Efficacy of Biologics in Patients with Ulcerative Colitis Exhibiting Non-Response or Insufficient Response to Cytapheresis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Evaluating the Efficacy of Biologics
2.3. Assessment of Endoscopic Improvement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
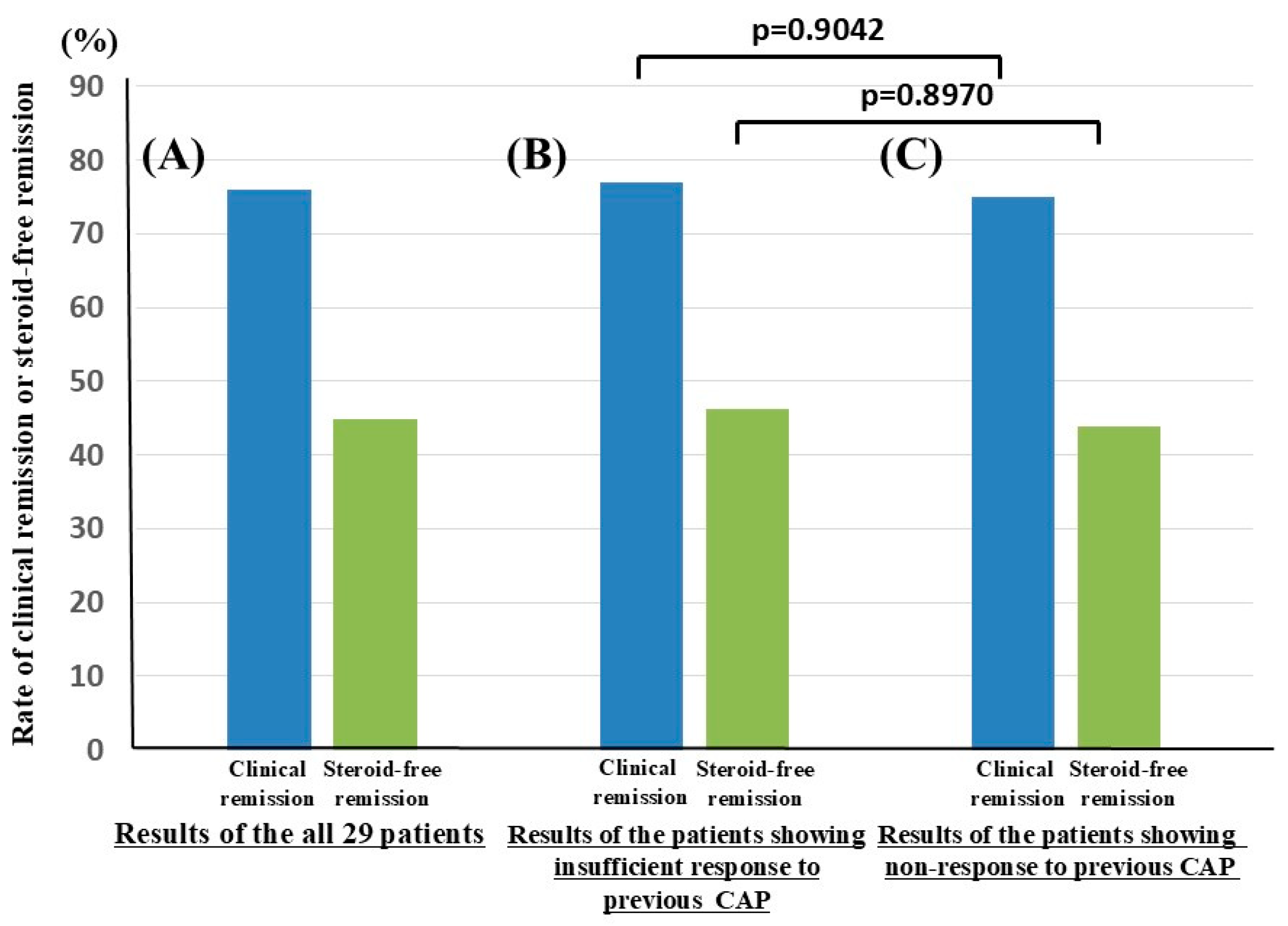
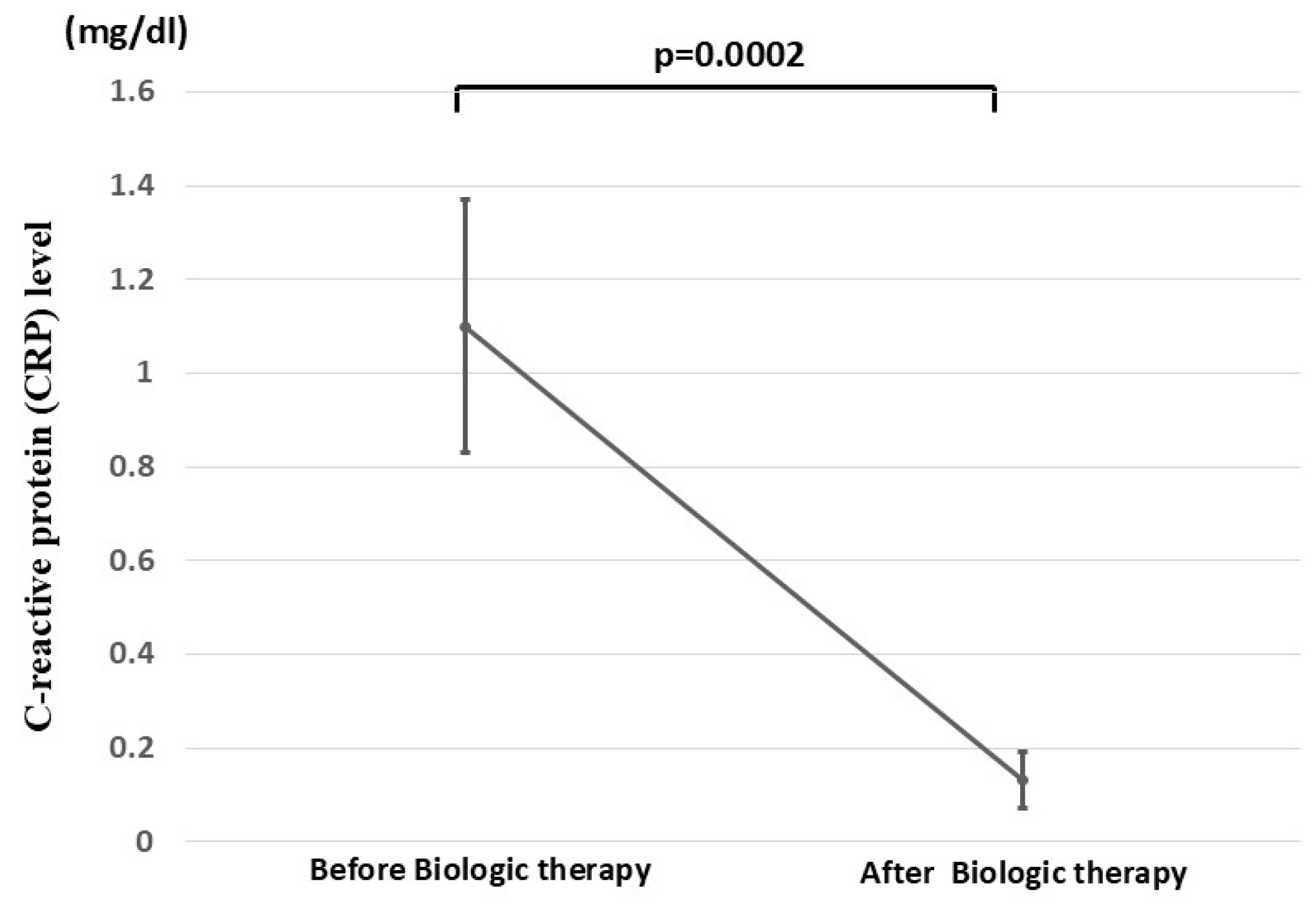
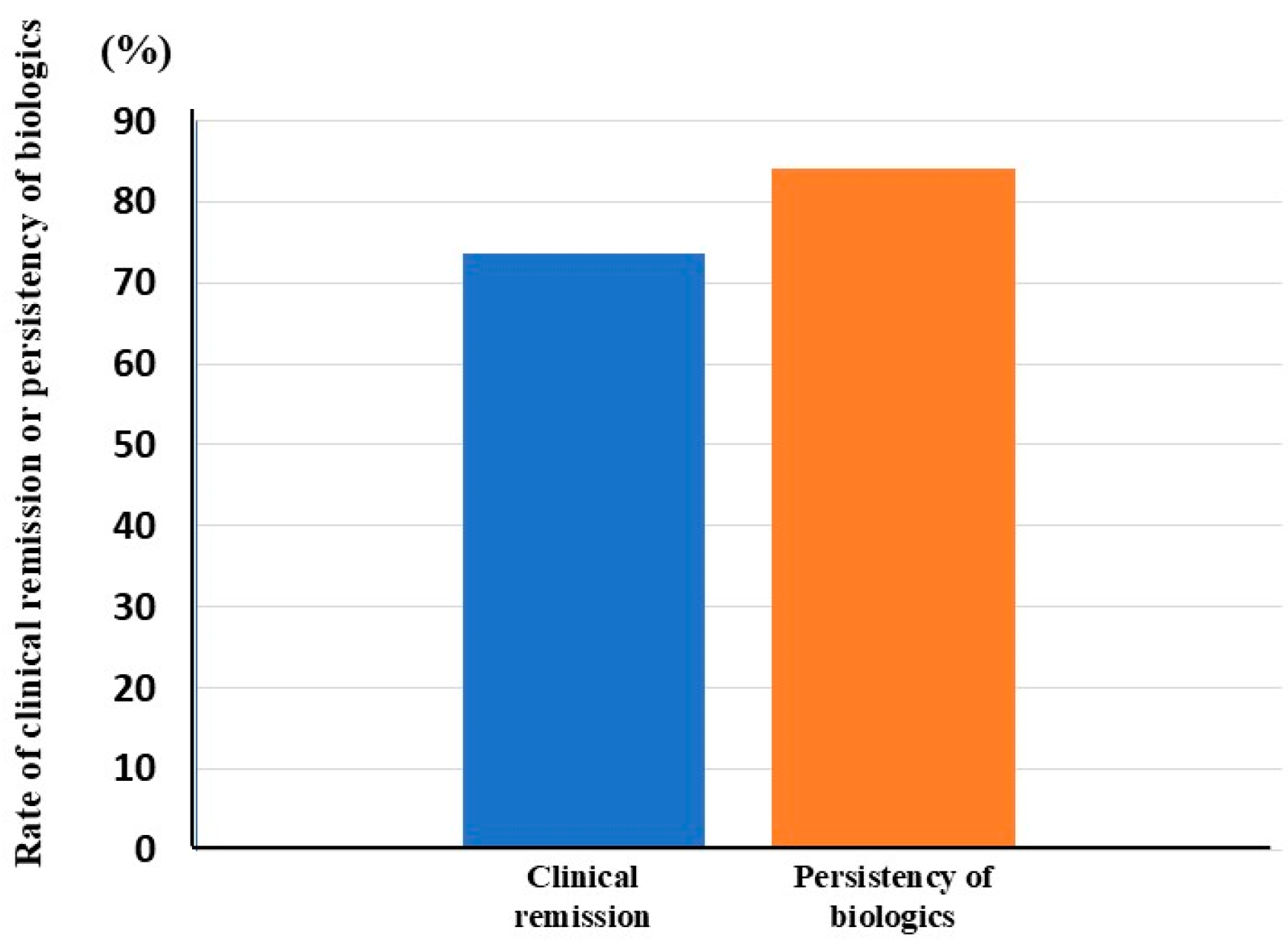
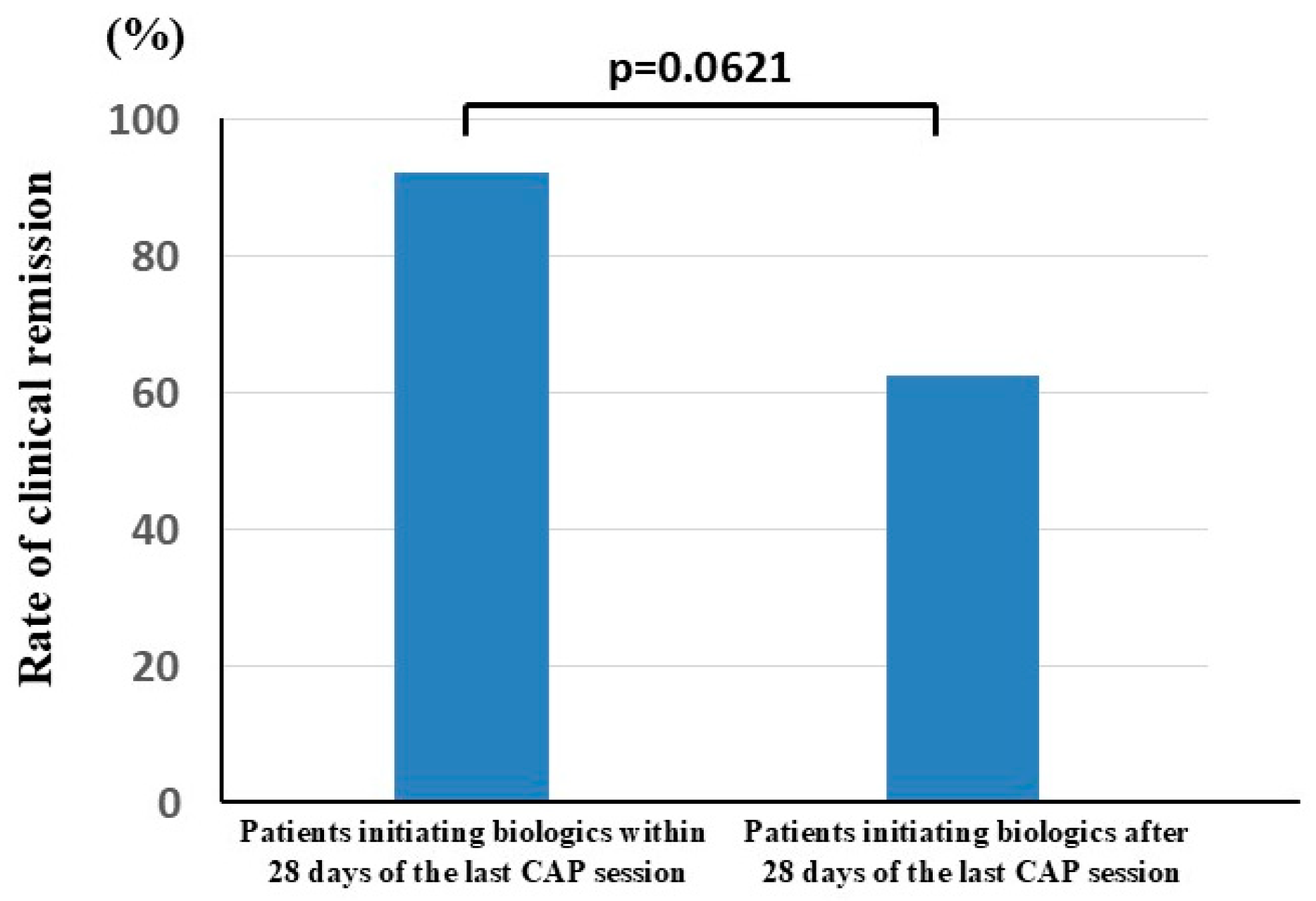
3.2. Clinical Efficacy of Biologics in Patients with UC Who Underwent CAP Before Biologic Therapy
3.3. Endoscopic Improvement After Biologic Therapy
3.4. Adverse Events
4. Discussion
| Biologics [Ref. No. *] | Rate of Clinical Remission (Biologic Naïve and Exposed Patients **) | Rate of Steroid-Free Remission (Biologic Naïve and Exposed Patients) | Rate of Endoscopic Improvement (Biologic Naïve and Exposed Patients) | |
|---|---|---|---|---|
| Iufliximab | [54] | 36.4% | ||
| [58] | 47.1% | |||
| [55] | 44.4% | 44.4% | 11.8% | |
| [56] | 48.6% *** | 80.6% *** | ||
| [57] | 39% | 60% | ||
| [57] | 34% | |||
| Adalimmab | [43] | 21.3% and 9.2% | ||
| [57] | 18% | 47% | ||
| [57] | 21% | 49% | ||
| [57] | 10% | 41% | ||
| [44] | 24.3% and 16.0% | 21.7% and 22.2% | 29.5% and 21.0% | |
| Vedolizumab | [54] | 32.4% | ||
| [58] | 65.6% | |||
| [55] | 67.5% | 67.5% | 29.5% | |
| [43] | 23.1% and 9.8% | |||
| [57] | 23% and 10% | 49% and 30% | ||
| [44] | 34.2% and 20.3% | 14.9% and 4.2% | 43.1% and 26.6% | |
| [46] | 46.9% and 36.1% | 44.6% and 26.7% | 60.0%and 44.6% | |
| [47] | 55.9% and 62.5% | 34.6% and 35.7% | 61.5% and 63.6% | |
| [56] | 65.9% | 86.6% | ||
| Ustekinumab | [54] | 43.3% | ||
| [43] | 18.4% and 12.7% | |||
| [49] | 72.2% and 62.6% | 70.9% and 60.4% | ||
| [50] | 80% and 54% | 77% and 48% | ||
5. Future Perspectives and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| IBD | Inflammatory bowel disease |
| CS | Corticosteroid |
| CAP | Cytapheretic |
| GMA | Granulocyte and monocyte adsorptive apheresis |
| LCAP | Leukocytapheresis |
| TNF-α | Tumor necrosis factor-α |
| CAI | Clinical activity index |
| IFX | Infliximab |
| ADA | Adalimumab |
| VDZ | Vedolizumab |
| UST | Ustekinumab |
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.M.; Mehmood, F.; Khan, N. Optimal management of steroid-dependent ulcerative colitis. Clin. Exp. Gastroenterol. 2015, 8, 293–302. [Google Scholar] [CrossRef]
- Faubion, W.A., Jr.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology 2001, 121, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.; Chiam, P.; Drummond, H.; Loane, J.; Arnott, I.D.; Satsangi, J. The efficacy of corticosteroid therapy in inflammatory bowel disease: Analysis of a 5-year UK inception cohort. Aliment. Pharmacol. Ther. 2006, 24, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Edwards, F.C.; Truelove, S.C. The course and prognosis of ulcerative colitis. Gut 1963, 4, 299–315. [Google Scholar] [CrossRef]
- Farmer, R.G.; Easley, K.A.; Rankin, G.B. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig. Dis. Sci. 1993, 38, 1137–1146. [Google Scholar] [CrossRef]
- Khan, N.; Abbas, A.; Williamson, A.; Balart, L. Prevalence of corticosteroids use and disease course after initial steroid exposure in ulcerative colitis. Dig. Dis. Sci. 2013, 58, 2963–2969. [Google Scholar] [CrossRef]
- Iizuka, M.; Etou, T.; Sagara, S. Efficacy of cytapheresis in patients with ulcerative colitis showing insufficient or lost response to biologics. World J. Gastroenterol. 2022, 28, 4959–4972. [Google Scholar] [CrossRef]
- Fudman, D.I.; McConnell, R.A.; Ha, C.; Singh, S. Modern Advanced Therapies for Inflammatory Bowel Diseases: Practical Considerations and Positioning. Clin. Gastroenterol. Hepatol. 2025, 23, 454–468. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, C.; Gu, Y.; Meng, F.; Qin, X.; Cao, H. Selection strategy of second-line biologic therapies in adult patients with ulcerative colitis following prior biologic treatment failure: Systematic review and meta-analysis. Pharmacol. Res. 2024, 202, 107108. [Google Scholar] [CrossRef]
- Zhao, M.; Sall Jensen, M.; Knudsen, T.; Kelsen, J.; Coskun, M.; Kjellberg, J.; Burisch, J. Trends in the use of biologicals and their treatment outcomes among patients with inflammatory bowel diseases—A Danish nationwide cohort study. Aliment. Pharmacol. Ther. 2022, 55, 541–557. [Google Scholar] [CrossRef]
- Kapizioni, C.; Desoki, R.; Lam, D.; Balendran, K.; Al-Sulais, E.; Subramanian, S.; Rimmer, J.E.; De La Revilla Negro, J.; Pavey, H.; Pele, L.; et al. Biologic Therapy for Inflammatory Bowel Disease: Real-World Comparative Effectiveness and Impact of Drug Sequencing in 13,222 Patients within the UK IBD BioResource. J. Crohn’s Colitis 2024, 18, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Hupé, M.; Rivière, P.; Nancey, S.; Roblin, X.; Altwegg, R.; Filippi, J.; Fumery, M.; Bouguen, G.; Peyrin-Biroulet, L.; Bourreille, A.; et al. Comparative efficacy and safety of vedolizumab and infliximab in ulcerative colitis after failure of a first subcutaneous anti-TNF agent: A multicentre cohort study. Aliment. Pharmacol. Ther. 2020, 51, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Favale, A.; Onali, S.; Caprioli, F.; Pugliese, D.; Armuzzi, A.; Macaluso, F.S.; Orlando, A.; Viola, A.; Fries, W.; Rispo, A.; et al. Comparative Efficacy of Vedolizumab and Adalimumab in Ulcerative Colitis Patients Previously Treated with Infliximab. Inflamm. Bowel Dis. 2019, 25, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Solitano, V.; Singh, S.; Ananthakrishnanm, A.N.; Jairath, V.; Syal, G.; Boland, B.S.; Ghosh, P.; Chang, J.T.; Singh, S. Differential Efficacy of Advanced Therapies in Inducing Remission in Ulcerative Colitis Based on Prior Exposure to TNF Antagonists. Clin. Gastroenterol. Hepatol. 2024, S1542–S3565, 01132–01137. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iida, T.; Ikeya, K.; Kato, M.; Matsuura, A.; Tamura, S.; Takano, R.; Tani, S.; Osawa, S.; Sugimoto, K.; et al. A multicenter retrospective study aiming to identify patients who respond well to adsorptive granulomonocytapheresis in moderately to severely active ulcerative colitis. Clin. Transl. Gastroenterol. 2018, 9, 170. [Google Scholar] [CrossRef]
- Dignass, A.; Akbar, A.; Hart, A.; Subramanian, S.; Bommelaer, G.; Baumgart, D.C.; Grimaud, J.C.; Cadiot, G.; Makins, R.; Hoque, S.; et al. Safety and Efficacy of Granulocyte/Monocyte Apheresis in Steroid-Dependent Active Ulcerative Colitis with Insufficient Response or Intolerance to Immunosuppressants and/or Biologics [the ART Trial]: 12-week Interim Results. J. Crohn’s Colitis 2016, 10, 812–820. [Google Scholar] [CrossRef]
- Fernández-Pérez, F.J.; Fernández-Moreno, N.; Soria-López, E.; Rodriguez-González, F.J.; Fernández-Galeote, F.J.; Lifante-Oliva, A.; Ruíz-Hernández, C.; Escalante-Quijaite, E.; Rivas-Ruiz, F. Granulocyte and monocyte adsorptive apheresis (GMA) in patients with inflammatory bowel disease: A useful therapeutic tool not just in ulcerative colitis but also in Crohn’s disease. Gastroenterol. Hepatol. 2024, 47, 502196. [Google Scholar] [CrossRef]
- Narula, N.; Peerani, F.; Meserve, J.; Kochhar, G.; Chaudrey, K.; Hartke, J.; Chilukuri, P.; Koliani-Pace, J.; Winters, A.; Katta, L.; et al. Vedolizumab for Ulcerative Colitis: Treatment Outcomes from the VICTORY Consortium. Am. J. Gastroenterol. 2018, 113, 1345. [Google Scholar] [CrossRef] [PubMed]
- Kotze, P.G.; Ma, C.; Almutairdi, A.; Al-Darmaki, A.; Devlin, S.M.; Kaplan, G.G.; Seow, C.H.; Novak, K.L.; Lu, C.; Ferraz, J.G.P.; et al. Real-world clinical, endoscopic and radiographic efficacy of vedolizumab for the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 48, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Motoya, S.; Tanaka, H.; Shibuya, T.; Osada, T.; Yamamoto, T.; Hongo, H.; Mizuno, C.; Saito, D.; Aoyama, N.; Kobayashi, T.; et al. Safety and effectiveness of granulocyte and monocyte adsorptive apheresis in patients with inflammatory bowel disease in special situations: A multicentre cohort study. BMC Gastroenterol. 2019, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Umegae, S.; Matsumoto, K. Mucosal healing in patients with ulcerative colitis during a course of selective leukocytapheresis therapy: A prospective cohort study. Inflamm. Bowel Dis. 2010, 16, 1905–1911. [Google Scholar] [CrossRef]
- Lindberg, A.; Eberhardson, M.; Karlsson, M.; Karlén, P. Long-term follow-up with Granulocyte and Monocyte Apheresis re-treatment in patients with chronically active inflammatory bowel disease. BMC Gastroenterol. 2010, 10, 73. [Google Scholar] [CrossRef]
- Sandborn, W.J. Preliminary data on the use of apheresis in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12 (Suppl. S1), S15–S21. [Google Scholar] [CrossRef]
- Hibi, T.; Sameshima, Y.; Sekiguchi, Y.; Hisatome, Y.; Maruyama, F.; Moriwaki, K.; Shima, C.; Saniabadi, A.R.; Matsumoto, T. Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: Clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan. Dig. Liver Dis. 2009, 41, 570–577. [Google Scholar] [CrossRef]
- Sakuraba, A.; Motoya, S.; Watanabe, K.; Nishishita, M.; Kanke, K.; Matsui, T.; Suzuki, Y.; Oshima, T.; Kunisaki, R.; Matsumoto, T.; et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am. J. Gastroenterol. 2009, 104, 2990–2995. [Google Scholar] [CrossRef]
- Takayama, T.; Kanai, T.; Matsuoka, K.; Okamoto, S.; Sujino, T.; Mikami, Y.; Hisamatsu, T.; Yajima, T.; Iwao, Y.; Ogata, H.; et al. Long-term prognosis of patients with ulcerative colitis treated with cytapheresis therapy. J. Crohn’s Colitis 2013, 7, e49–e54. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Matsuoka, K.; Kobayashi, T.; Sawada, K.; Fujiyoshi, T.; Ando, T.; Ohnishi, Y.; Ishida, T.; Oka, M.; Yamada, M.; et al. A large-scale, prospective, observational study of leukocytapheresis for ulcerative colitis: Treatment outcomes of 847 patients in clinical practice. J. Crohn’s Colitis 2014, 8, 981–991. [Google Scholar] [CrossRef]
- Iizuka, M.; Etou, T.; Shimodaira, Y.; Hatakeyama, T.; Sagara, S. Cytapheresis re-induces high-rate steroid-free remission in patients with steroid-dependent and steroid-refractory ulcerative colitis. World J. Gastroenterol. 2021, 27, 1194–1212. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Nakase, H.; Minami, N.; Yamada, S.; Matsuura, M.; Yazumi, S.; Chiba, T. Efficacy and safety of granulocyte and monocyte adsorption apheresis for ulcerative colitis: A meta-analysis. Dig. Liver Dis. 2014, 46, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xu, X.; Nie, F.; Tong, J.; Xiao, S.; Ran, Z. The efficacy and safety of selective leukocytapheresis in the treatment of ulcerative colitis: A meta-analysis. Int. J. Colorectal Dis. 2011, 26, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Ricart, E.; Esteve, M.; Andreu, M.; Casellas, F.; Monfort, D.; Sans, M.; Oudovenko, N.; Lafuente, R.; Panes, J. Evaluation of 5 versus 10 granulocyteaphaeresis sessions in steroid-dependent ulcerative colitis: A pilot, prospective, multicenter, randomized study. World J. Gastroenterol. 2007, 13, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Cabriada, J.L.; Ibargoyen, N.; Hernández, A.; Bernal, A.; Castiella, A. Sustained remission after steroids and leukocytapheresis induced response in steroid-dependent ulcerative colitis: Results at 1 year. Dig. Liver Dis. 2010, 42, 432–435. [Google Scholar] [CrossRef]
- Cabriada, J.L.; Domènech, E.; Ibargoyen, N.; Hernández, V.; Clofent, J.; Ginard, D.; Gutiérrez-Ibarluzea, I.; Hinojosa, J. Leukocytapheresis for steroid-dependent ulcerative colitis in clinical practice: Results of a nationwide Spanish registry. J. Gastroenterol. 2012, 47, 359–365. [Google Scholar] [CrossRef]
- Imperiali, G.; Amato, A.; Terpin, M.M.; Beverina, I.; Bortoli, A.; Devani, M.; Viganò, C.; Study Group on IBD (GSMII). Granulocyte-Monocyte Apheresis in Steroid-Dependent, Azathioprine-Intolerant/Resistant Moderate Ulcerative Colitis: A Prospective Multicenter Study. Gastroenterol. Res. Pract. 2017, 2017, 9728324. [Google Scholar] [CrossRef]
- Dignass, A.; Akbar, A.; Baumgart, D.C.; Bommelaer, G.; Bouguen, G.; Cadiot, G.; Gillessen, A.; Grimaud, J.C.; Hart, A.; Hoque, S.; et al. Granulocyte/monocyte adsorptive apheresis for the treatment of therapy-refractory chronic active ulcerative colitis. Scand. J. Gastroenterol. 2018, 53, 442–448. [Google Scholar] [CrossRef]
- Domènech, E.; Panés, J.; Hinojosa, J.; Annese, V.; Magro, F.; Sturniolo, G.C.; Bossa, F.; Fernández, F.; González-Conde, B.; García-Sánchez, V.; et al. Addition of granulocyte/monocyte apheresis to oral prednisone for steroid-dependent ulcerative colitis: A randomized, multicentre, clinical trial. J. Crohn’s Colitis 2018, 12, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Bressler, B. Is there an optimal sequence of biologic therapies for inflammatory bowel disease? Therap. Adv. Gastroenterol. 2023, 16, 17562848231159452. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Danese, S.; Colombel, J.F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Bessissow, T.; Nguyen, G.C.; Tarabain, O.; Peyrin-Biroulet, L.; Foucault, N.; McHugh, K.; Ruel, J. Impact of adalimumab on disease burden in moderate-to-severe ulcerative colitis patients: The one-year, real-world UCanADA study. World J. Gastroenterol. 2022, 28, 5058–5075. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rubin, D.T.; Danese, S.; Vermeire, S.; Abhyankar, B.; Sankoh, S.; James, A.; Smyth, M. Efficacy of Vedolizumab Induction and Maintenance Therapy in Patients With Ulcerative Colitis, Regardless of Prior Exposure to Tumor Necrosis Factor Antagonists. Clin. Gastroenterol. Hepatol. 2017, 15, 229–239.e5. [Google Scholar] [CrossRef]
- Lin, W.C.; Tai, W.C.; Chang, C.H.; Tu, C.H.; Feng, I.C.; Shieh, M.J.; Chung, C.S.; Yen, H.H.; Chou, J.W.; Wong, J.M.; et al. Real-World Evidence of Effectiveness and Safety of Vedolizumab for Inflammatory Bowel Disease in Taiwan: A Prospective Nationwide Registry (VIOLET) Study. Inflamm. Bowel Dis. 2023, 29, 1730–1740. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Rowbotham, D.S.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Tikhonov, I.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension. J. Crohn’s Colitis 2022, 16, 1222–1234. [Google Scholar] [CrossRef]
- Ando, K.; Fujiya, M.; Ueno, N.; Ito, T.; Maemoto, A.; Nasuno, M.; Tanaka, H.; Sakurai, K.; Katsurada, T.; Orii, F.; et al. Effectiveness and Persistency of Ustekinumab Treatment for Ulcerative Colitis: A Phoenix retrospective Cohort Study. Crohn’s Colitis 360 2024, 6, otae024. [Google Scholar] [CrossRef] [PubMed]
- Thunberg, J.; Björkqvist, O.; Hedin, C.R.H.; Forss, A.; Söderman, C.; Bergemalm, D.; SWIBREG Study Group; Olén, O.; Hjortswang, H.; Strid, H.; et al. Ustekinumab treatment in ulcerative colitis: Real-world data from the Swedish inflammatory bowel disease quality register. United Eur. Gastroenterol. J. 2022, 10, 631–639. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Mocci, G.; Tursi, A.; Onidi, F.M.; Usai-Satta, P.; Pes, G.M.; Dore, M.P. Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms. J. Clin. Med. 2024, 13, 1519. [Google Scholar] [CrossRef]
- Naganuma, M.; Shiga, H.; Shimoda, M.; Matsuura, M.; Takenaka, K.; Fujii, T.; Yamamoto, S.; Matsubayashi, M.; Kobayashi, T.; Aoyama, N.; et al. First-line biologics as a treatment for ulcerative colitis: A multicenter randomized control study. J. Gastroenterol. 2025, 60, 430–441. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, J.; Wu, R.; Long, S.; Chen, W.; Lu, T.; Xia, Q.; Wu, Y.; Yang, H.; Yang, Q.; et al. Comparison of clinical and endoscopic efficacy between vedolizumab and infliximab in bio-naïve patients with ulcerative colitis: A multicenter, real-world study. Therap. Adv. Gastroenterol. 2024, 17, 17562848241281218. [Google Scholar] [CrossRef] [PubMed]
- Bressler, B.; Yarur, A.; Silverberg, M.S.; Bassel, M.; Bellaguarda, E.; Fourment, C.; Gatopoulou, A.; Karatzas, P.; Kopylov, U.; Michalopoulos, G.; et al. Vedolizumab and Anti-Tumour Necrosis Factor α Real-World Outcomes in Biologic-Naïve Inflammatory Bowel Disease Patients: Results from the EVOLVE Study. J. Crohn’s Colitis 2021, 15, 1694–1706. [Google Scholar] [CrossRef]
- Vickers, A.D.; Ainsworth, C.; Mody, R.; Bergman, A.; Ling, C.S.; Medjedovic, J.; Smyth, M. Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis. PLoS ONE 2016, 11, e0165435. [Google Scholar] [CrossRef]
- Meng, R.P.; Huang, B.B.; Wei, Y.L.; Lyu, L.; Yang, H.; Liu, C.; Zhou, H.L.; Liao, X.P.; Zhou, J.Y.; Xie, X. Effectiveness and safety of vedolizumab and infliximab in biologic-naïve patients with moderate-to-severe ulcerative colitis: A multicenter, retrospective cohort study. J. Dig. Dis. 2024, 25, 230–237. [Google Scholar] [CrossRef]
- Domènech, E.; Grífols, J.R.; Akbar, A.; Dignass, A.U. Use of granulocyte/monocytapheresis in ulcerative colitis: A practical review from a European perspective. World J. Gastroenterol. 2021, 27, 908–918. [Google Scholar] [CrossRef]
- Goss Sawhney, T.; Dobes, A.; O’Charoen, S. Real-World Persistency for Inflammatory Bowel Disease Biologics Using Patient Registry Data. Crohn’s Colitis 360 2023, 5, otad051. [Google Scholar] [CrossRef]
- Rodríguez-Lago, I.; Sempere, L.; Gutiérrez, A.; Núñez, A.; Leo Carnerero, E.; Hinojosa, E.; Mora, M.; Cañete, F.; Mañosa, M.; Herrera, C.; et al. Granulocyte-monocyte apheresis: An alternative combination therapy after loss of response to anti-TNF agents in ulcerative colitis. Scand. J. Gastroenterol. 2019, 54, 459–464. [Google Scholar] [CrossRef]
- Hanai, H.; Watanabe, F.; Yamada, M.; Sato, Y.; Takeuchi, K.; Iida, T.; Tozawa, K.; Tanaka, T.; Maruyama, Y.; Matsushita, I.; et al. Correlation of serum soluble TNF-alpha receptors I and II levels with disease activity in patients with ulcerative colitis. Am. J. Gastroenterol. 2004, 99, 1532–1538. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kamikozuru, K.; Watanabe, K.; Nakamura, S. Inflammatory bowel disease patients experiencing a loss of response to infliximab regain long-term response after undergoing granulocyte/monocyte apheresis: A case series. Cytokine 2018, 103, 25–28. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sawada, K.; Aoyama, N.; Yoshimura, N.; Sako, M.; Hirai, F.; Kashiwagi, N.; Suzuki, Y. Efficacy of Granulocyte and Monocyte Adsorptive Apheresis in Patients with Inflammatory Bowel Disease Showing Lost Response to Infliximab. J. Crohn’s Colitis 2020, 14, 1264–1273. [Google Scholar] [CrossRef]

| Age (years, mean ± SD) | 18–85 (47.0 ± 17.7) | |
| Sex | Male 13, Female 17 | |
| Disease duration from diagnosis (month, mean ± SD) | 10–276 (102.6 ± 81.6) | |
| Disease extent | Left-sided colitis | 8 |
| Pancolitis | 22 | |
| Disease refractory type | Steroid-dependent | 18 |
| Steroid-refractory | 12 | |
| Efficacy of the previous CAP | ||
| Non-response | 16 | |
| Insufficient response | 14 | |
| Mean CAI * (mean ± SE) at CAP initiation | 9.70±0.45 | |
| Duration between CAP failure and biologic initiation | 1–4015 days (median 56 days) | |
| Mean CAI (mean ± SE) at initiation of biologics | 8.07 ± 0.52 | |
| Mean serum albumin concentration (mean ± SE) at initiation of biologics | 3.66 ± 0.10 g/dL | |
| Name of the biologics used | ||
| Infliximab | 11 | |
| Adalimumab | 11 | |
| Vedolizumab | 7 | |
| Ustekinumab | 1 | |
| Medication (before biologic therapy) | ||
| PSL ** | Yes 30, No 0 | |
| 5-ASA *** | Yes 28, No 2 | |
| Thiopurines | Yes 19, No 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iizuka, M.; Etou, T.; Yorozu, H.; Kato, B.; Edagawa, T.; Sagara, S.; Matsushita, H.-O.; Yoshikawa, K. Efficacy of Biologics in Patients with Ulcerative Colitis Exhibiting Non-Response or Insufficient Response to Cytapheresis. J. Clin. Med. 2025, 14, 6574. https://doi.org/10.3390/jcm14186574
Iizuka M, Etou T, Yorozu H, Kato B, Edagawa T, Sagara S, Matsushita H-O, Yoshikawa K. Efficacy of Biologics in Patients with Ulcerative Colitis Exhibiting Non-Response or Insufficient Response to Cytapheresis. Journal of Clinical Medicine. 2025; 14(18):6574. https://doi.org/10.3390/jcm14186574
Chicago/Turabian StyleIizuka, Masahiro, Takeshi Etou, Haruka Yorozu, Bunichiro Kato, Takeya Edagawa, Shiho Sagara, Hiro-O Matsushita, and Kenjiro Yoshikawa. 2025. "Efficacy of Biologics in Patients with Ulcerative Colitis Exhibiting Non-Response or Insufficient Response to Cytapheresis" Journal of Clinical Medicine 14, no. 18: 6574. https://doi.org/10.3390/jcm14186574
APA StyleIizuka, M., Etou, T., Yorozu, H., Kato, B., Edagawa, T., Sagara, S., Matsushita, H.-O., & Yoshikawa, K. (2025). Efficacy of Biologics in Patients with Ulcerative Colitis Exhibiting Non-Response or Insufficient Response to Cytapheresis. Journal of Clinical Medicine, 14(18), 6574. https://doi.org/10.3390/jcm14186574







