Atrial Fibrillation Ablation After Three Decades: Mechanistic Insight or Just a Technological Race?
Abstract
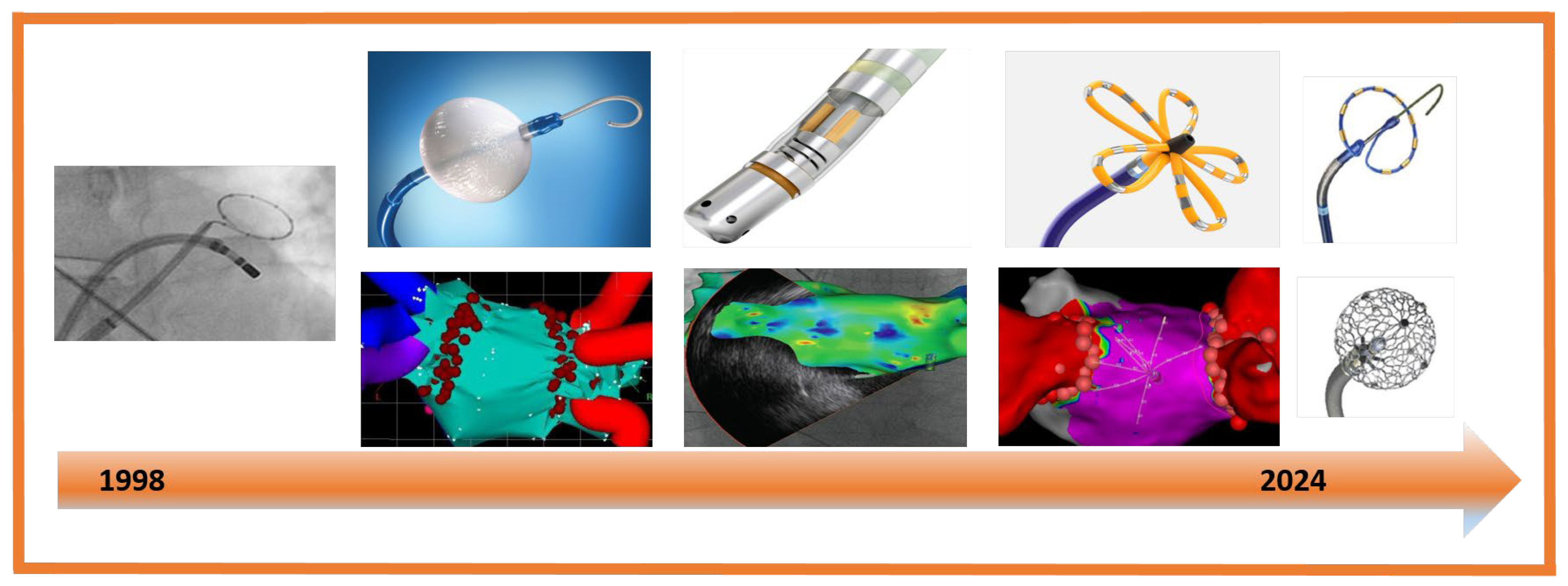
1. Introduction
2. Atrial Fibrillation Ablation
2.1. Radiofrequency Ablation
2.2. Cryoballon Ablation (CBA)
2.3. Pulse Field Ablation (PFA)
3. Tailored Therapy
3.1. Paroxysmal AF: Targeting Ectopic Triggers
3.2. Persistent AF: Substrate Modification
3.3. Autonomic Nervous System and Atrial Fibrillation: Translational Therapeutic Perspectives
3.4. Atrial Fibrillation in a Specific Population: Athletes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Blomstrom Lundqvist, C.; Lip, G.Y.H.; Kirchhof, P. What are the costs of atrial fibrillation? Europace 2011, 13 (Suppl. 2), 9–12. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. Available online: https://www.nejm.org/doi/pdf/10.1056/NEJM199809033391003 (accessed on 7 July 2025). [CrossRef]
- Jones, J.; Stanbury, M.; Haynes, S.; Bunting, K.V.; Lobban, T.; Camm, A.J.; Calvert, M.J.; Kotecha, D.; on behalf of the RAte control Therapy Evaluation in permanent Atrial Fibrillation (RATE-AF) trial group (2020). Importance and Assessment of Quality of Life in Symptomatic Permanent Atrial Fibrillation: Patient Focus Groups from the RATE-AF Trial. Cardiology 2020, 145, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. J. Am. Med. Assoc. 2019, 321, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Boersma, L.; Andrade, J.G.; Betts, T.; Duytschaever, M.; Pürerfellner, H.; Santoro, F.; Tzeis, S.; Verma, A. Progress in atrial fibrillation ablation during 25 years of Europace journal. Europace 2023, 25, euad244. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Oreto, G.; Lamberti, F.; Vicedomini, G.; Loricchio, M.L.; Shpun, S.; Rillo, M.; Calabrò, M.P.; Conversano, A.; Ben-Haim, S.A.; et al. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation 1999, 100, 1203–1208. [Google Scholar] [CrossRef]
- Pappone, C.; Rosanio, S.; Oreto, G.; Tocchi, M.; Gugliotta, F.; Vicedomini, G.; Salvati, A.; Dicandia, C.; Mazzone, P.; Santinelli, V.; et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation 2000, 102, 2619–2628. [Google Scholar] [CrossRef]
- Thomas, S.P.; Aggarwal, G.; Boyd, A.C.; Jin, Y.; Ross, D.L. A comparison of open irrigated and non-irrigated tip catheter ablation for pulmonary vein isolation. Europace 2004, 6, 330–335. [Google Scholar] [CrossRef]
- Natale, A.; Reddy, V.Y.; Monir, G.; Wilber, D.J.; Lindsay, B.D.; McElderry, H.T.; Kantipudi, C.; Mansour, M.C.; Melby, D.P.; Packer, D.L.; et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: Results of the prospective, multicenter SMART-AF trial. J. Am. Coll. Cardiol. 2014, 64, 647–656. [Google Scholar] [CrossRef]
- Scarà, A.; Sciarra, L.; De Ruvo, E.; Borrelli, A.; Grieco, D.; Palamà, Z.; Golia, P.; De Luca, L.; Rebecchi, M.; Calò, L. Safety and feasibility of atrial fibrillation ablation using Amigo® system versus manual approach: A pilot study. Indian Pacing Electrophysiol. J. 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; d’Avila, A.; Dukkipati, S.R.; Koruth, J.S.; Viles-Gonzalez, J.; Napolitano, C.; Eggert, C.; Fischer, A.; Gomes, J.A.; Reddy, V.Y. Acute electrical isolation is a necessary but insufficient endpoint for achieving durable PV isolation: The importance of closing the visual gap. Europace 2012, 14, 653–660. [Google Scholar] [CrossRef]
- Jahangir, A.; Lee, V.; Friedman, P.A.; Trusty, J.M.; Hodge, D.O.; Kopecky, S.L.; Packer, D.L.; Hammill, S.C.; Shen, W.K.; Gersh, B.J. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation 2007, 115, 3050–3056. [Google Scholar] [CrossRef]
- Khakpour, H.; Shemin, R.J.; Lee, J.M.; Buch, E.; Boyle, N.G.; Shivkumar, K.; Bradfield, J.S. Atrioesophageal fistula after atrial fibrillation ablation: A single center series. J. Atr. Fibrillation. 2017, 10, 1654. [Google Scholar] [CrossRef]
- Yacoub, M.; Sheppard, R.C. Cryoballoon Pulmonary Vein Catheter Ablation of Atrial Fibrillation; StatPearls: Tampa, FL, USA, 2018. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30521225 (accessed on 24 July 2023).
- Di Biase, L.; Diaz, J.C.; Zhang, X.D.; Romero, J. Pulsed field catheter ablation in atrial fibrillation. Trends Cardiovasc. Med. 2022, 32, 378–387. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1050173821000839 (accessed on 27 June 2025). [CrossRef]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Packer, D.L.; Kowal, R.C.; Wheelan, K.R.; Irwin, J.M.; Champagne, J.; Guerra, P.G.; Dubuc, M.; Reddy, V.; Nelson, L.; Holcomb, R.G.; et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American arctic front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013, 61, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Macle, L.; Wells, G.A.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; et al. Progression of Atrial Fibrillation after Cryoablation or Drug Therapy. N. Engl. J. Med. 2023, 388, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, M.A.; Hoffmann, B.A.; Schaeffer, B.; Chung, D.-U.; Moser, J.; Akbulak, R.O.; Jularic, M.; Eickholt, C.; Nuehrich, J.; Meyer, C.; et al. Influence of energy source on early atrial fibrillation recurrences: A comparison of cryoballoon vs. Radiofrequency current energy ablation with the endpoint of unexcitability in pulmonary vein isolation. Europace 2018, 20, 43–49. [Google Scholar] [CrossRef]
- Gasperetti, A.; Assis, F.; Tripathi, H.; Suzuki, M.; Gonuguntla, A.; Shah, R.; Sampognaro, J.; Schiavone, M.; Karmarkar, P.; Tandri, H. Determinants of acute irreversible electroporation lesion characteristics after pulsed field ablation: The role of voltage, contact, and adipose interference. Europace 2023, 25, euad257. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef]
- Sawada, M.; Nagashima, K.; Watanabe, R.; Saito, Y.; Wakamatsu, Y.; Otsuka, N.; Hirata, S.; Hirata, M.; Kurokawa, S.; Ito, S.; et al. Acute kidney injury after pulsed field ablation in a patient with Waldenström’s macroglobulinemia: A case report. Heart Case Rep. 2025; in press. [Google Scholar] [CrossRef]
- Boyle, T.A.; Frankel, D.S. Severe acute kidney injury from limited pulsed field ablation. Heart Rhythm. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Venier, S.; Vaxelaire, N.; Jacon, P.; Carabelli, A.; Desbiolles, A.; Garban, F.; Defaye, P. Severe acute kidney injury related to haemolysis after pulsed field ablation for atrial fibrillation. Europace 2023, 26, euad371. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.A.; Venier, S.; Menè, R.; Della Rocca, D.G.; Sacher, F.; Derval, N.; Hocini, M.; Dulucq, S.; Caluori, G.; Combes, S.; et al. Characterization and Clinical Significance of Hemolysis After Pulsed Field Ablation for Atrial Fibrillation: Results of a Multicenter Analysis. Circ. Arrhythm. Electrophysiol. 2024, 17, e012732. [Google Scholar] [CrossRef]
- Higuchi, S.; Gerstenfeld, E.P. Coronary artery injury in pulsed field ablation. Curr. Opin. Cardiol. 2025, 40, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ruwald, M.H.; Johannessen, A.; Hansen, M.L.; Haugdal, M.; Worck, R.; Hansen, J. Pulsed field ablation in real-world atrial fibrillation patients: Clinical recurrence, operator learning curve and re-do procedural findings. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing. 2023, 66, 1837–1848. [Google Scholar] [CrossRef]
- Johnson & Johnson MedTech. Varipulse Bi-Directional Ablation Catheter Recall. 2025. Available online: https://www.fda.gov/medical-devices/medical-device-recalls/ablation-catheter-correction-biosense-webster-updates-use-instructions-varipulse-due-high-rate (accessed on 28 February 2025).
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.-H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Palamà, Z.; Nesti, M.; Robles, A.G.; Scarà, A.; Romano, S.; Cavarretta, E.; Penco, M.; Delise, P.; Rillo, M.; Calò, L.; et al. Tailoring the Ablative Strategy for Atrial Fibrillation: A State-of-the-Art Review. Cardiol. Res. Pract. 2022, 2022, 9295326. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Kueffer, T.; Badertscher, P.; Jüni, P.; Knecht, S.; Thalmann, G.; Kozhuharov, N.; Krisai, P.; Jufer, C.; Maurhofer, J.; et al. Pulsed Field or Cryoballoon Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Shah, D.C.; Jaïs, P.; Hocini, M.; Yamane, T.; Deisenhofer, I.; Chauvin, M.; Garrigue, S.; ClémEnty, J. Electrophysiological Breakthroughs from the Left Atrium to the Pulmonary Veins. Circulation 2000, 102, 2463–2465. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Scharf, C.; Chugh, A.; Hall, B.; Cheung, P.; Good, E.; Veerareddy, S.; Pelosi, F., Jr.; Morady, F. Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003, 108, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, P.; Hocini, M.; Hsu, L.F.; Sanders, P.; Scavee, C.; Weerasooriya, R.; Macle, L.; Raybaud, F.; Garrigue, S.; Shah, D.C.; et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004, 110, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.C.; Santinelli, V.; Augello, G.; Ferro, A.; Gugliotta, F.; Gulletta, S.; Vicedomini, G.; Mesas, C.; Paglino, G.; Sala, S.; et al. Transcatheter radiofrequency ablation of atrial fibrillation in patients with mitral valve prostheses and enlarged atria: Safety, feasibility, and efficacy. J. Am. Coll. Cardiol. 2005, 45, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Marrouche, N.F.; Khaykin, Y.; Gillinov, A.M.; Wazni, O.; Martin, D.O.; Rossillo, A.; Verma, A.; Cummings, J.; Erciyes, D.; et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J. Am. Coll. Cardiol. 2004, 43, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Mantovan, R.; Macle, L.; De Martino, G.; Chen, J.; Morillo, C.A.; Novak, P.; Calzolari, V.; Guerra, P.G.; Nair, G.; et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur. Heart J. 2010, 31, 1344–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddy, V.Y.; Pollak, S.; Lindsay, B.D.; McElderry, H.T.; Natale, A.; Kantipudi, C.; Mansour, M.; Melby, D.P.; Lakkireddy, D.; Levy, T.; et al. Relationship Between Catheter Stability and 12-Month Success After Pulmonary Vein Isolation: A Subanalysis of the SMART-AF Trial. JACC Clin. Electrophysiol. 2016, 2, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Taghji, P.; El Haddad, M.; Phlips, T.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; Duytschaever, M. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins With Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation: A Pilot Study. JACC Clin. Electrophysiol. 2018, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Musikantow, D.R.; Neuzil, P.; Anic, A.; Balin, P.; Petru, J.; Funasako, M.; Lisica, L.; Jurisic, Z.; Jais, P.; Reddy, V.Y. Long-Term Clinical Outcomes of Pulsed Field Ablation in the Treatment of Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Hussein, A.A.; Delaughter, M.C.; Monir, G.; Natale, A.; Dukkipati, S.; Oza, S.; Daoud, E.; Di Biase, L.; Mansour, M.; et al. Very High-Power Short-Duration, Temperature-Controlled Radiofrequency Ablation in Paroxysmal Atrial Fibrillation: The Prospective Multicenter Q-FFICIENCY Trial. JACC Clin. Electrophysiol. 2023, 9, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Raatikainen, M.P.; Hakalahti, A.; Uusimaa, P.; Nielsen, J.C.; Johannessen, A.; Hindricks, G.; Walfridsson, H.; Pehrson, S.; Englund, A.; Hartikainen, J.; et al. Radiofrequency catheter ablation maintains its efficacy better than antiarrhythmic medication in patients with paroxysmal atrial fibrillation: On-treatment analysis of the randomized controlled MANTRA-PAF trial. Int. J. Cardiol. 2015, 198, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; Natale, A.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. J. Am. Med. Assoc. 2014, 311, 692–700. [Google Scholar] [CrossRef]
- Sciarra, L.; Rebecchi, M.; De Ruvo, E.; De Luca, L.; Zuccaro, L.M.; Fagagnini, A.; Corò, L.; Allocca, G.; Lioy, E.; Delise, P.; et al. How many atrial fibrillation ablation candidates have an underlying supraventricular tachycardia previously unknown? Efficacy of isolated triggering arrhythmia ablation. EP Eur. 2010, 12, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Marchlinski, F.E. Techniques for the provocation, localization, and ablation of non–pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017, 14, 1087–1096. [Google Scholar] [CrossRef]
- Bressi, E.; Rebecchi, M.; Sgueglia, M.; Crescenzi, C.; Panattoni, G.; Martino, A.; Casalese, A.; Sangiorgi, C.; Politano, A.; Cicogna, F.; et al. Atrial fibrillation and sport: Need for monitoring. Minerva Cardiol. Angiol. 2022, 70, 594–605. [Google Scholar] [CrossRef]
- Ioannidis, P.; Zografos, T.; Christoforatou, E.; Kouvelas, K.; Tsoumeleas, A.; Vassilopoulos, C.; Chen, R. The Electrophysiology of Atrial Fibrillation: From Basic Mechanisms to Catheter Ablation. Cardiol. Res. Pract. 2021, 2021, 4109269. [Google Scholar] [CrossRef]
- Aryana, A. Rationale and Outcomes of Cryoballoon Ablation of the Left Atrial Posterior Wall in Conjunction with Pulmonary Vein Isolation. J. Innov. Card. Rhythm. Manag. 2021, 12, 4633–4646. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation: The DECAAF Study. J. Am. Med. Assoc. 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dagres, N.; Hocini, M.; Fauchier, L.; Bongiorni, M.G.; Defaye, P.; Hernandez-Madrid, A.; Estner, H.; Sciaraffia, E.; Blomström-Lundqvist, C.; et al. Catheter ablation for atrial fibrillation: Results from the first European Snapshot Survey on Procedural Routines for Atrial Fibrillation Ablation (ESS-PRAFA) Part II. EP Eur. 2015, 17, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Scarà, A.; Palamà, Z.; Robles, A.G.; Dei, L.-L.; Borrelli, A.; Zanin, F.; Pignalosa, L.; Romano, S.; Sciarra, L. Non-Pharmacological Treatment of Heart Failure-From Physical Activity to Electrical Therapies: A Literature Review. J. Cardiovasc. Dev. Dis. 2024, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Raveendra, K.; Larvin, H.; Farooq, M.; Mohammed, H.; Wu, J.; Gale, C.; Nadarajah, R. Primary prevention of atrial fibrillation with pharmacological therapy. Europace 2024, 26, euae102-829. [Google Scholar] [CrossRef]
- Fedele, D.; Alvarez, M.C.; Maida, A.; Vasumini, N.; Amicone, S.; Canton, L.; Di Leo, M.; Basile, M.; Manaresi, T.; Angeli, F.; et al. Prevention of atrial fibrillation with SGLT2 inhibitors across the spectrum of cardiovascular disorders: A meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 441–450. [Google Scholar] [CrossRef]
- Allessie, M.A.; Boyden, P.A.; Camm, A.J.; Kléber, A.G.; Lab, M.J.; Legato, M.J.; Rosen, M.R.; Schwartz, P.J.; Spooner, P.M.; Van Wagoner, D.R.; et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001, 103, 769–777. [Google Scholar] [CrossRef]
- Coumel, P. Autonomic influences in atrial tachyarrhythmias. J. Cardiovasc. Electrophysiol. 1996, 7, 999–1007. [Google Scholar] [CrossRef]
- Scherlag, B.J.; Yamanashi, W.; Patel, U.; Lazzara, R.; Jackman, W.M. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J. Am. Coll. Cardiol. 2005, 45, 1878–1886. [Google Scholar] [CrossRef]
- Patterson, E.; Po, S.S.; Scherlag, B.J.; Lazzara, R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005, 2, 624–631. [Google Scholar] [CrossRef]
- Pappone, C.; Santinelli, V.; Manguso, F.; Vicedomini, G.; Gugliotta, F.; Augello, G.; Mazzone, P.; Tortoriello, V.; Landoni, G.; Zangrillo, A.; et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004, 109, 327–334. [Google Scholar] [CrossRef]
- Driessen, A.H.G.; Berger, W.R.; Krul, S.P.J.; van den Berg, N.W.E.; Neefs, J.; Piersma, F.R.; Chan Pin Yin, D.R.P.P.; de Jong, J.S.S.G.; van Boven, W.P.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, J.; Po, S.S.; Wang, H.; Zhang, L.; Zhang, F.; Wang, K.; Zhou, Q. Catheter-based renal sympathetic denervation significantly inhibits atrial fibrillation induced by electrical stimulation of the left stellate ganglion and rapid atrial pacing. PLoS ONE 2013, 8, e78218. [Google Scholar] [CrossRef]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2010, 182, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef]
- Roberts, J.D.; Marcus, G.M. Ablatogenomics: Can genotype guide catheter ablation for cardiac arrhythmias? Pharmacogenomics 2016, 17, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Petrungaro, M.; Fusco, L.; Cavarretta, E.; Scarà, A.; Borrelli, A.; Romano, S.; Petroni, R.; D’ascenzi, F.; Sciarra, L. Long-Term Sports Practice and Atrial Fibrillation: An Updated Review of a Complex Relationship. J. Cardiovasc. Dev. Dis. 2023, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, J.; Nielsen, J.R. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. EP Eur. 2009, 11, 1156–1159. [Google Scholar] [CrossRef]
- Karjalainen, J.; Kujala, U.M.; Kaprio, J.; Sarna, S.; Viitasalo, M. Lone atrial fibrillation in vigorously exercising middle aged men: Case-control study. BMJ 1998, 316, 1784–1785. [Google Scholar] [CrossRef]
- Mont, L.; Sambola, A.; Brugada, J.; Vacca, M.; Marrugat, J.; Elosua, R.; Paré, C.; Azqueta, M.; Sanz, G. Long-lasting sport practice and lone atrial fibrillation. Eur. Heart J. 2002, 23, 477–482. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Anselmi, F.; Focardi, M.; Mondillo, S. Atrial Enlargement in the Athlete’s Heart: Assessment of Atrial Function May Help Distinguish Adaptive from Pathologic Remodeling. J. Am. Soc. Echocardiogr. 2018, 31, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Flannery, M.D.; Kalman, J.M.; Sanders, P.; La Gerche, A. State of the Art Review: Atrial Fibrillation in Athletes. Heart Lung Circ. 2017, 26, 983–989. [Google Scholar] [CrossRef]
- Raju, H.; Kalman, J.M. Management of Atrial Fibrillation in the Athlete. Heart Lung Circ. 2018, 27, 1086–1092. [Google Scholar] [CrossRef]
- Lampert, R.; Chung, E.H.; Ackerman, M.J.; Arroyo, A.R.; Darden, D.; Deo, R.; Dolan, J.; Etheridge, S.P.; Gray, B.R.; Harmon, K.G.; et al. 2024 HRS expert consensus statement on arrhythmias in the athlete: Evaluation, treatment, and return to play. Heart Rhythm 2024, 21, e151–e252. [Google Scholar] [CrossRef] [PubMed]


| Year | Par AF | Pers AF | Study |
|---|---|---|---|
| 2000 | 75% | n/a | Haïssaguerre et al. (Circulation) [36] |
| 2003 | 75% | 55% | Oral et al. (Circulation) [37] |
| 2004 | 73% | 55% | Jais et al. (Circulation) [38] |
| 2005 | 74% | 65% | Lang et al. (JACC) [39] |
| 2006 | 75% | 55% | Chen et al. (JACC) [40] |
| 2010 | 75% | 55% | Verma et al. [41] |
| 2015 | 78% | 68% | Reddy et al. (JACC: Clinical EP) [42] |
| 2016 | 75% | 68% | Fire and ICE [43] |
| 2018 | 88% | 78% | Close [44] |
| 2019 | 82% | 77% | Reddy et al. (JACC) [45] |
| 2023 | 75% | 65% | ADVENT [25] |
| 2023 | 82% | 68% | Musikantow et al. (JACC EP) [46] |
| 2024 | 81% | 73% | QDOT MICRO REGISTRY [47] |
| Clinical Scenario | Dominant Mechanism | Preferred Lesion Strategy | Energy Considerations |
|---|---|---|---|
| Symptomatic paroxysmal AF, structurally normal atria | PV triggers | Wide antral PVI | CBA or PFA for efficiency; RFA for anatomy variants |
| Paroxysmal AF with suspected non-PV triggers | Focal ectopy | Map + focal ablation ± PVI | RFA (versatile); focal PFA emerging |
| Early persistent AF, mild substrate | PV + limited substrate | PVI ± targeted LVA/driver ablation | RFA (tailored); investigational PFA |
| Advanced persistent AF, atrial dilation/fibrosis | Substrate-dominant | PVI + voltage-guided homogenization ± lines ± hybrid posterior wall | RFA backbone; add surgical/hybrid; PFA future |
| Vagal AF phenotype | Autonomic modulation | PVI ± GP ablation; lifestyle | RFA platform for GP; investigational neuromodulation |
| Endurance athlete | PV triggers + vagal modulation | Efficient PVI; selective adjuncts; return-to-play focus | CBA for speed; RFA for adjunct; PFA under study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiriti, G.; Scarà, A.; Borrelli, A.; Zanin, F.; Pignalosa, L.; Buzzelli, L.; Palamà, Z.; Robles, A.G.; Nesti, M.; Sciarra, L. Atrial Fibrillation Ablation After Three Decades: Mechanistic Insight or Just a Technological Race? J. Clin. Med. 2025, 14, 6601. https://doi.org/10.3390/jcm14186601
Spiriti G, Scarà A, Borrelli A, Zanin F, Pignalosa L, Buzzelli L, Palamà Z, Robles AG, Nesti M, Sciarra L. Atrial Fibrillation Ablation After Three Decades: Mechanistic Insight or Just a Technological Race? Journal of Clinical Medicine. 2025; 14(18):6601. https://doi.org/10.3390/jcm14186601
Chicago/Turabian StyleSpiriti, Giulia, Antonio Scarà, Alessio Borrelli, Federico Zanin, Leonardo Pignalosa, Lorenzo Buzzelli, Zefferino Palamà, Antonio Gianluca Robles, Martina Nesti, and Luigi Sciarra. 2025. "Atrial Fibrillation Ablation After Three Decades: Mechanistic Insight or Just a Technological Race?" Journal of Clinical Medicine 14, no. 18: 6601. https://doi.org/10.3390/jcm14186601
APA StyleSpiriti, G., Scarà, A., Borrelli, A., Zanin, F., Pignalosa, L., Buzzelli, L., Palamà, Z., Robles, A. G., Nesti, M., & Sciarra, L. (2025). Atrial Fibrillation Ablation After Three Decades: Mechanistic Insight or Just a Technological Race? Journal of Clinical Medicine, 14(18), 6601. https://doi.org/10.3390/jcm14186601








