Is Less More? Limited Surgery Is Insufficient in the Treatment of Spinal Hydatid Cysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Collection of Clinical and Surgical Data
2.3. Radiological and Laboratory Assessments
2.4. Methods of Surgery
2.5. Follow-Up and Outcome Measures
2.6. Statistical Analysis
2.7. Ethical Approval and Informed Consent
3. Results
3.1. Patient Demographics and Clinical Presentation
3.2. Radiological Findings
3.3. Surgical Outcomes and Perioperative Parameters
3.4. Long-Term Outcomes and Recurrence Patterns
3.5. Laboratory and Serological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLDS | Cystectomy, laminectomy, decompression, and stabilization |
| CRP | C-reactive protein |
| CT | Computed tomography |
| ELISA | Enzyme-linked immunosorbent assay |
| ESR | Erythrocyte sedimentation rate |
| IgG | Immunoglobulin G |
| IHA | Indirect hemagglutination |
| MRI | Magnetic resonance imaging |
| SD | Standard deviation |
| TES | Total en bloc spondylectomy |
References
- Mijiti, W.; Wang, X.; Jia, Q.; Li, Y.; Zhou, Z.; Reheman, T.; Ayiheng, Y.; Dong, S.; Xie, Z. Challenges and pitfalls in managing lumbosacral hydatid disease: Lessons learned from clinical practice. Diagn. Microbiol. Infect. Dis. 2024, 110, 116542. [Google Scholar] [CrossRef] [PubMed]
- Inayat, F.; Azam, S.; Baig, A.S.; Nawaz, G.; Ullah, W. Presentation Patterns, Diagnostic Modalities, Management Strategies, and Clinical Outcomes in Patients with Hydatid Disease of the Pelvic Bone: A Comparative Review of 31 Cases. Cureus 2019, 11, e4178. [Google Scholar] [CrossRef] [PubMed]
- Sioutis, S.; Reppas, L.; Bekos, A.; Soulioti, E.; Saranteas, T.; Koulalis, D.; Sapkas, G.; Mavrogenis, A.F. Echinococcosis of the spine: A comprehensive review. EFORT Open Rev. 2021, 6, 288–296. [Google Scholar] [CrossRef]
- Meng, Y.; Ren, Q.; Xiao, J.; Sun, H.; Huang, Y.; Liu, Y.; Wang, S.; Wang, S. Progress of research on the diagnosis and treatment of bone cystic echinococcosis. Front. Microbiol. 2023, 14, 1273870. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Prasad, G.; Rustagi, T.; Bhojraj, S.Y. Hydatid disease of spine: Multiple meticulous surgeries and a long term followup. Indian J. Orthop. 2014, 48, 529–532. [Google Scholar] [CrossRef]
- Gezercan, Y.; Ökten, A.I.; Çavuş, G.; Açık, V.; Bilgin, E. Spinal Hydatid Cyst Disease. World Neurosurg. 2017, 108, 407–417. [Google Scholar] [CrossRef]
- Neumayr, A.; Tamarozzi, F.; Goblirsch, S.; Blum, J.; Brunetti, E. Spinal cystic echinococcosis—A systematic analysis and review of the literature: Part 1. Epidemiology and anatomy. PLoS Negl. Trop. Dis. 2013, 7, e2450. [Google Scholar] [CrossRef]
- Braithwaite, P.A.; Lees, R.F. Vertebral hydatid disease: Radiological assessment. Radiology 1981, 140, 763–766. [Google Scholar] [CrossRef]
- Song, X.H.; Ding, L.W.; Wen, H. Bone hydatid disease. Postgrad. Med. J. 2007, 83, 536–542. [Google Scholar] [CrossRef]
- Pamir, M.N.; Ozduman, K.; Elmaci, I. Spinal hydatid disease. Spinal Cord 2002, 40, 153–160. [Google Scholar] [CrossRef]
- Herrera, A.; Martinez, A.A.; Rodriguez, J. Spinal hydatidosis. Spine 2005, 30, 2439–2444. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Liang, Q.; Xiang, H.; Xu, L.; Wen, H.; Tian, Z.; Yunus, A.; Wang, C.; Jiang, D.; Abuduwaili, M.; Chen, J.; et al. Treatment experiences of thoracic spinal hydatidosis: A single-center case-series study. Int. J. Infect. Dis. 2019, 89, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Somay, H.; Ayan, E.; Turk, C.C.; Emon, S.T.; Berkman, M.Z. Long-term disseminated recurrence in spinal hydatid cyst: A case report. Turk. Neurosurg. 2014, 24, 78–81. [Google Scholar]
- Phan, K.; Leung, V.; Scherman, D.B.; Tan, A.R.; Rao, P.J.; Mobbs, R.J. Bilateral versus unilateral instrumentation in spinal surgery: Systematic review and trial sequential analysis of prospective studies. J. Clin. Neurosci. 2016, 30, 15–23. [Google Scholar] [CrossRef]
- Kaloostian, P.E.; Gokaslan, Z.L. Spinal hydatid disease: A multidisciplinary pathology. World Neurosurg. 2014, 80, 620–626. [Google Scholar] [CrossRef]
- Caglar, Y.S.; Ozgural, O.; Zaimoglu, M.; Kilinc, C.; Eroglu, U.; Dogan, I.; Kahilogullari, G. Spinal hydatid cyst disease: Challenging surgery—An institutional experience. J. Korean Neurosurg. Soc. 2019, 62, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Akhaddar, A.; Boucetta, M. A new surgical technique for the removal of extensive spinal epidural hydatid cyst. Surg. Neurol. Int. 2018, 9, 96. [Google Scholar] [CrossRef]
- Sharma, J.K.; Tandon, V.; Marathe, N.A.; Agrahari, Y.; Mallepally, A.R.; Chhabra, H.S. Hydatid cyst of the spine: A rare case report and review of literature. J. Orthop. Case Rep. 2020, 10, 57–59. [Google Scholar]
- Smith, I.; Bleibleh, S.; Hartley, L.J.; Rehousek, P.; Hughes, S.; Grainger, M.; Jones, M. Blood loss in total en bloc spondylectomy for primary spinal bone tumours: A comparison of estimated blood loss versus actual blood loss in a single centre over 10 years. J. Spine Surg. 2022, 8, 353–361. [Google Scholar] [CrossRef] [PubMed]
- De la Garza Ramos, R.; Ryvlin, J.; Yassari, R. Blood loss after total en bloc spondylectomy. J. Spine Surg. 2022, 8, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Naumov, D.G.; Tkach, S.G.; Aliev, G.B.; Vishnevsky, A.A.; Yablonsky, P.K. Surgical Treatment of Chronic Infectious Cervicothoracic Spondylitis. Traumatol. Orthop. Russ. 2025, 31, 43–54. [Google Scholar] [CrossRef]
- Majmundar, N.; Patel, P.D.; Dodson, V.; Tran, A.; Goldstein, I.; Assina, R. Parasitic infections of the spine: Case series and review of the literature. Neurosurg. Focus 2019, 46, E12. [Google Scholar] [CrossRef]
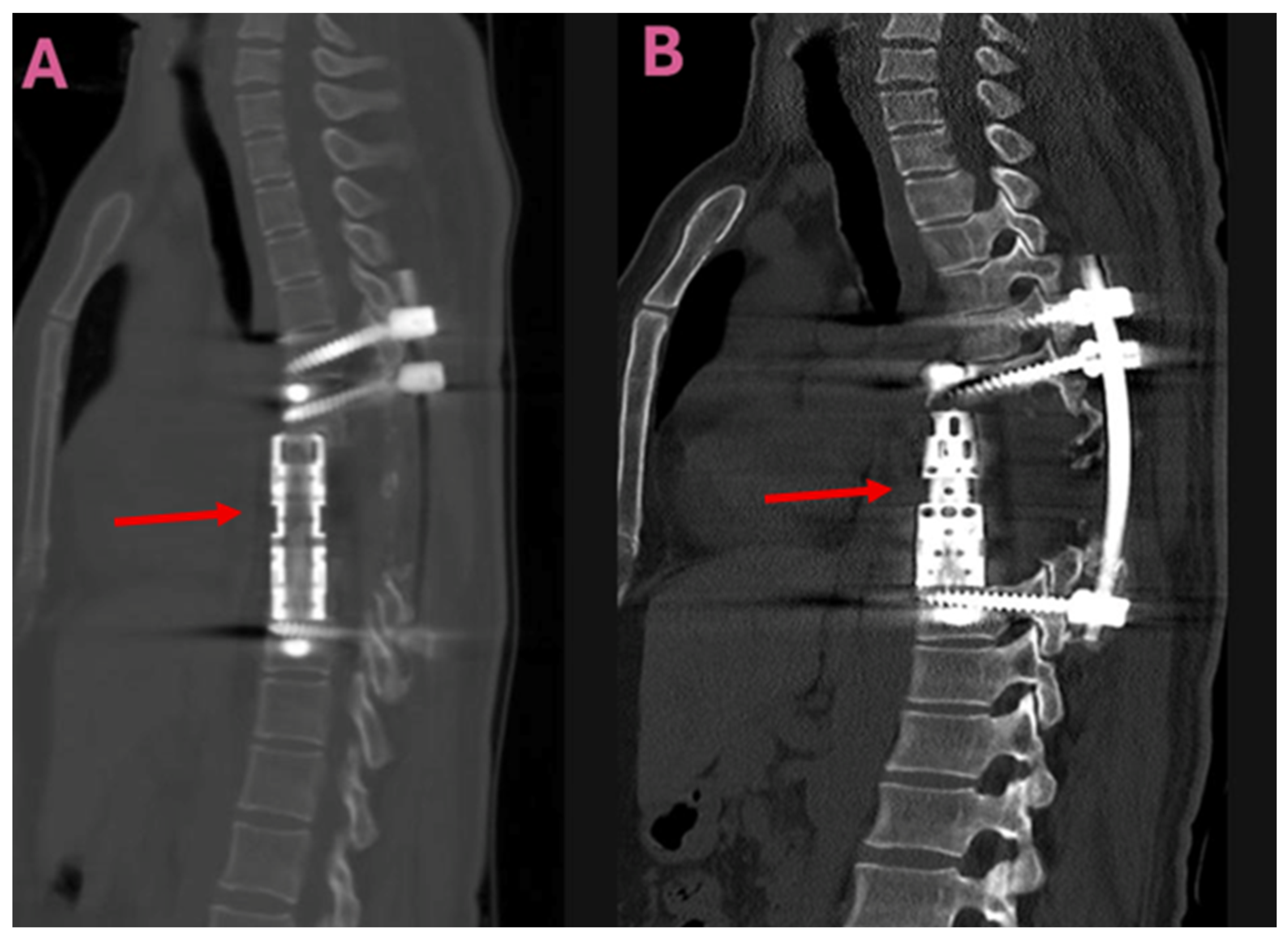
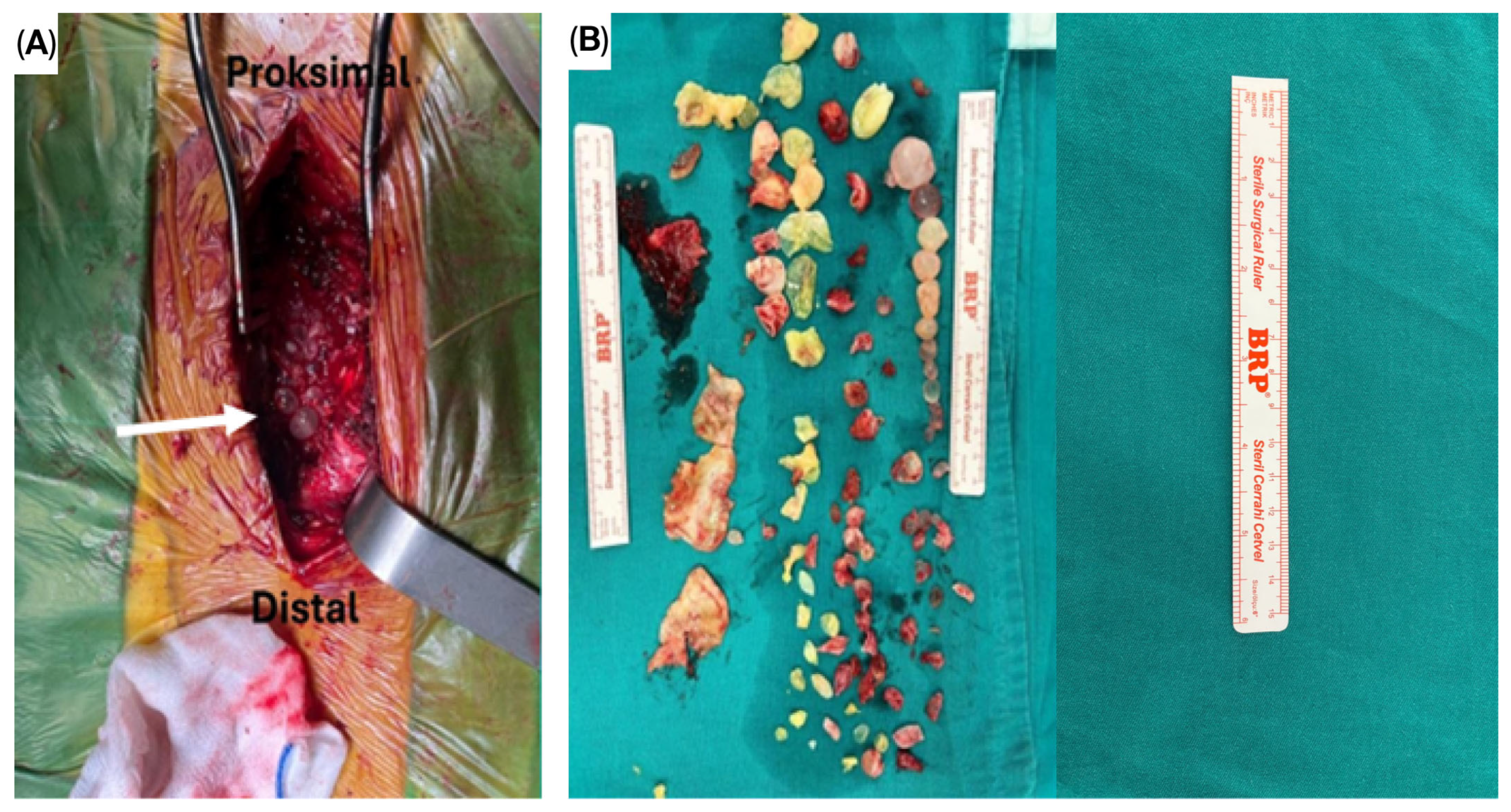

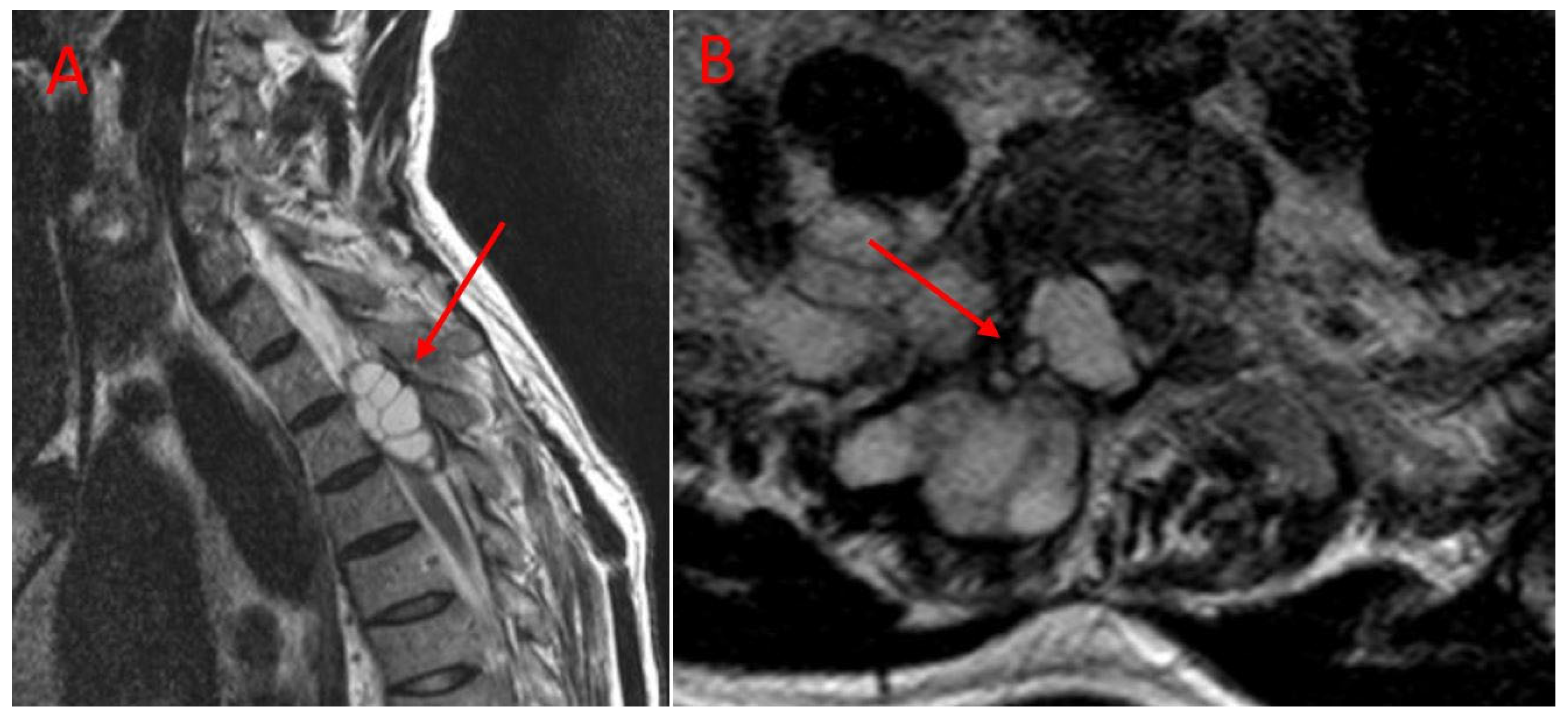

| Parameter | TES Group (n = 9) | CLDS Group (n = 12) | p Value |
|---|---|---|---|
| Age, years * | 40.5 (20–65) | 44.2 (22–68) | 0.342 ᵃ |
| Gender, n (%) | 0.685 ᵇ | ||
| Male | 6 (66.7) | 7 (58.3) | |
| Female | 3 (33.3) | 5 (41.7) | |
| Symptom duration, months * | 8.5 (3–24) | 10.2 (4–36) | 0.456 ᵃ |
| Presenting symptoms, n (%) | |||
| Back pain | 9 (100) | 12 (100) | 1.000 ᵇ |
| Motor deficit | 5 (55.6) | 7 (58.3) | 0.898 ᵇ |
| Sensory deficit | 3 (33.3) | 5 (41.7) | 0.695 ᵇ |
| Sphincter dysfunction | 1 (11.1) | 2 (16.7) | 0.721 ᵇ |
| Preoperative Frankel grade, n (%) | 0.812 ᵃ | ||
| A, B | 1 (11.1) | 2 (16.7) | |
| C | 3 (33.3) | 4 (33.3) | |
| D | 4 (44.5) | 5 (41.7) | |
| E | 1 (11.1) | 1 (8.3) | |
| Hepatic hydatid cyst | 2 (22.2) | 3 (25.0) | 0.883 ᵇ |
| Parameter | TES Group (n = 9) | CLDS Group (n = 12) | p Value |
|---|---|---|---|
| Lesion location, n (%) | 0.754 ᵇ | ||
| Thoracic | 7 (77.8) | 10 (83.3) | |
| Lumbar | 1 (11.1) | 2 (16.7) | |
| Thoracolumbar | 1 (11.1) | 0 (0) | |
| Affected vertebrae * | 2 (1–3) | 2 (1–4) | 0.234 ᵃ |
| Vertebral body involvement | 9 (100) | 0 (0) | <0.001 ᵇ |
| Posterior element involvement | 7 (77.8) | 12 (100) | 0.075 ᵇ |
| Paravertebral extension | 3 (33.3) | 6 (50.0) | 0.449 ᵇ |
| Spinal canal invasion >50% | 5 (55.6) | 8 (66.7) | 0.606 ᵇ |
| Parameter | TES Group (n = 9) | CLDS Group (n = 12) | p Value |
|---|---|---|---|
| Operative time, minutes † | 275 ± 30 | 185 ± 25 | <0.001 ᵃ |
| Intraoperative blood loss, mL † | 700 ± 150 | 450 ± 100 | 0.002 ᵃ |
| Transfusion requirement | 9 (100) | 4 (33.3) | 0.002 ᵇ |
| Controlled cyst rupture | 1 (11.1) | 4 (33.3) | 0.238 ᵇ |
| Dural injury | 1 (11.1) | 2 (16.7) | 0.222 ᵇ |
| Anterior column reconstruction | <0.001 ᵇ | ||
| Titanium cage | 9 (100) | 0 (0) |
| Parameter | TES Group (n = 9) | CLDS Group (n = 12) | p Value |
|---|---|---|---|
| Follow-up duration, months † | 38.1 ± 4.5 | 38.1 ± 4.5 | 0.998 ᵃ |
| Postoperative Frankel grade, n (%) | 0.445 ᵃ | ||
| C | 0 (0) | 1 (8.3) | |
| D | 3 (33.3) | 5 (41.7) | |
| E | 6 (66.7) | 6 (50.0) | |
| Neurological improvement | 6 (66.7) | 7 (58.3) | 0.698 ᵇ |
| Recurrence | 0 (0) | 7 (58.3) | 0.004 ᵇ |
| Operations in recurrent cases † | - | 2.8 ± 0.6 (2–4) | - |
| Time to recurrence, months * | - | 14 (6–28) | - |
| Total operations | 9 | 26 | - |
| Wound infection | 1 (11.1) | 2 (16.7) | 0.721 ᵇ |
| Deep infection | 0 (0) | 1 (8.3) | 0.379 ᵇ |
| Implant failure | 0 (0) | 1 (8.3) | 0.379 ᵇ |
| Parameter | TES Group (n = 9) | CLDS Group (n = 12) | p Value |
|---|---|---|---|
| Postoperative albendazole duration, months * | 6 (6–6) | 6 (6–6) | 1.000 ᵃ |
| Preoperative eosinophilia (>4%) | 4 (44.4) | 6 (50.0) | 0.801 ᵇ |
| Preoperative IgG-ELISA positivity | 7 (77.8) | 9 (75.0) | 0.883 ᵇ |
| Indirect hemagglutination positivity | 6 (66.7) | 8 (66.7) | 1.000 ᵇ |
| ESR, mm/hour * | 28 (12–65) | 32 (15–72) | 0.567 ᵃ |
| CRP, mg/L * | 12 (3–45) | 15 (5–52) | 0.445 ᵃ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarac, M.E.; Boga, Z.; Olguner, S.K.; Arslan, A.; Çınkı, A.H.; Ozer, M.; Gezercan, Y. Is Less More? Limited Surgery Is Insufficient in the Treatment of Spinal Hydatid Cysts. J. Clin. Med. 2025, 14, 6540. https://doi.org/10.3390/jcm14186540
Sarac ME, Boga Z, Olguner SK, Arslan A, Çınkı AH, Ozer M, Gezercan Y. Is Less More? Limited Surgery Is Insufficient in the Treatment of Spinal Hydatid Cysts. Journal of Clinical Medicine. 2025; 14(18):6540. https://doi.org/10.3390/jcm14186540
Chicago/Turabian StyleSarac, Mustafa Emre, Zeki Boga, Semih Kivanc Olguner, Ali Arslan, Ahmet Hamit Çınkı, Mehmet Ozer, and Yurdal Gezercan. 2025. "Is Less More? Limited Surgery Is Insufficient in the Treatment of Spinal Hydatid Cysts" Journal of Clinical Medicine 14, no. 18: 6540. https://doi.org/10.3390/jcm14186540
APA StyleSarac, M. E., Boga, Z., Olguner, S. K., Arslan, A., Çınkı, A. H., Ozer, M., & Gezercan, Y. (2025). Is Less More? Limited Surgery Is Insufficient in the Treatment of Spinal Hydatid Cysts. Journal of Clinical Medicine, 14(18), 6540. https://doi.org/10.3390/jcm14186540





