrillators |
| CIEDs | Cardiac Implantable Electronic Devices |
| CT | Computed tomography |
| ICE | Intra-Cardiac Echography |
| 3D-EAM | Three-Dimensional Electroanatomic Map |
| EMI | Electro Magnetic Interference |
References
- Tomasoni, D.; Vishram-Nielsen, J.K.K.; Pagnesi, M.; Adamo, M.; Lombardi, C.M.; Gustafsson, F.; Metra, M. Advanced heart failure: Guideline-directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022, 9, 1507–1523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peichl, P.; Bayes-Genis, A.; Deneke, T.; Chioncel, O.; deRiva, M.; Crespo-Leiro, M.G.; Frontera, A.; Gustafsson, F.; Martins, R.P.; Pagnesi, M.; et al. Drug therapy and catheter ablation for management of arrhythmias in continuous flow left ventricular assist device’s patients: A Clinical Consensus Statement of the European Heart Rhythm Association and the Heart Failure Association of the ESC. Europace 2024, 26, euae272, Erratum in Europace 2025, 27, euaf086. https://doi.org/10.1093/europace/euaf086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, N.; Arnaoutakis, G.J.; George, T.J.; Allen, J.G.; Ju, D.G.; Schaffer, J.M.; Russell, S.D.; Shah, A.S.; Conte, J.V. The spectrum of complications following left ventricular assist device placement. J. Card. Surg. 2012, 27, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, S.P.; Lachat, M.L.; Zünd, G.; Turina, M.I. Left ventricular assist device (LVAD) enables survival during 7 h of sustained ventricular fibrillation. Eur. J. Cardiothorac. Surg. 2004, 26, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.B.; Rosner, G.; Uriel, N.; González-Costello, J.; Ehlert, F.A.; Jorde, U.P. Twelve hours of sustained ventricular fibrillation supported by a continuous-flow left ventricular assist device. Pacing Clin. Electrophysiol. 2012, 35, e144–e148. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, M.; Derr, C.; Keffeler, J.; Jelic, T. Organized cardiac activity in an awake LVAD patient during ventricular fibrillation. Am. J. Emerg. Med. 2017, 35, 1041.e1–1041.e3. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.C.; Rose, E.A.; Slater, J.; Kuiper, J.J.; Catanese, K.A.; Levin, H.R. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. J. Am. Coll. Cardiol. 1994, 24, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Kormos, R.L.; Stevenson, L.W.; Pagani, F.D.; Miller, M.A.; Ulisney, K.L.; Baldwin, J.T.; Young, J.B. Second INTERMACS annual report: More than 1,000 primary left ventricular assist device implants. J. Heart Lung Transplant. 2010, 29, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoruk, A.; Sherazi, S.; Massey, H.T.; Kutyifa, V.; McNitt, S.; Hallinan, W.; Huang, D.T.; Chen, L.; Aktas, M.K. Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm 2016, 13, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, Z.; Cogswell, R.; Schultz, J.; Von Wald, L.; John, R.; Roukoz, H. Association between early ventricular arrhythmias and mortality in destination vs. bridge patients on continuous flow LVAD support. Sci. Rep. 2021, 11, 19196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopinathannair, R.; Cornwell, W.K.; Dukes, J.W.; Ellis, C.R.; Hickey, K.T.; Joglar, J.A.; Pagani, F.D.; Roukoz, H.; Slaughter, M.S.; Patton, K.K. Device Therapy and Arrhythmia Management in Left Ventricular Assist Device Recipients: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e967–e989. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Xie, R.; Cowger, J.; de By, T.M.M.H.; Nakatani, T.; Schueler, S.; Taylor, R.; Lannon, J.; Mohacsi, P.; Gummert, J.; et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support Registry. J. Heart Lung Transplant. 2018, 37, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. MOMENTUM3 Investigators AFully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- John, R. Current axial-flow devices—The HeartMate II and Jarvik 2000 left ventricular assist devices. Semin. Thorac. Cardiovasc. Surg. 2008, 20, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Nof, E.; Peichl, P.; Stojadinovic, P.; Arceluz, M.; Maury, P.; Katz, M.; Tedrow, U.B.; Singh, R.M.; Narui, R.; John, R.M.; et al. HeartMate 3: New challenges in ventricular tachycardia ablation. Europace 2022, 24, 598–605. [Google Scholar] [CrossRef]
- Loring, Z.; Sen, S.; Black-Maier, E.; Atwater, B.D.; Russell, S.D.; DeVore, A.D.; Piccini, J.P. Reducing ECG Artefact From Left Ventricular Assist Device Electromagnetic Interference. J. Am. Heart Assoc. 2020, 9, e017563. [Google Scholar] [CrossRef]
- Bedi, M.; Kormos, R.; Winowich, S.; McNamara, D.M.; Mathier, M.A.; Murali, S. Ventricular arrhythmias during left ventricular assist device support. Am. J. Cardiol. 2007, 99, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.D.; Piacentino, V., 3rd; Gaughan, J.P.; Houser, S.R.; Margulies, K.B. Electrophysiological alterations after mechanical circulatory support in patients with advanced cardiac failure. Circulation 2001, 104, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.S.; Maynes, E.J.; Choi, J.H.; Wood, C.T.; Weber, M.P.; Morris, R.J.; Massey, H.T.; Tchantchaleishvili, V. Ventricular arrhythmias following continuous-flow left ventricular assist device implantation: A systematic review. Artif. Organs 2020, 44, E313–E325. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.D.; Calenda, B.; Miller, M.A.; Anyanwu, A.C.; Pinney, S.P. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices. Circ. Arrhythm. Electrophysiol. 2013, 6, 668–674, Erratum in Circ. Arrhythm. Electrophysiol. 2014, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Sacher, F.; Reichlin, T.; Zado, E.S.; Field, M.E.; Viles-Gonzalez, J.F.; Peichl, P.; Ellenbogen, K.A.; Maury, P.; Dukkipati, S.R.; Picard, F.; et al. Characteristics of ventricular tachycardia ablation in patients with continuous flow left ventricular assist devices. Circ. Arrhythm. Electrophysiol. 2015, 8, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.D.; Lee, G.; Virk, S.; Bennett, R.G.; Hayward, C.S.; Muthiah, K.; Kalman, J.; Kumar, S. Catheter Ablation of Ventricular Tachycardia in Patients With a Ventricular Assist Device: A Systematic Review of Procedural Characteristics and Outcomes. JACC Clin. Electrophysiol. 2019, 5, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Cantillon, D.J.; Bianco, C.; Wazni, O.M.; Kanj, M.; Smedira, N.G.; Wilkoff, B.L.; Starling, R.C.; Saliba, W.I. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm 2012, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.S.; Salamonsen, R.; Keogh, A.M.; Woodard, J.; Ayre, P.; Prichard, R.; Walker, R.; Kotlyar, E.; Macdonald, P.S.; Jansz, P.; et al. Effect of alteration in pump speed on pump output and left ventricular filling with continuous-flow left ventricular assist device. ASAIO J. 2011, 57, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Galand, V.; Flécher, E.; Auffret, V.; Boulé, S.; Vincentelli, A.; Dambrin, C.; Mondoly, P.; Sacher, F.; Nubret, K.; Kindo, M.; et al. ASSIST-ICD Investigators. Predictors and Clinical Impact of Late Ventricular Arrhythmias in Patients With Continuous-Flow Left Ventricular Assist Devices. JACC Clin. Electrophysiol. 2018, 4, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Efimova, E.; Fischer, J.; Bertagnolli, L.; Dinov, B.; Kircher, S.; Rolf, S.; Sommer, P.; Bollmann, A.; Richter, S.; Meyer, A.; et al. Predictors of ventricular arrhythmia after left ventricular assist device implantation: A large single-center observational study. Heart Rhythm 2017, 14, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.P.; Leclercq, C.; Bourenane, H.; Auffret, V.; Boulé, S.; Loobuyck, V.; Dambrin, C.; Mondoly, P.; Sacher, F.; Bordachar, P.; et al. Incidence, predictors, and clinical impact of electrical storm in patients with left ventricular assist devices: New insights from the ASSIST-ICD study. Heart Rhythm 2019, 16, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Sisti, N.; Mandoli, G.E.; Sciaccaluga, C.; Valente, S.; Mondillo, S.; Cameli, M. Insight into Atrial Fibrillation in LVAD Patients: From Clinical Implications to Prognosis. Pulse 2020, 8, 2–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garan, A.R.; Iyer, V.; Whang, W.; Mody, K.P.; Yuzefpolskaya, M.; Colombo, P.C.; Te-Frey, R.; Takayama, H.; Naka, Y.; Garan, H.; et al. Catheter ablation for ventricular tachyarrhythmias in patients supported by continuous-flow left ventricular assist devices. ASAIO J. 2014, 60, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Pompa, A.G.; Beerman, L.B.; Feingold, B.; Zinn, M.D.; Arora, G. Arrhythmia Burden in Pediatric Patients With a Ventricular Assist Device. Circ. Heart Fail. 2022, 15, e009566. [Google Scholar] [CrossRef] [PubMed]
- Vakil, K.; Kazmirczak, F.; Sathnur, N.; Adabag, S.; Cantillon, D.J.; Kiehl, E.L.; Koene, R.; Cogswell, R.; Anand, I.; Roukoz, H. Implantable Cardioverter-Defibrillator Use in Patients With Left Ventricular Assist Devices: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2016, 4, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Cikes, M.; Jakus, N.; Claggett, B.; Brugts, J.J.; Timmermans, P.; Pouleur, A.C.; Rubis, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Grundmann, S.; et al. PCHF-VADregistry Cardiac implantable electronic devices with a defibrillator component all-cause mortality in left ventricular assist device carriers: Results from the PCHF-VAD registry. Eur. J. Heart Fail. 2019, 21, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Topkara, V.K.; Demmer, R.T.; Dizon, J.M.; Yuzefpolskaya, M.; Fried, J.A.; Mai, X.; Mancini, D.M.; Takeda, K.; Takayama, H.; et al. Implantable Cardioverter-Defibrillators in Patients With a Continuous-Flow Left Ventricular Assist Device: An Analysis of the INTERMACS Registry. JACC Heart Fail. 2017, 5, 916–926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agrawal, S.; Garg, L.; Nanda, S.; Sharma, A.; Bhatia, N.; Manda, Y.; Singh, A.; Fegley, M.; Shirani, J. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices—A meta-analysis. Int. J. Cardiol. 2016, 222, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. ESC Scientific Document Group 2022 ESC Guidelines for the management of patients with ventricular arrhythmias the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.E.; Johnson, G.W.; Hellkamp, A.S.; Anderson, J.; Callans, D.J.; Raitt, M.H.; Reddy, R.K.; Marchlinski, F.E.; Yee, R.; Guarnieri, T.; et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, B.B.; Grinstein, J.S.; Imamura, T.; Kruse, E.; Nguyen, A.B.; Narang, N.; Holzhauser, L.H.; Burkhoff, D.; Lang, R.M.; Sayer, G.T.; et al. Biventricular Pacing Versus Right Ventricular Pacing in Patients Supported With LVAD. JACC Clin. Electrophysiol. 2021, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Tomashitis, B.; Baicu, C.F.; Butschek, R.A.; Jackson, G.R.; Winterfield, J.; Tedford, R.J.; Zile, M.R.; Gold, M.R.; Houston, B.A. Acute Hemodynamic Effects of Cardiac Resynchronization Therapy Versus Alternative Pacing Strategies in Patients With Left Ventricular Assist Devices. J. Am. Heart Assoc. 2021, 10, e018127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hohnloser, S.H.; Dorian, P.; Roberts, R.; Gent, M.; Israel, C.W.; Fain, E.; Champagne, J.; Connolly, S.J. Effect of amiodarone and sotalol on ventricular defibrillation threshold: The optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation 2006, 114, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Kadish, A.; Morady, F. A prospective comparison of class IA, B, and C antiarrhythmic agents in combination with amiodarone in patients with inducible, sustained ventricular tachycardia. Circulation 1991, 84, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Hallstrom, A.P.; Cappato, R.; Schron, E.B.; Kuck, K.H.; Zipes, D.P.; Greene, H.L.; Boczor, S.; Domanski, M.; Follmann, D.; et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur. Heart J. 2000, 21, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Pothineni, N.V.K.; Trivedi, J.R.; Roukoz, H.; Cowger, J.; Ahmed, M.M.; Bhan, A.; Ravichandran, A.K.; Bhat, G.; Al Ahmad, A.; et al. Amiodarone use and all-cause mortality in patients with a continuous-flow left ventricular assist device. J. Am. Heart Assoc. 2022, 11, e023762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chinnadurai, T.; Patel, S.R.; Saeed, O.; Hanif, W.; Rivas-Lasarte, M.; Farooq, M.; Castillo, C.; Taveras, M.; Fauel, D.; Shin, J.J.; et al. The interaction of amiodarone and continuous-flow left ventricular assist device use in risk of severe primary graft dysfunction following heart transplantation. Transplant. Direct 2022, 8, e1281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graboyes, S.D.T.; Kline, T.M.; Harris, T.N.; Iyer, P.S.; Hollis, I.B. Safety and efficacy of prophylactic amiodarone after left ventricular assist device. ASAIO J. 2023, 69, 96–100. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, P.; Coelho, C.; Costa-Oliveira, C.; Rocha, S. The Effect of Sacubitril-Valsartan on Ventricular Arrhythmia Burden in Patients With Heart Failure With Reduced Ejection Fraction. Cureus 2023, 15, e34508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Biase, L.; Santangeli, P.; Burkhardt, D.J.; Bai, R.; Mohanty, P.; Carbucicchio, C.; Dello Russo, A.; Casella, M.; Mohanty, S.; Pump, A.; et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Michowitz, Y.; Yu, R.; Mathuria, N.; Vaseghi, M.; Buch, E.; Bradfield, J.; Fujimura, O.; Gima, J.; Discepolo, W.; et al. Epicardial ablation of ventricular tachycardia: An institutional experience of safety and efficacy. Heart Rhythm 2013, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Cerrud-Rodriguez, R.C.; Di Biase, L.; Diaz, J.C.; Alviz, I.; Grupposo, V.; Cerna, L.; Avendano, R.; Tedrow, U.; Natale, A.; et al. Combined Endocardial-Epicardial Versus Endocardial Catheter Ablation Alone for Ventricular Tachycardia in Structural Heart Disease: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2019, 5, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Emaminia, A.; Nagji, A.S.; Ailawadi, G.; Bergin, J.D.; Kern, J.A. Concomitant left ventricular assist device placement and cryoablation for treatment of ventricular tachyarrhythmias associated with heart failure. Ann. Thorac. Surg. 2011, 92, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, D.P.; Bhamidipati, C.M.; Stone, M.L.; Ailawadi, G.; Bergin, J.D.; Mahapatra, S.; Kern, J.A. Cryoablation during left ventricular assist device implantation reduces postoperative ventricular tachyarrhythmias. J. Thorac. Cardiovasc. Surg. 2013, 145, 1207–1213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, M.; Rojas, F.; Shabari, F.R.; Simpson, L.; Cohn, W.; Frazier, O.H.; Mallidi, H.; Cheng, J.; Mathuria, N. Safety and Feasibility of Open Chest Epicardial Mapping and Ablation of Ventricular Tachycardia During the Period of Left Ventricular Assist Device Implantation. J. Cardiovasc. Electrophysiol. 2016, 27, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.D.; Oesterle, A.; Raiman, M.; Flatley, E.E.; Beaser, A.D.; Jeevanandam, V.; Klein, L.; Ota, T.; Wieselthaler, G.; Uriel, N.; et al. Feasibility and utility of intraoperative epicardial scar characterization during left ventricular assist device implantation. J. Cardiovasc. Electrophysiol. 2019, 30, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, M.; Rothstein, P.; Sauer, P.; Zipse, M.M.; Sandhu, A.; Tumolo, A.Z.; Borne, R.T.; Aleong, R.G.; Cleveland, J.C., Jr.; Fullerton, D.; et al. Open surgical ablation of ventricular tachycardia: Utility and feasibility of contemporary mapping and ablation tools. Heart Rhythm O2 2021, 2, 271–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tankut, S.; Gosev, I.; Yoruk, A.; Younis, A.; McNitt, S.; Bjelic, M.; Vidula, H.; Wu, I.; Aktas, M.K.; Goldenberg, I.; et al. Intraoperative Ventricular Tachycardia Ablation During Left Ventricular Assist Device Implantation in High-Risk Heart Failure Patients. Circ. Arrhythm. Electrophysiol. 2022, 15, e010660. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.T.; Gosev, I.; Wood, K.L.; Vidula, H.; Stevenson, W.; Marchlinski, F.; Supple, G.; Zalawadiya, S.K.; Weiss, J.P.; Tung, R.; et al. Design and characteristics of the prophylactic intra-operative ventricular arrhythmia ablation in high-risk LVAD candidates (PIVATAL) trial. Ann. Noninvasive Electrocardiol. 2023, 28, e13073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Englert, F.; Bahlke, F.; Erhard, N.; Krafft, H.; Popa, M.A.; Risse, E.; Lennerz, C.; Lengauer, S.; Telishevska, M.; Reents, T.; et al. VT ablation based on CT imaging substrate visualization: Results from a large cohort of ischemic and non-ischemic cardiomyopathy patients. Clin. Res. Cardiol. 2024, 113, 1478–1484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devabhaktuni, S.R.; Shirazi, J.T.; Miller, J.M. Mapping and Ablation of Ventricle Arrhythmia in Patients with Left Ventricular Assist Devices. Card. Electrophysiol. Clin. 2019, 11, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Chien, C.; Gelow, J.; Dalouk, K.; Henrikson, C.A.; Mudd, J.; Stecker, E.C. Ventricular arrhythmias after left ventricular assist device. Circ. Arrhythm. Electrophysiol. 2013, 6, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Snipelisky, D.; Reddy, Y.N.; Manocha, K.; Patel, A.; Dunlay, S.M.; Friedman, P.A.; Munger, T.M.; Asirvatham, S.J.; Packer, D.L.; Cha, Y.M.; et al. Effect of Ventricular Arrhythmia Ablation in Patients With Heart Mate II Left Ventricular Assist Devices: An Evaluation of Ablation Therapy. J. Cardiovasc. Electrophysiol. 2017, 28, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Labedi, M.R.; Alharethi, R.; Kfoury, A.G.; Budge, D.; Bruce, R.; Rasmusson, B.; Bunch, T.J. Electromagnetic interference of automatic implantable cardioverter defibrillator and HeartWare left ventricular assist device. ASAIO J. 2013, 59, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.L.H.K.; Meyer, D.; Pless, T. Minimizing magnetic interaction between an electroanatomic navigation system and a left ventricular assist device. J. Innov. Card. Rhythm. Manag. 2013, 4, 1440–1446. [Google Scholar] [CrossRef]
- Liang, J.J.; Canterbury, A.; Kancharla, K.; Santangeli, P. Catheter and surgical ablation for ventricular tachycardia in patients with left ventricular assist devices. Heart Rhythm 2023, 20, 927–932. [Google Scholar] [CrossRef] [PubMed]
- van den Bruck, J.H.; Hohendanner, F.; Heil, E.; Albert, K.; Duncker, D.; Estner, H.; Deneke, T.; Parwani, A.; Potapov, E.; Seuthe, K.; et al. Characterization of ventricular tachycardia ablation in end-stage heart failure patients with left ventricular assist device (CHANNELED registry). Europace 2025, 27, euaf054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Silva, K.; Haqqani, H.; Mahajan, R.; Qian, P.; Chik, W.; Voskoboinik, A.; Kistler, P.M.; Lee, G.; Jackson, N.; Kumar, S. Catheter Ablation vs Antiarrhythmic Drug Therapy for Treatment of Premature Ventricular Complexes: A Systematic Review. JACC Clin. Electrophysiol. 2023, 9, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Grinstein, J.; Garan, A.R.; Oesterle, A.; Fried, J.; Imamura, T.; Mai, X.; Kalantari, S.; Sayer, G.; Kim, G.H.; Sarswat, N.; et al. Increased Rate of Pump Thrombosis and Cardioembolic Events Following Ventricular Tachycardia Ablation in Patients Supported With Left Ventricular Assist Devices. ASAIO J. 2020, 66, 1127–1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moss, J.D.; Flatley, E.E.; Beaser, A.D.; Shin, J.H.; Nayak, H.M.; Upadhyay, G.A.; Burke, M.C.; Jeevanandam, V.; Uriel, N.; Tung, R. Characterization of Ventricular Tachycardia After Left Ventricular Assist Device Implantation as Destination Therapy: A Single-Center Ablation Experience. JACC Clin. Electrophysiol. 2017, 3, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mainville, D.J.; Vacaru, A.; Pasca, I. Iatrogenic Hypoxemia and Atrial Septal Defect Due to Electrical Storm Ablation After Left Ventricular Assist Device: A Case Report. Cureus 2023, 15, e39418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamura, S.; Shimeno, K.; Abe, Y.; Naruko, T. A right-to-left atrial shunt via an iatrogenic atrial septal defect after atrial fibrillation ablation induced by a percutaneous left ventricular assist device. Eur. Heart J. 2022, 43, 839. [Google Scholar] [CrossRef] [PubMed]

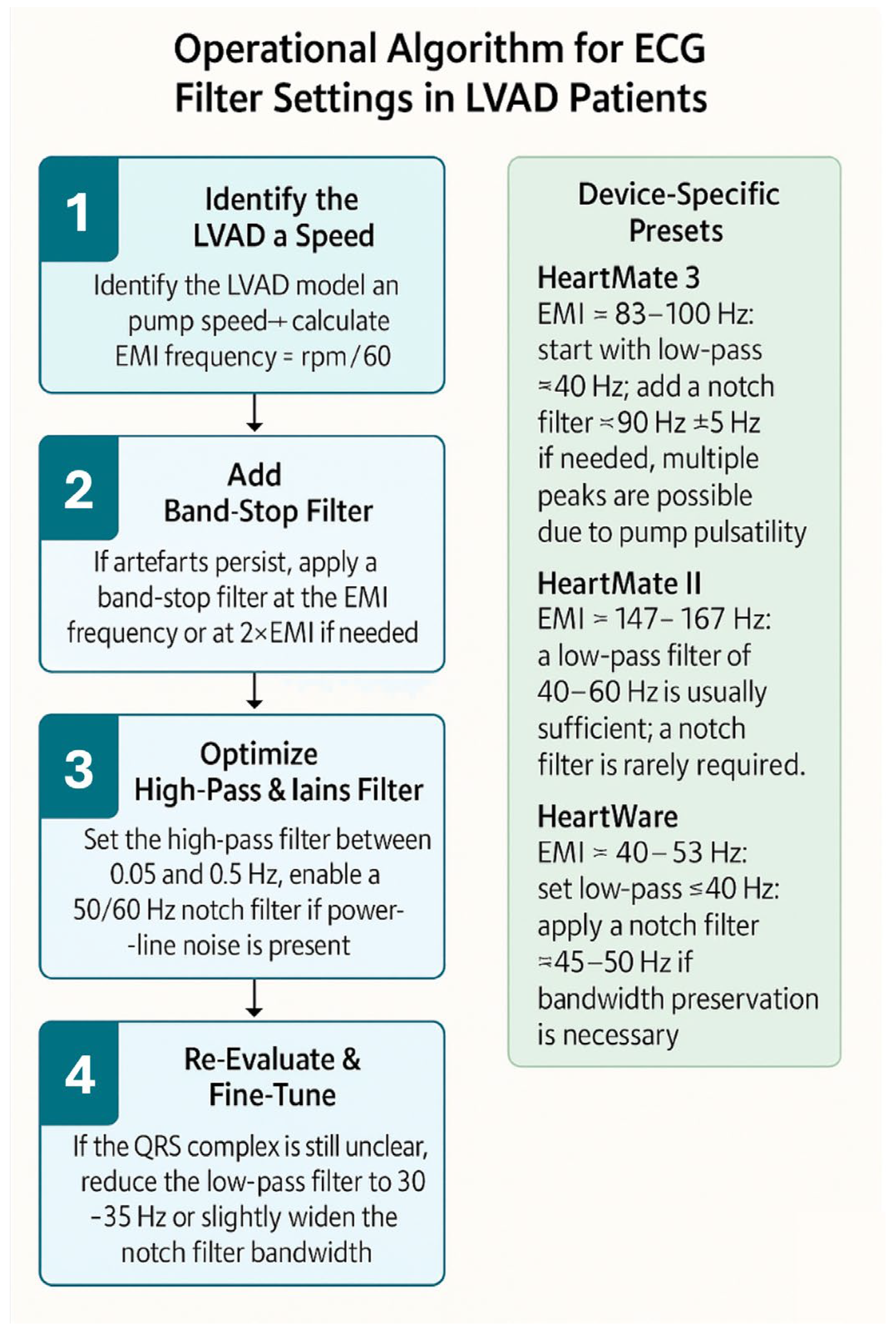




| LVAD Model | Typical Pump Speed(rpm) | Main EMI Frequency (Hz) | Recommended Low-Pass Filter | Band-Stop Filter Option | Notes/Tips |
|---|---|---|---|---|---|
| HeartMate 3 (Abbott) | 5000–6000 rpm | ~83–100 Hz | Set low-pass ≈40 Hz; preserves QRS morphology but may attenuate ST segments | Band-stop at pump-specific peak (~83–100 Hz) | Multiple peaks may occur due to the pump’s artificial pulsatility; more than one notch filter may be required |
| HeartMate II (Abbott) | 8800–10,000 rpm | ~147–167 Hz | Low-pass 40–60 Hz usually sufficient; artefact occurs at high frequencies | Notch filter rarely needed unless peaks overlap power-line frequency | High-frequency artefacts; lowering the low-pass filter to ≈40 Hz almost always improves ECG clarity |
| HeartWare (Medtronic) | 2400–3200 rpm | ~40–53 Hz | Set low-pass ≤40 Hz to suppress primary artefact | Optional: notch filter around 40–50 Hz | Artefacts usually occur at lower frequencies and are easily managed by lowering the low-pass filter |
| Complication | Incidence % (n) | Reference |
|---|---|---|
| Groin hematoma | 3.6–4.4% (4/110) | Anderson et al. [22] |
| Vascular access surgically treated | 1.8% (2/110) | Anderson et al. [22] |
| Cerebrovascular accidents | 1.8% (2/110) | Anderson et al. [22] |
| Cardiogenic shock | 0.9% (1/110) | Anderson et al. [22] |
| Pump thrombosis | Rare to 11% | Anderson et al. [22] Grinstein et al. [66] |
| Persistent ASD with right-to-left shunt | Rare | Wang et al. [68] Tamura et al. [69] |
| Catheter entrapment in LVAD cannula | Not yet reported for LVAD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgarito, G.; Campo, F.; Sciacca, S.; Pilato, M.; Cipriani, M.; Conti, S. Management of Ventricular Arrhythmias in Patients with Left Ventricular Assist Devices: Pathophysiology, Risk Stratification, and Ablation Strategies. J. Clin. Med. 2025, 14, 6604. https://doi.org/10.3390/jcm14186604
Sgarito G, Campo F, Sciacca S, Pilato M, Cipriani M, Conti S. Management of Ventricular Arrhythmias in Patients with Left Ventricular Assist Devices: Pathophysiology, Risk Stratification, and Ablation Strategies. Journal of Clinical Medicine. 2025; 14(18):6604. https://doi.org/10.3390/jcm14186604
Chicago/Turabian StyleSgarito, Giuseppe, Francesco Campo, Sergio Sciacca, Michele Pilato, Manlio Cipriani, and Sergio Conti. 2025. "Management of Ventricular Arrhythmias in Patients with Left Ventricular Assist Devices: Pathophysiology, Risk Stratification, and Ablation Strategies" Journal of Clinical Medicine 14, no. 18: 6604. https://doi.org/10.3390/jcm14186604
APA StyleSgarito, G., Campo, F., Sciacca, S., Pilato, M., Cipriani, M., & Conti, S. (2025). Management of Ventricular Arrhythmias in Patients with Left Ventricular Assist Devices: Pathophysiology, Risk Stratification, and Ablation Strategies. Journal of Clinical Medicine, 14(18), 6604. https://doi.org/10.3390/jcm14186604







