Robotic-Assisted Total Pelvic Exenteration for Rectal Cancer Using the Hugo™ RAS System: First Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient
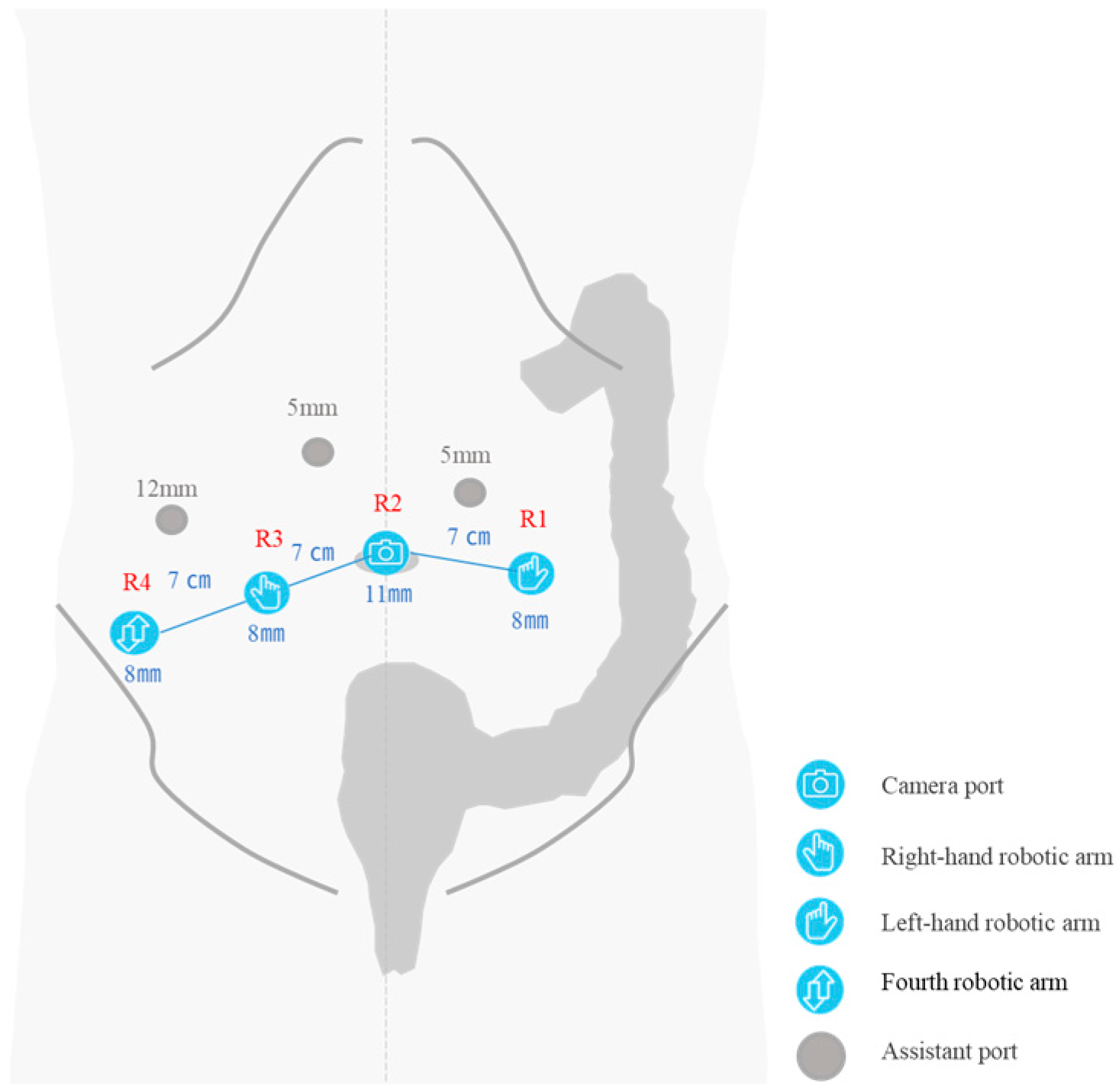
2.2. Surgical Setup
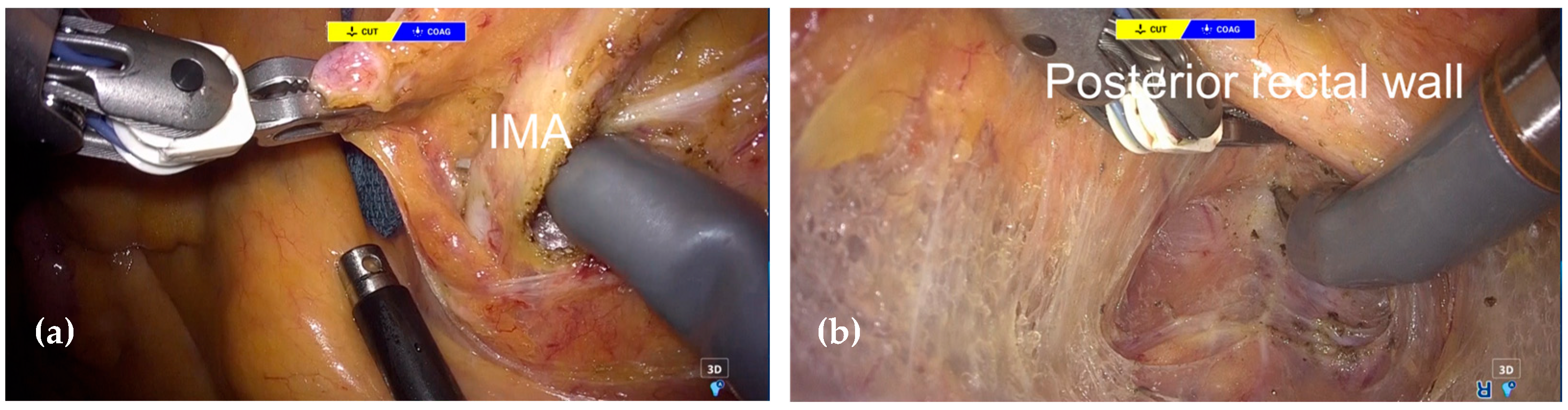
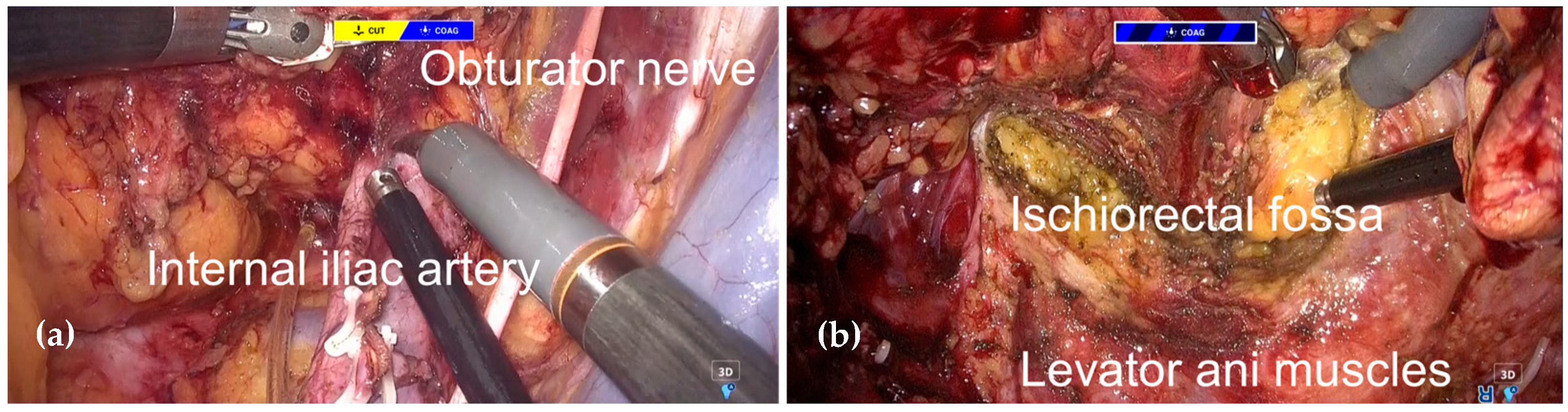
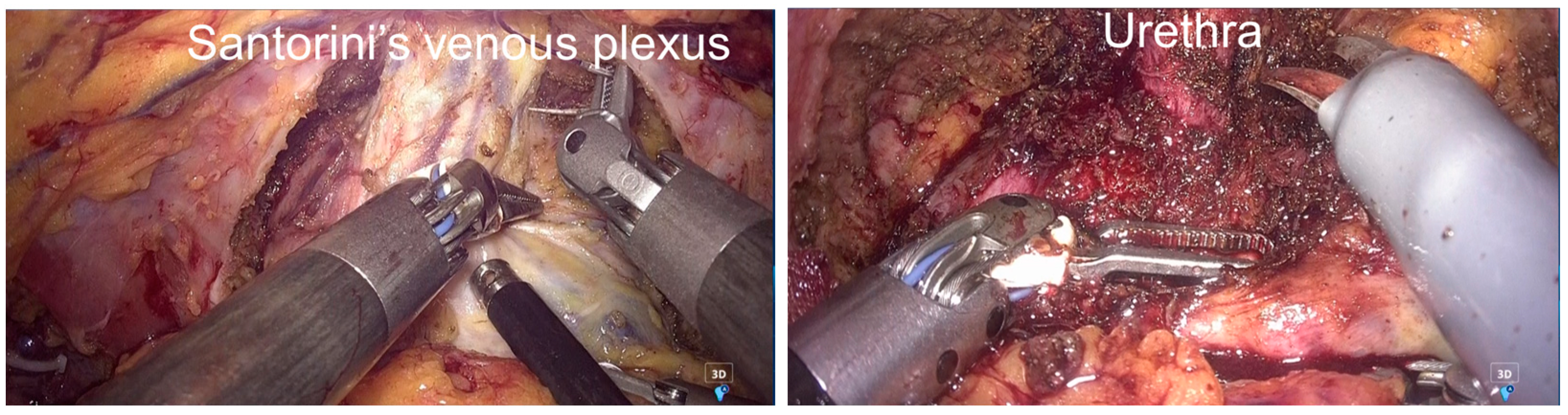
2.3. Surgical Procedure
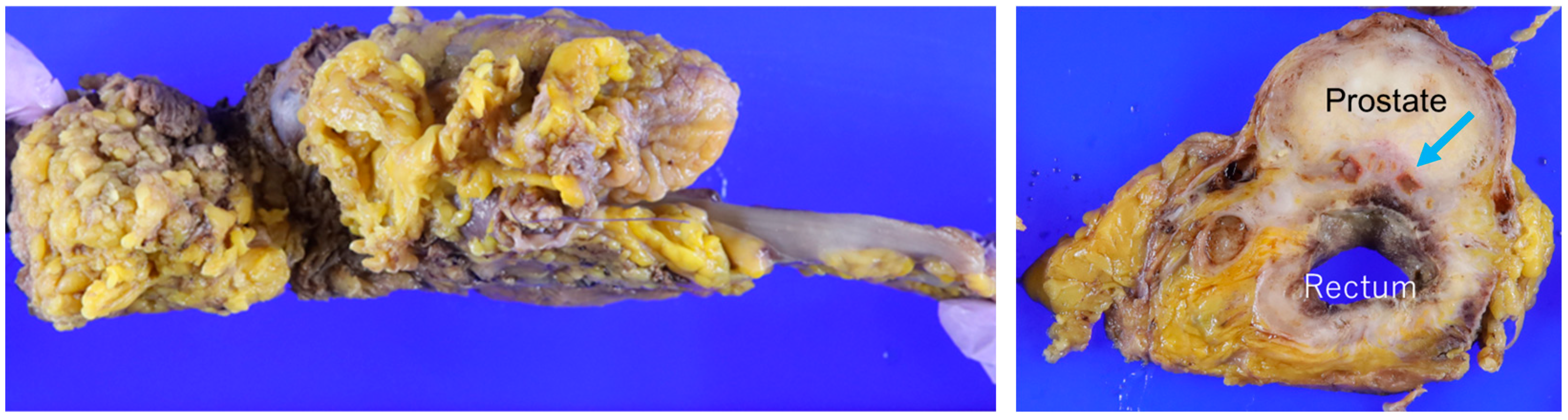
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomized clinical trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, G.S.; Park, S.Y.; Kim, H.J.; Ryuk, J.P. Comparison of laparoscopic versus robot-assisted surgery for rectal cancers: The COLRAR randomized controlled trial. Ann. Surg. 2022, 275, e600–e608. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): Short-term outcomes of a multicentre randomized controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Miyo, M.; Okuya, K.; Ito, T.; Akizuki, E.; Noda, A.; Ogawa, T.; Ishii, M.; Miura, R.; Ichihara, M.; et al. Cylindrical abdominoperineal resection for rectal cancer using the Hugo RAS system: The first ever case report for rectal cancer. Asian J. Endosc. Surg. 2024, 17, e13321. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: The 3rd English edition [secondary publication]. J. Anus Rectum Colon 2019, 3, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, T.; Eguchi, T.; Kiyama, M.; Matoba, S.; Hanaoka, Y.; Toda, S.; Kuroyanagi, H. Fascia lata grafting combined with gluteal flaps for pelvic floor reconstruction after oncologic resection. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4528. [Google Scholar] [CrossRef] [PubMed]
- Brunschwig, A. Complete excision of the pelvic viscera for advanced carcinoma. Cancer 1948, 1, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Pomel, C.; Rouzier, R.; Pocard, M.; Thoury, A.; Sideris, L.; Morice, P.; Duvillard, P.; Bourgain, J.; Castaigne, D. Laparoscopic total pelvic exenteration for cervical cancer relapse. Gynecol. Oncol. 2003, 91, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.C.W. Robotic assisted total pelvic exenteration: A case report. Gynecol. Oncol. 2009, 115, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Stefan, S.; Dumitrascu, T.; Ionescu, M.; Stanescu, D.; Dinu, M.; Bratu, O. Robotic total pelvic exenteration for locally advanced rectal cancer: Case report and literature review. Ann. R. Coll. Surg. Engl. 2022, 104, e74–e78. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Keeley, J.; Collins, J.W.; Sheikh, N.; Al-Dujaily, S.; Stewart, G.D. Robotic pelvic exenteration and extended pelvic resections for locally advanced or synchronous rectal and urological malignancy. Investig. Clin. Urol. 2021, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kihara, K.; Koyama, Y.; Hanaki, T.; Miyatani, K.; Matsunaga, T.; Yamamoto, M.; Morizane, S.; Tokuyasu, N.; Sakamoto, T.; Fujiwara, Y. Robot-assisted total pelvic exenteration for rectal cancer after neoadjuvant chemoradiotherapy: A case report. Surg. Case Rep. 2022, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.S.; Cooper, A.B.; Lavoie, A.; Brown, C.J.; Smith, A.J. Robotic beyond total mesorectal excision for locally advanced rectal cancers: Perioperative and oncological outcomes. Eur. J. Surg. Oncol. 2024, 50, 108308. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Hanaoka, M.; Hanazawa, R.; Hirakawa, A.; Mukai, T.; Kimura, K.; Yamanoi, K.; Kono, J.; Yokota, M.; Takahashi, H.; et al. Robotic versus open and laparoscopic pelvic exenterations for pelvic cancer: A multicenter propensity matched analysis in Japan. Surg. Endosc. 2024, 38, 4390–4401. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiramatsu, K.; Toda, S.; Matoba, S.; Tomita, D.; Maeda, Y.; Okazaki, N.; Fukui, Y.; Hanaoka, Y.; Ueno, M.; Oka, S.; et al. Robotic-Assisted Total Pelvic Exenteration for Rectal Cancer Using the Hugo™ RAS System: First Case Report. J. Clin. Med. 2025, 14, 6603. https://doi.org/10.3390/jcm14186603
Hiramatsu K, Toda S, Matoba S, Tomita D, Maeda Y, Okazaki N, Fukui Y, Hanaoka Y, Ueno M, Oka S, et al. Robotic-Assisted Total Pelvic Exenteration for Rectal Cancer Using the Hugo™ RAS System: First Case Report. Journal of Clinical Medicine. 2025; 14(18):6603. https://doi.org/10.3390/jcm14186603
Chicago/Turabian StyleHiramatsu, Kosuke, Shigeo Toda, Shuichiro Matoba, Daisuke Tomita, Yusuke Maeda, Naoto Okazaki, Yudai Fukui, Yutaka Hanaoka, Masashi Ueno, Suguru Oka, and et al. 2025. "Robotic-Assisted Total Pelvic Exenteration for Rectal Cancer Using the Hugo™ RAS System: First Case Report" Journal of Clinical Medicine 14, no. 18: 6603. https://doi.org/10.3390/jcm14186603
APA StyleHiramatsu, K., Toda, S., Matoba, S., Tomita, D., Maeda, Y., Okazaki, N., Fukui, Y., Hanaoka, Y., Ueno, M., Oka, S., Eguchi, T., & Kuroyanagi, H. (2025). Robotic-Assisted Total Pelvic Exenteration for Rectal Cancer Using the Hugo™ RAS System: First Case Report. Journal of Clinical Medicine, 14(18), 6603. https://doi.org/10.3390/jcm14186603





