Predictive Measurement of Urethral Mobility for Successful Transurethral Bulkamid Application in Women with Stress Urinary Incontinence
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEKNO | North Rhine Westphalia Medical Association |
| ALPP | Abdominal leak point pressure |
| CI | Confidence interval |
| EC | Endoscopic colposuspension |
| ICIQ-UI SF | International Consultation on Incontinence Modular Questionnaire Urinary Incontinence short form |
| LDM | Linear dorsocaudal movement |
| SUI | Stress urinary incontinence |
| TVT | Tension-free vaginal tape |
| UI | Urinary incontinence |
References
- Naumann, G.; Aigmuller, T.; Bader, W.; Bauer, R.; Beilecke, K.; Betschart Meier, C.; Bruer, G.; Bschleipfer, T.; Deniz, M.; Fink, T.; et al. Diagnosis and Therapy of Female Urinary Incontinence. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry No. 015/091, January 2022): Part 1 with Recommendations on Diagnostics and Conservative and Medical Treatment. Geburtshilfe Frauenheilkd. 2023, 83, 377–409. [Google Scholar] [CrossRef]
- Naumann, G.; Aigmuller, T.; Bader, W.; Bauer, R.; Beilecke, K.; Betschart Meier, C.; Bruer, G.; Bschleipfer, T.; Deniz, M.; Fink, T.; et al. Diagnosis and Therapy of Female Urinary Incontinence. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No. 015/091, January 2022): Part 2 with Recommendations on Interventional/Surgical Therapy of Overactive Bladder, Surgical Treatment of Stress Urinary Incontinence and Diagnosis and Therapy of Iatrogenic Urogenital Fistula. Geburtshilfe Frauenheilkd. 2023, 83, 410–436. [Google Scholar] [CrossRef]
- Toozs-Hobson, P.; Al-Singary, W.; Fynes, M.; Tegerstedt, G.; Lose, G. Two-year follow-up of an open-label multicenter study of polyacrylamide hydrogel (Bulkamid®) for female stress and stress-predominant mixed incontinence. Int. Urogynecol. J. 2012, 23, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.R.; Karram, M.M.; Dmochowski, R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: A randomized, prospective, multicenter North American study. J. Urol. 2014, 192, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.P. Ultrasound imaging of the pelvic floor. Part I: Two-dimensional aspects. Ultrasound Obstet. Gynecol. 2004, 23, 80–92. [Google Scholar] [CrossRef]
- Viereck, V.; Pauer, H.U.; Hesse, O.; Bader, W.; Tunn, R.; Lange, R.; Hilgers, R.; Emons, G. Urethral hypermobility after anti-incontinence surgery—A prognostic indicator? Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006, 17, 586–592. [Google Scholar] [CrossRef]
- Yune, J.J.; Quiroz, L.; Nihira, M.A.; Siddighi, S.; O’Leary, D.E.; Santiago, A.; Shobeiri, S.A. The Location and Distribution of Transurethral Bulking Agent: 3-Dimensional Ultrasound Study. Female Pelvic Med. Reconstr. Surg. 2016, 22, 98–102. [Google Scholar] [CrossRef]
- Wlazlak, E.; Viereck, V.; Kociszewski, J.; Kuszka, A.; Rautenberg, O.; Walser, C.; Surkont, G.; Gamper, M.; Fehr, M.K. Role of intrinsic sphincter deficiency with and without urethral hypomobility on the outcome of tape insertion. Neurourol. Urodyn. 2017, 36, 1910–1916. [Google Scholar] [CrossRef]
- Viereck, V.; Nebel, M.; Bader, W.; Harms, L.; Lange, R.; Hilgers, R.; Emons, G. Role of bladder neck mobility and urethral closure pressure in predicting outcome of tension-free vaginal tape (TVT) procedure. Ultrasound Obstet. Gynecol. 2006, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, C.; Shek, K.L.; Martin, A.J.; Dietz, H.P. What is normal bladder neck anatomy? Int. Urogynecol. J. 2016, 27, 945–950. [Google Scholar] [CrossRef]
- Robinson, D.; Anders, K.; Cardozo, L.; Bidmead, J.; Dixon, A.; Balmforth, J.; Rufford, J. What Do Women Want?: Interpretation of the Concept of Cure. Female Pelvic Med. Reconstr. Surg. 2003, 9, 273–277. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Giammo, A.; Geretto, P.; Ammirati, E.; Manassero, A.; Squintone, L.; Vercelli, D.; Carone, R. Urethral bulking with Bulkamid: An analysis of efficacy, safety profile, and predictors of functional outcomes in a single-center cohort. Neurourol. Urodyn. 2020, 39, 1523–1528. [Google Scholar] [CrossRef]
- Dietz, H.P.; Clarke, B.; Herbison, P. Bladder neck mobility and urethral closure pressure as predictors of genuine stress incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2002, 13, 289–293. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.E.; Apostolidis, A.; Birder, L.; Bliss, D.; Brubaker, L.; Cardozo, L.; Castro-Diaz, D.; O’Connell, P.R.; Cottenden, A.; et al. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and Treatment of Urinary Incontinence, Pelvic Organ Prolapse and Faecal Incontinence. Neurourol. Urodyn. 2018, 37, 2271–2272. [Google Scholar] [CrossRef]
- Ghoniem, G.; Boctor, N. Update on urethral bulking agents for female stress urinary incontinence due to intrinsic sphincter deficiency. J. Urol. Res. 2014, 1, 1009. [Google Scholar]
- Kirchin, V.; Page, T.; Keegan, P.E.; Atiemo, K.O.; Cody, J.D.; McClinton, S.; Aluko, P. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst. Rev. 2017, 7, CD003881. [Google Scholar] [CrossRef]
- Kerr, L.A. Bulking agents in the treatment of stress urinary incontinence: History, outcomes, patient populations, and reimbursement profile. Rev. Urol. 2005, 7 (Suppl. S1), S3–S11. [Google Scholar] [PubMed]
- Klarskov, N.; Lose, G. Urethral injection therapy: What is the mechanism of action? Neurourol. Urodyn. 2008, 27, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Appell, R.A.; Dmochowski, R.R.; Herschorn, S. Urethral injections for female stress incontinence. BJU Int. 2006, 98 (Suppl. S1), 27–30; discussion 31. [Google Scholar] [CrossRef] [PubMed]
- Ulmsten, U.; Falconer, C. Connective tissue in female urinary incontinence. Curr. Opin. Obstet. Gynecol. 1999, 11, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.aekno.de/aerzte/berufsordnung#_15 (accessed on 28 March 2025).
| Post Hoc Subgroups | |||||
|---|---|---|---|---|---|
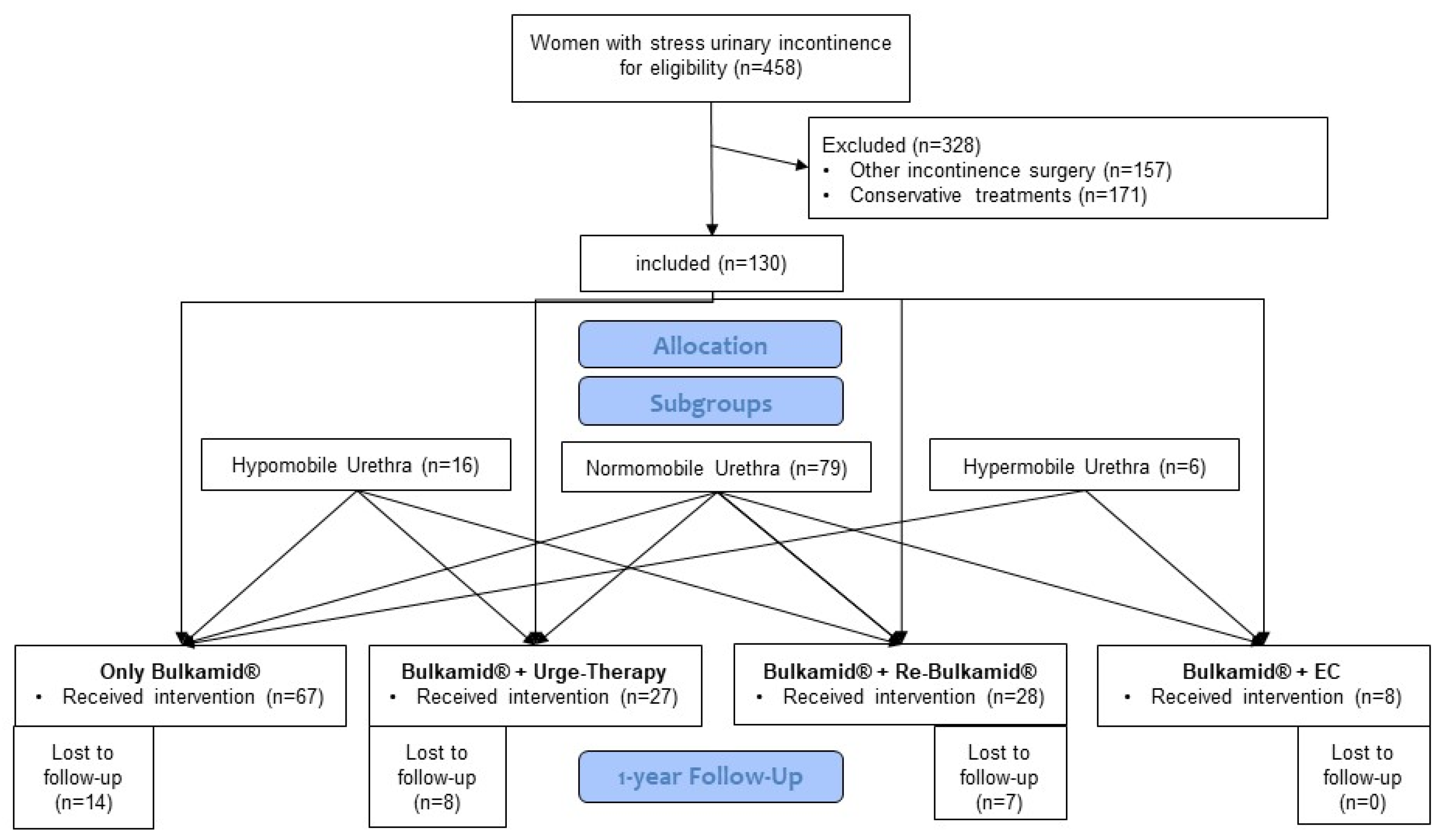
| Overall Cohort (n = 130) | Bulkamid (n = 67) | Urge-Therapy (n = 27) | Re-Bulkamid (n = 28) | Endoscopic Colposuspension (n = 8) | |
| Age in years (median, Q1, Q3) | 69 (61; 77) | 69 (63; 74) | 71 (66; 80) | 73.5 (64; 79) | 56 (48; 63) |
| Preoperative ICIQ-UI SF (mean, SD) | 14.7 (SD 11.2–18.2) | 14.1 (10.7; 17.5) | 14.9 (11.5; 18.3) | 15.4 (11.4; 19.4) | 16.0 (13.2; 18.8) |
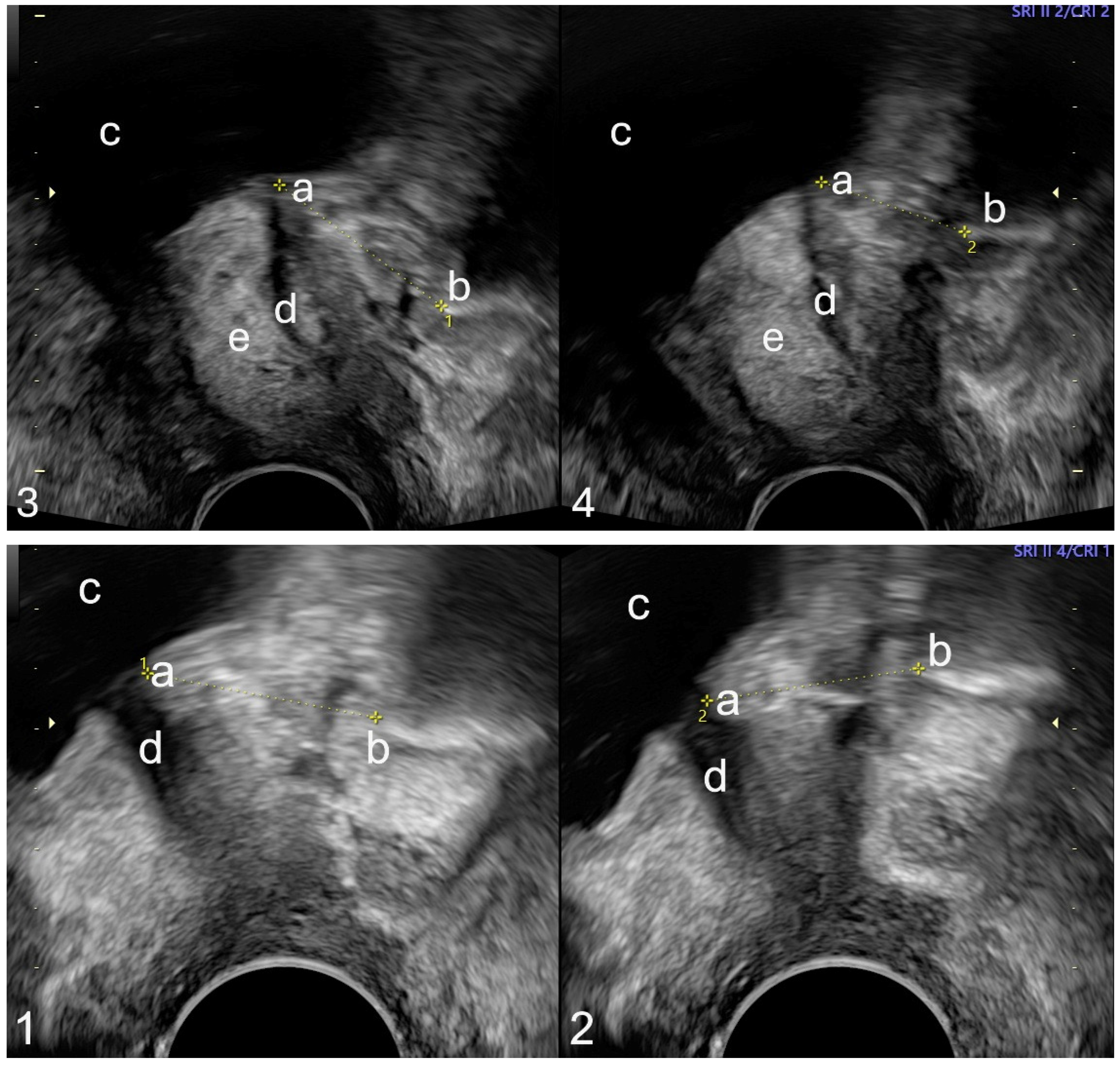
| Urethra length in mm (median, Q1, Q3) | 28 (26; 31) | 28 (27; 31) | 29 (26; 30) | 28.5 (26.5; 30.5) | 28.5 (26.5; 30.5) |
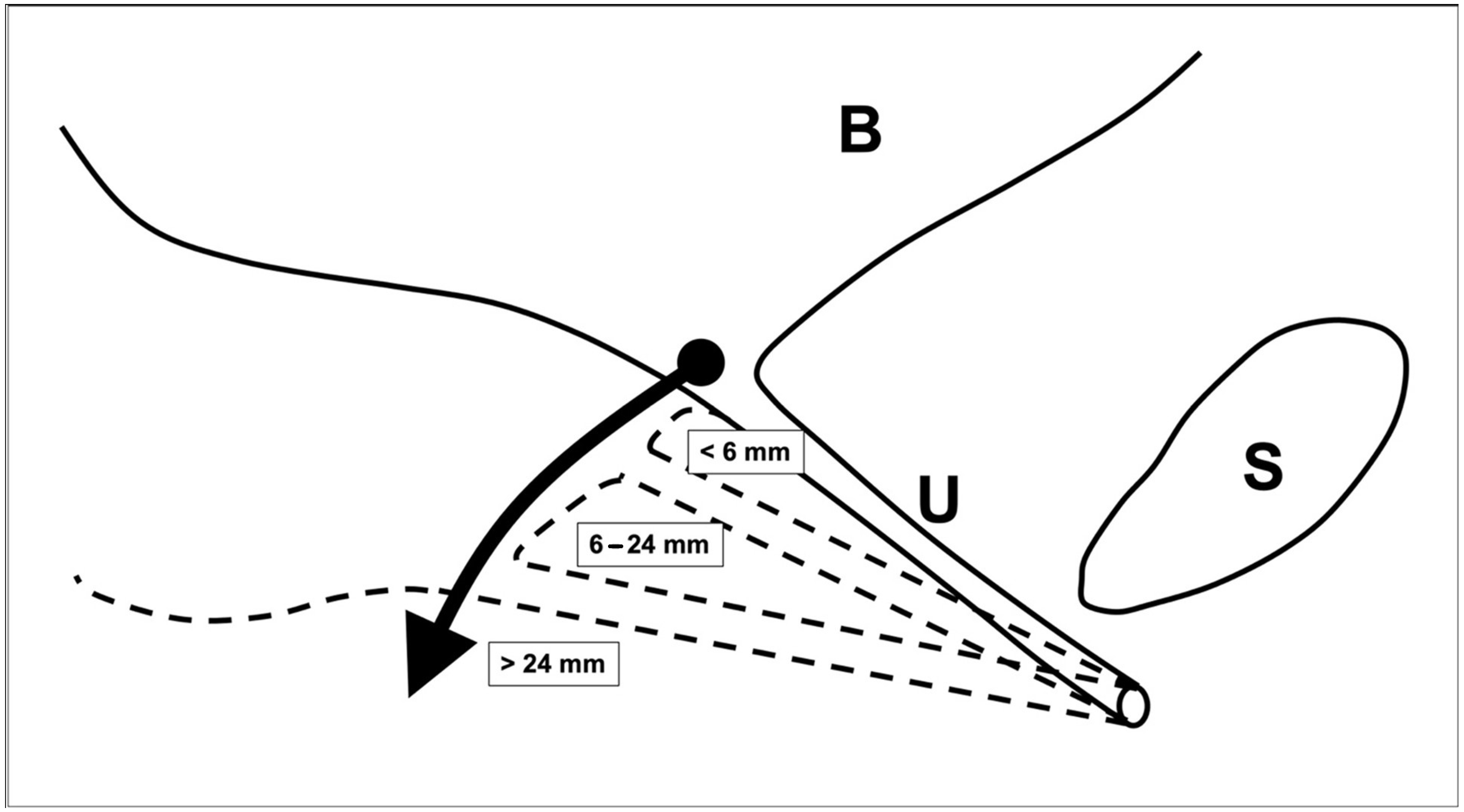
| Urethra mobility in mm (median, Q1, Q3) (n = 107) | 11 (6; 17) | 8.5 (7.0; 13.5) | 11 (4; 20) | 10 (6; 16) | 24 (15; 30) |
| Pretreated with TVT (n) | 13 | 5 | 2 | 5 | 1 |
| OP-time (min) (median, Q1, Q3) | 9 (7; 10) | 9 (7; 10) | 8.5 (7; 11) | 9 (7; 11) | 8 (7; 11) |
| Anesthetics | |||||
| Local (n) | 29 | 15 | 6 | 6 | 1 |
| General (n) | 108 | 52 | 21 | 22 | 7 |
| Type of Surgery | Time Points of Measurement | n | ICIQ-UI SF Score Mean Value (95%CI) | Absolute Difference from Initial Value (95% CI) | Relative Difference from Initial Value (95% CI) |
|---|---|---|---|---|---|
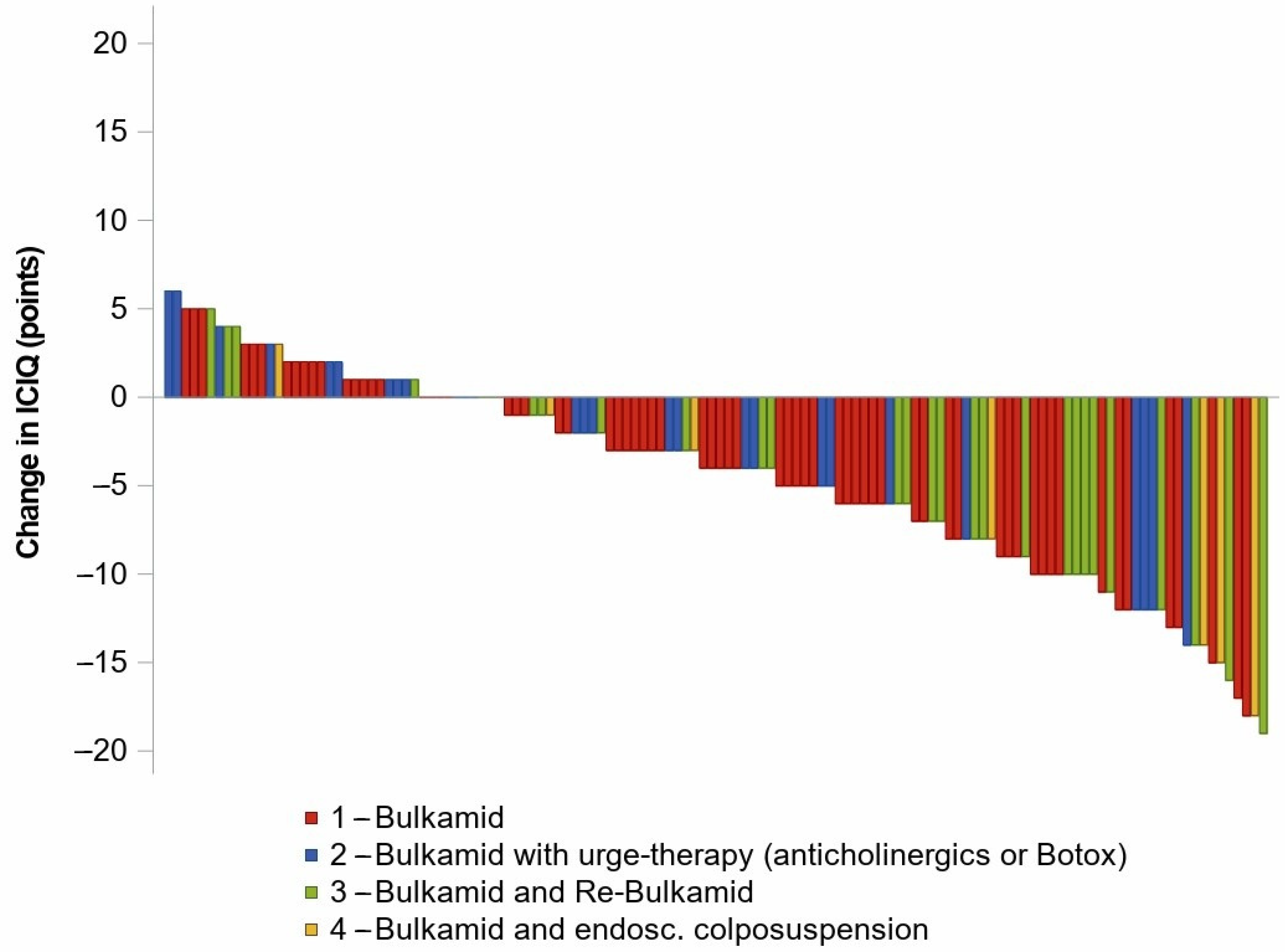
| Bulkamid | before Bulkamid | 67 | 14.1 (13.2; 14.9) | - | - |
| after Bulkamid | 67 | 9.9 (8.6; 11.2) | −4.1 (−5.5; −2.8) | −0.28 (−0.38; −0.18) | |
| Re-Bulkamid | before Bulkamid | 28 | 15.4 (13.9; 17.0) | - | - |
| after Bulkamid | 28 | 12.1 (10.1; 14.2) | −3.3 (−5.4; −1.2) | −0.19 (−0.33; −0.05) | |
| after Re-Therapy | 28 | 9.6 (7.3; 11.9) | −5.6 (−8.2; −3.5) | −0.36 (−0.52; −0.21) |
| Mobility | n (Total) | Cured (n) | ICIQ-UI SF Score Change After Bulkamid® (95% CI) | Improved (n) | n (After Re-Bulkamid®) | ICIQ-UI SF Score Change After Re-Bulkamid® (95% CI) |
|---|---|---|---|---|---|---|
| Hypomobile (<6 mm) | 16 | 0 | −0.8 (−2.7; 1.1) | 8 | 5 | −0.8 (−6.5; 4.9) |
| Normomobile (6–24 mm) | 79 | 13 | −3.8 (−5.0; −2.5) | 41 | 28 | −6.3 (−8.7; −3.9) |
| Hypermobile (>24 mm) | 6 | 0 | −2.5 (−7.7; 2.7) | 3 | 2 | −4.5 (−62.7; 51.7) |
| Lost for follow-up | 29 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosal, N.; Gerling, A.; Kuhn, A.; Pfleiderer, M.; Baek, S.; Ludwig, S. Predictive Measurement of Urethral Mobility for Successful Transurethral Bulkamid Application in Women with Stress Urinary Incontinence. J. Clin. Med. 2025, 14, 6555. https://doi.org/10.3390/jcm14186555
Nosal N, Gerling A, Kuhn A, Pfleiderer M, Baek S, Ludwig S. Predictive Measurement of Urethral Mobility for Successful Transurethral Bulkamid Application in Women with Stress Urinary Incontinence. Journal of Clinical Medicine. 2025; 14(18):6555. https://doi.org/10.3390/jcm14186555
Chicago/Turabian StyleNosal, Norbert, Andrea Gerling, Annette Kuhn, Mathieu Pfleiderer, Sunhwa Baek, and Sebastian Ludwig. 2025. "Predictive Measurement of Urethral Mobility for Successful Transurethral Bulkamid Application in Women with Stress Urinary Incontinence" Journal of Clinical Medicine 14, no. 18: 6555. https://doi.org/10.3390/jcm14186555
APA StyleNosal, N., Gerling, A., Kuhn, A., Pfleiderer, M., Baek, S., & Ludwig, S. (2025). Predictive Measurement of Urethral Mobility for Successful Transurethral Bulkamid Application in Women with Stress Urinary Incontinence. Journal of Clinical Medicine, 14(18), 6555. https://doi.org/10.3390/jcm14186555







