Long-Term Effects of Tolvaptan Therapy on Total Kidney Volume and Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Data
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
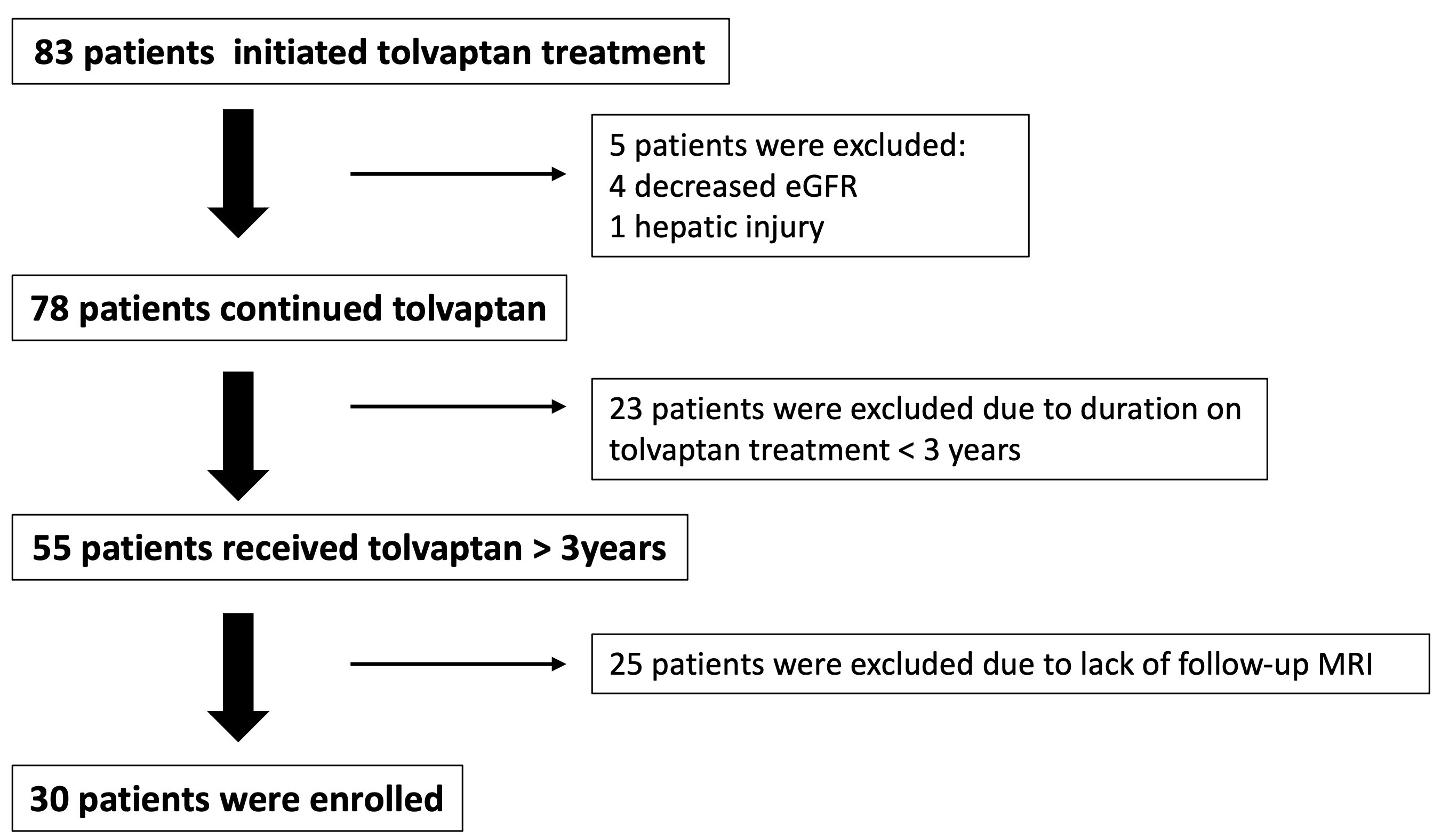
3.1. Study Cohort
3.2. Baseline Patient Characteristics
3.3. Tolvaptan Dosing
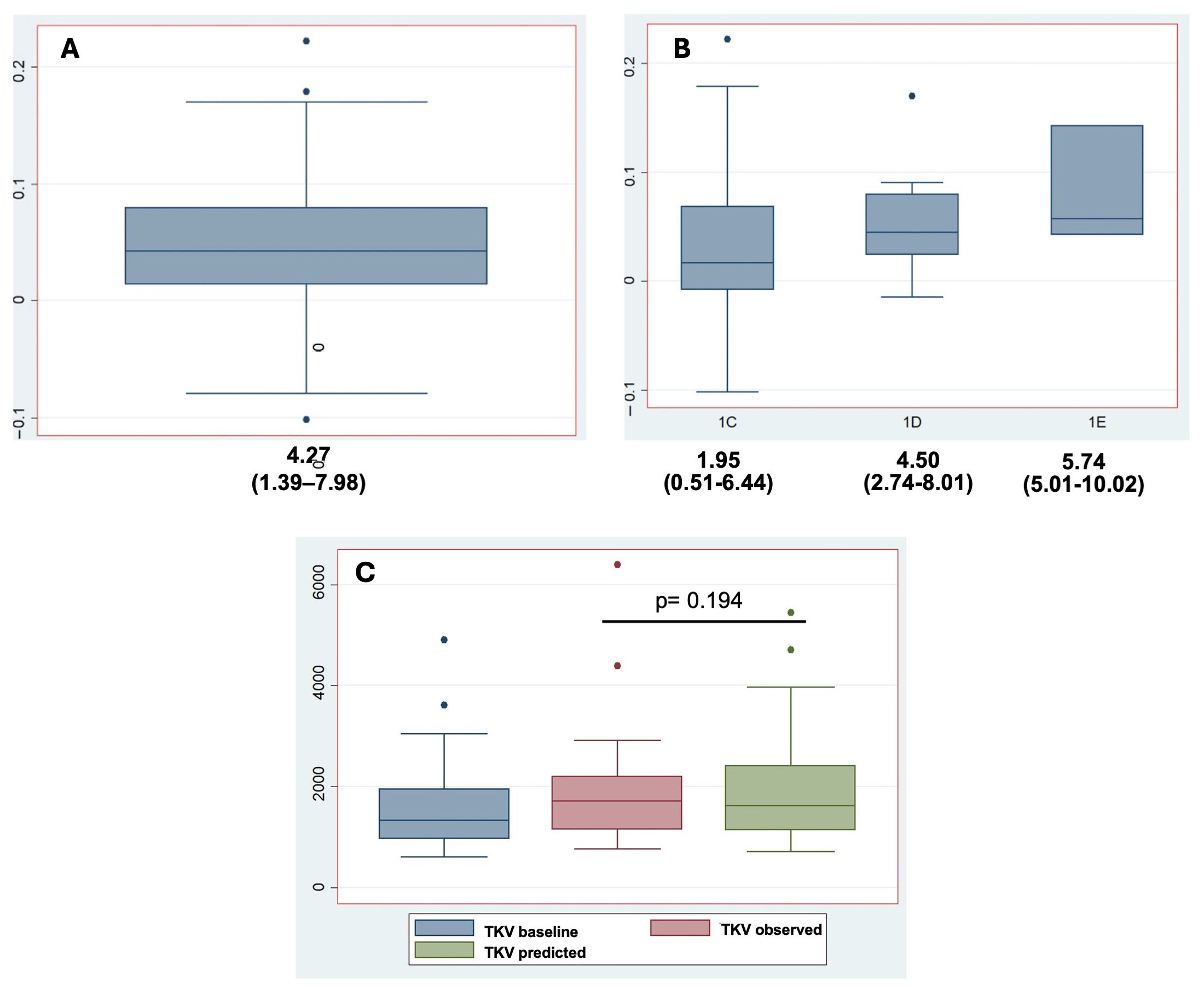
3.4. Tolvaptan Effects on TKV Growth
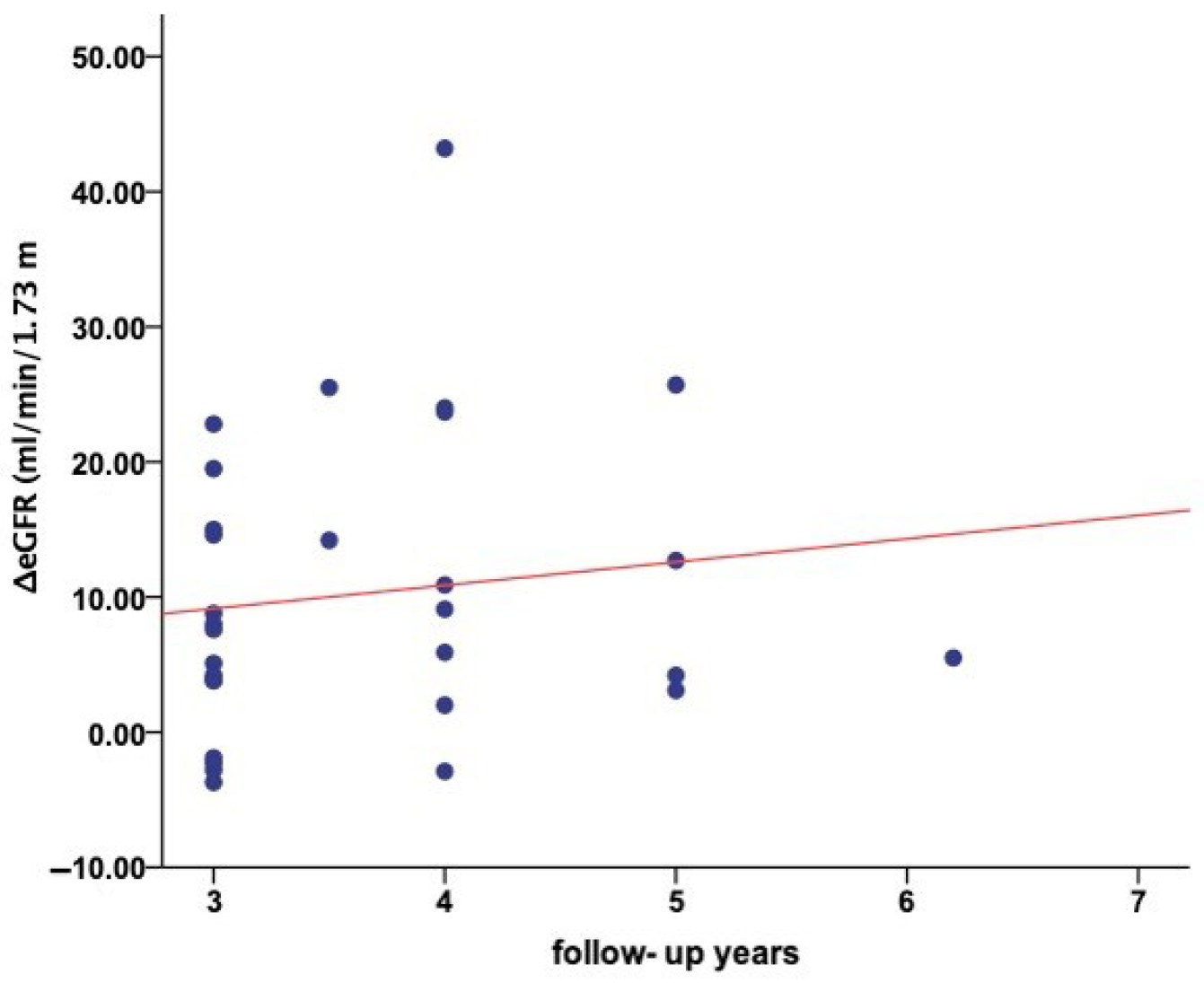
3.5. Tolvaptan Effects on eGFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADPKD | Autosomal Dominant Polycystic Kidney Disease |
| TKV | Total Kidney Volume |
| IQR | Interquartile Ranges |
| eGFR | estimated Glomerular Filtration Rate |
| ESKD | End-Stage Kidney Disease |
| cAMP | Cyclic Adenosine Monophosphate |
| MCIC | Mayo Clinic Imaging Classification |
| MRI | Magnetic Resonance Imaging |
| BMI | Body Mass Index |
References
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Grantham, J.J. Polycystic kidney disease: From the bedside to the gene and back. Curr. Opin. Nephrol. Hypertens. 2001, 10, 533–542. [Google Scholar] [CrossRef]
- Chapin, H.C.; Caplan, M.J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef]
- Reif, G.A.; Yamaguchi, T.; Nivens, E.; Fujiki, H.; Pinto, C.S.; Wallace, D.P. Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am. J. Physiol. Ren. Physiol. 2011, 301, F1005–F1013. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015, 26, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Torres, V.E.; Chapman, A.B.; Guay-Woodford, L.M.; Bae, K.T.; King, B.F.; Wetzel, L.H.; Baumgarten, D.A.; Kenney, P.J.; Harris, P.C.; et al. Volume Progression in Polycystic Kidney Disease. N. Engl. J. Med. 2006, 354, 2122–2130. [Google Scholar] [CrossRef]
- Chapman, A.B.; Bost, J.E.; Torres, V.E.; Guay-Woodford, L.; Bae, K.T.; Landsittel, D.; Li, J.; King, B.F.; Martin, D.; Wetzel, L.H.; et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 479–486. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Dandurand, A.; Ouyang, J.; Czerwiec, F.S.; Blais, J.D. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol. Dial. Transplant. 2018, 33, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, E.; Nutahara, K.; Itoh, M.; Okegawa, T.; Tambo, M.; Yamaguchi, T.; Nakamura, Y.; Taguchi, S.; Kaname, S.; Yokoyama, K.; et al. Long-Term Outcomes of Longitudinal Efficacy Study with Tolvaptan in ADPKD. Kidney Int. Rep. 2022, 7, 270–281. [Google Scholar] [CrossRef]
- Zhou, X.; Davenport, E.; Ouyang, J.; Hoke, M.E.; Garbinsky, D.; Agarwal, I.; Krasa, H.B.; Oberdhan, D. Pooled Data Analysis of the Long-Term Treatment Effects of Tolvaptan in ADPKD. Kidney Int. Rep. 2022, 7, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Gkika, V.; Louka, M.; Tsagkatakis, M.; Tsirpanlis, G.; Efficacy, T. the Treatment Response and the Aquaretic Effects of a Three-Year Tolvaptan Regimen in Polycystic Kidney Disease Patients. Clin. Pract. 2023, 13, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, P.; Bais, T.; Kuiken, V.; Knol, M.G.E.; Casteleijn, N.F.; Vart, P.; Meijer, E.; Gansevoort, R.T. The long-term effect of tolvaptan treatment on kidney function and volume in patients with ADPKD. Nephrol. Dial. Transplant. 2025, 40, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.T.; Goto, S.; Schreiber, K.; Torp-Pedersen, C. Why do we need observational studies of everyday patients in the real-life setting? Eur. Heart J. Suppl. 2015, 17, D2–D8. [Google Scholar] [CrossRef]
- Chebib, F.T.; Perrone, R.D.; Chapman, A.B.; Dahl, N.K.; Harris, P.C.; Mrug, M.; Mustafa, R.A.; Rastogi, A.; Watnick, T.; Yu, A.S.L.; et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J. Am. Soc. Nephrol. 2018, 29, 2458–2470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. Authors Current Mailing address: 8. Paul Eggers Kidney & Urology Branch, NIDDK, National Institutes of Health, 6707 Democracy Blvd, 2081. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar]
- Available online: https://www.mayo.edu/research/documents/pkd-center-adpkd-classification/doc-20094754 (accessed on 1 July 2024).
- Park, H.C.; Hong, Y.; Yeon, J.H.; Ryu, H.; Kim, Y.C.; Lee, J.; Kim, Y.H.; Chae, D.W.; Chung, W.; Ahn, C.; et al. Mayo imaging classification is a good predictor of rapid progress among Korean patients with autosomal dominant polycystic kidney disease: Results from the KNOW-CKD study. Kidney Res. Clin. Pract. 2022, 41, 432–441. [Google Scholar] [CrossRef]
- Bais, T.; Geertsema, P.; Knol, M.G.E.; Van Gastel, M.D.A.; De Haas, R.J.; Meijer, E.; Gansevoort, R.T.; Drenth, J.P.H.; Peters, D.J.M.; Zietse, R.; et al. Validation of the Mayo Imaging Classification System for Predicting Kidney Outcomes in ADPKD. Clin. J. Am. Soc. Nephrol. 2024, 19, 591–601. [Google Scholar] [CrossRef]
- Higashihara, E.; Torres, V.E.; Chapman, A.B.; Grantham, J.J.; Bae, K.; Watnick, T.J.; Horie, S.; Nutahara, K.; Ouyang, J.; Krasa, H.B.; et al. Tolvaptan in autosomal dominant polycystic kidney disease: Three years’ experience. Clin. J. Am. Soc. Nephrol. 2011, 6, 2499–2507. [Google Scholar] [CrossRef]
- Dev, H.; Hu, Z.; Blumenfeld, J.D.; Sharbatdaran, A.; Kim, Y.; Zhu, C.; Shimonov, D.; Chevalier, J.M.; Donahue, S.; Wu, A.; et al. The Role of Baseline Total Kidney Volume Growth Rate in Predicting Tolvaptan Efficacy for ADPKD Patients: A Feasibility Study. J. Clin. Med. 2025, 14, 1449. [Google Scholar] [CrossRef]
- Gregory, A.V.; Chebib, F.T.; Poudyal, B.; Holmes, H.L.; Yu, A.S.L.; Landsittel, D.P.; Bae, K.T.; Chapman, A.B.; Frederic, R.O.; Mrug, M.; et al. Utility of new image-derived biomarkers for autosomal dominant polycystic kidney disease prognosis using automated instance cyst segmentation. Kidney Int. 2023, 104, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Kawano, H.; Miyoshi, M.; Kimura, T.; Takahashi, K.; Muto, S.; Horie, S. Long-Term Effects of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Predictors of Treatment Response and Safety over 6 Years of Continuous Therapy. Int. J. Mol. Sci. 2024, 25, 2088. [Google Scholar] [CrossRef]
- Boertien, W.E.; Meijer, E.; De Jong, P.E.; Bakker, S.J.L.; Czerwiec, F.S.; Struck, J.; Oberdhan, D.; Shoaf, S.E.; Krasa, H.B.; Gansevoort, R.T. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013, 84, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E.; Hogan, M.C.; Glockner, J.; King, B.F.; Ofstie, T.G.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011, 80, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.E.; Chebib, F.T.; Irazabal, M.V.; Ofstie, T.G.; Bungum, L.A.; Metzger, A.J.; Senum, S.R.; Hogan, M.C.; El-Zoghby, Z.M.; Kline, T.L.; et al. Long-term administration of tolvaptan in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Saitta, C.; Afari, J.A.; Autorino, R.; Capitanio, U.; Porpiglia, F.; Amparore, D.; Piramide, F.; Cerrato, C.; Meagher, M.F.; Noyes, S.L.; et al. Development of a novel score (RENSAFE) to determine probability of acute kidney injury and renal functional decline post surgery: A multicenter analysis. Urol. Oncol. Semin. Orig. Investig. 2023, 41, e15–e487. [Google Scholar] [CrossRef]
| n = 30 | |
|---|---|
| Female, n (%) | 17 (56.7) |
| Age, years, median (IQR) | 35 (29.5–44.5) |
| Body weight, kg, median (IQR) | 77.2 (57.5–87.5) |
| Height, m, median (IQR) | 1.75 (1.67–1.82) |
| BMI, kg/m2, median (IQR) | 24.8 (21.1–26.9) |
| Family Hx of ADPKD, n (%) | 27 (90) |
| Hypertension, n (%) | 18 (60) |
| Smoking, n (%) | 11 (36.7) |
| eGFR mL/min/1.73 m2 (at tolvaptan initiation), median (IQR) | 77 (54–97) |
| TKV, mL (at tolvaptan initiation), median (IQR) | 1323 (940–1956) |
| MAYO classification n, (%) | |
| 1C | 13 (43.3) |
| 1D | 14 (46.7) |
| 1E | 3 (10) |
| All (n = 30) | 1C (n = 13) | 1D (n = 14) | 1E (n = 3) | |
|---|---|---|---|---|
| TKV baseline, mL, median (IQR) | 1323 (961–1948) | 1293 (982–1570) | 1565 (921–2163) | 1469 (949–4894) |
| Follow-up, years, median (IQR) | 3.5 (3–4) | 3.25 (3–4) | 3.75 (3–4) | 4 (3–5) |
| TKV observed, mL, median (IQR) | 1704 (1149–2202) | 1270 (956–1767) | 1810 (1490–2829) | 1811 (1149–6394) |
| TKV growth rate *, %/year, median (IQR) | 4.27 (1.39–7.98) | 1.95 (0.51–6.44) | 4.50 (2.74–8.01) | 5.74 (5.01–10.02) |
| eGFR Baseline, mL/min/1.73 m2, Median (IQR) | Predicted eGFR, mL/min/1.73 m2, Median (IQR) | Observed eGFR mL/min/1.73 m2, Median (IQR) | p-Value * | 95% CI | |
|---|---|---|---|---|---|
| All (n = 30) | 77 (54–97) | 60.8 (38.3–79.3) | 65 (46–105.5) | <0.001 | −14.60 to −6.18 |
| 1C (n = 13) | 65 (49.5–82) | 48.5 (41.1–70) | 62 (45.7–88) | 0.004 | −15.60 to −3.69 |
| 1D (n = 14) | 84 (56.2–93.7) | 60 (39.6–84.3) | 64.05 (48.2–90.2) | 0.008 | −12.29 to −2.23 |
| 1E (n = 3) | 98 (72–109) | 61 (45.9–72.5) | 104 (75–107) | 0.001 | −63.33 to −7.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filiopoulos, V.; Kofotolios, I.; Vallianou, K.; Karavasilis, E.; Ntounas, G.; Melexopoulou, C.; Marinaki, S. Long-Term Effects of Tolvaptan Therapy on Total Kidney Volume and Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease: A Single-Center Experience. J. Clin. Med. 2025, 14, 6537. https://doi.org/10.3390/jcm14186537
Filiopoulos V, Kofotolios I, Vallianou K, Karavasilis E, Ntounas G, Melexopoulou C, Marinaki S. Long-Term Effects of Tolvaptan Therapy on Total Kidney Volume and Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease: A Single-Center Experience. Journal of Clinical Medicine. 2025; 14(18):6537. https://doi.org/10.3390/jcm14186537
Chicago/Turabian StyleFiliopoulos, Vassilis, Ioannis Kofotolios, Kalliopi Vallianou, Efstratios Karavasilis, Georgios Ntounas, Christina Melexopoulou, and Smaragdi Marinaki. 2025. "Long-Term Effects of Tolvaptan Therapy on Total Kidney Volume and Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease: A Single-Center Experience" Journal of Clinical Medicine 14, no. 18: 6537. https://doi.org/10.3390/jcm14186537
APA StyleFiliopoulos, V., Kofotolios, I., Vallianou, K., Karavasilis, E., Ntounas, G., Melexopoulou, C., & Marinaki, S. (2025). Long-Term Effects of Tolvaptan Therapy on Total Kidney Volume and Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease: A Single-Center Experience. Journal of Clinical Medicine, 14(18), 6537. https://doi.org/10.3390/jcm14186537






