Cell-Free Circulating Mitochondrial DNA Levels Following High-Frequency Jet Ventilation—A Post Hoc Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. Protocol
2.3. Measurements
2.4. Statistical Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Singer, M.; Young, P.J.; Laffey, J.G.; Asfar, P.; Taccone, F.S.; Skrifvars, M.B.; Meyhoff, C.S.; Radermacher, P. Dangers of hyperoxia. Crit. Care 2021, 25, 440. [Google Scholar] [CrossRef]
- Machado-Junior, P.A.; Dias, M.S.S.; de Souza, A.B.F.; Lopes, L.S.E.; Menezes, T.P.; Talvani, A.; Brochard, L.; Bezerra, F.S. A short duration of mechanical ventilation alters redox status in the diaphragm and aggravates inflammation in septic mice. Respir. Physiol. Neurobiol. 2025, 331, 104361. [Google Scholar] [CrossRef]
- Wrigge, H.; Uhlig, U.; Baumgarten, G.; Menzenbach, J.; Zinserling, J.; Ernst, M.; Dromann, D.; Welz, A.; Uhlig, S.; Putensen, C. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: A prospective randomized clinical trial. Intensive Care Med. 2005, 31, 1379–1387. [Google Scholar] [CrossRef]
- Jing, R.; Hu, Z.K.; Lin, F.; He, S.; Zhang, S.S.; Ge, W.Y.; Dai, H.J.; Du, X.K.; Lin, J.Y.; Pan, L.H. Mitophagy-Mediated mtDNA Release Aggravates Stretching-Induced Inflammation and Lung Epithelial Cell Injury via the TLR9/MyD88/NF-kappaB Pathway. Front. Cell Dev. Biol. 2020, 8, 819. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Jing, R.; Lin, F.; Ge, W.Y.; Dai, H.J.; Pan, L. High Tidal Volume Induces Mitochondria Damage and Releases Mitochondrial DNA to Aggravate the Ventilator-Induced Lung Injury. Front. Immunol. 2018, 9, 1477. [Google Scholar] [CrossRef]
- Alva, R.; Mirza, M.; Baiton, A.; Lazuran, L.; Samokysh, L.; Bobinski, A.; Cowan, C.; Jaimon, A.; Obioru, D.; Al Makhoul, T.; et al. Oxygen toxicity: Cellular mechanisms in normobaric hyperoxia. Cell Biol. Toxicol. 2023, 39, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Budinger, G.R.; Tso, M.; McClintock, D.S.; Dean, D.A.; Sznajder, J.I.; Chandel, N.S. Hyperoxia-induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl-2 proteins. J. Biol. Chem. 2002, 277, 15654–15660. [Google Scholar] [CrossRef]
- Ruchko, M.; Gorodnya, O.; LeDoux, S.P.; Alexeyev, M.F.; Al-Mehdi, A.B.; Gillespie, M.N. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L530–L535. [Google Scholar] [CrossRef] [PubMed]
- Xuefei, Y.; Xinyi, Z.; Qing, C.; Dan, Z.; Ziyun, L.; Hejuan, Z.; Xindong, X.; Jianhua, F. Effects of Hyperoxia on Mitochondrial Homeostasis: Are Mitochondria the Hub for Bronchopulmonary Dysplasia? Front. Cell Dev. Biol. 2021, 9, 642717. [Google Scholar] [CrossRef]
- Joelsson, J.P.; Asbjarnarson, A.; Sigurdsson, S.; Kricker, J.; Valdimarsdottir, B.; Thorarinsdottir, H.; Starradottir, E.; Gudjonsson, T.; Ingthorsson, S.; Karason, S. Ventilator-induced lung injury results in oxidative stress response and mitochondrial swelling in a mouse model. Lab. Anim. Res. 2022, 38, 23. [Google Scholar] [CrossRef]
- Bacher, A.; Lang, T.; Weber, J.; Aloy, A. Respiratory efficacy of subglottic low-frequency, subglottic combined-frequency, and supraglottic combined-frequency jet ventilation during microlaryngeal surgery. Anesth. Analg. 2000, 91, 1506–1512. [Google Scholar] [CrossRef]
- Bacher, A.; Pichler, K.; Aloy, A. Supraglottic combined frequency jet ventilation versus subglottic monofrequent jet ventilation in patients undergoing microlaryngeal surgery. Anesth. Analg. 2000, 90, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Lanzenberger-Schragl, E.; Donner, A.; Grasl, M.C.; Zimpfer, M.; Aloy, A. Superimposed high-frequency jet ventilation for laryngeal and tracheal surgery. Arch. Otolaryngol. -Head Neck Surg. 2000, 126, 40–44. [Google Scholar] [CrossRef]
- Rezaie-Majd, A.; Bigenzahn, W.; Denk, D.M.; Burian, M.; Kornfehl, J.; Grasl, M.; Ihra, G.; Aloy, A. Superimposed high-frequency jet ventilation (SHFJV) for endoscopic laryngotracheal surgery in more than 1500 patients. Br. J. Anaesth. 2006, 96, 650–659. [Google Scholar] [CrossRef]
- Heinze, H.; Eichler, W.; Karsten, J.; Sedemund-Adib, B.; Heringlake, M.; Meier, T. Functional residual capacity-guided alveolar recruitment strategy after endotracheal suctioning in cardiac surgery patients. Crit. Care Med. 2011, 39, 1042–1049. [Google Scholar] [CrossRef]
- Rubin, J.S.; Patel, A.; Lennox, P. Subglottic jet ventilation for suspension microlaryngoscopy. J. Voice Off. J. Voice Found. 2005, 19, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.C. Mitochondrial damage pathways in ventilator induced lung injury (VILI): An update. J. Lung Health Dis. 2018, 2, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Gong, R.; Wang, Q.; Zhang, D.; Huang, C. Role of released mitochondrial DNA in acute lung injury. Front. Immunol. 2022, 13, 973089. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Zhang, X.; Li, X.; Wu, X.; Zhao, Y.; Ren, J. Circulating mitochondrial DNA-triggered autophagy dysfunction via STING underlies sepsis-related acute lung injury. Cell Death Dis. 2021, 12, 673. [Google Scholar] [CrossRef]
- Kandasamy, J.; Rezonzew, G.; Jilling, T.; Ballinger, S.; Ambalavanan, N. Mitochondrial DNA variation modulates alveolar development in newborn mice exposed to hyperoxia. Am. J. physiology. Lung Cell. Mol. Physiol. 2019, 317, L740–L747. [Google Scholar] [CrossRef]
- Windpassinger, M.; Prusak, M.; Gemeiner, J.; Edlinger-Stanger, M.; Roesner, I.; Denk-Linnert, D.M.; Plattner, O.; Khattab, A.; Kaniusas, E.; Wang, L.; et al. Regional lung ventilation during supraglottic and subglottic jet ventilation: A randomized cross-over trial. J. Clin. Anesth. 2025, 102, 111773. [Google Scholar] [CrossRef]
- Trappl, M.; Vostatek, R.; Salzmann, M.; Kraemmer, D.; Gebhart, J.; Hohensinner, P.; Pabinger, I.; Ay, C. Hemophilia is associated with accelerated biological aging. Haematologica 2025, 110, 2055–2063. [Google Scholar] [CrossRef]
- Kim, Y.E.; Ahn, S.Y.; Sung, S.I.; Yang, M.; Sung, D.K.; Park, W.S.; Chang, Y.S. Mesenchymal Stem Cells Reduce the Extracellular Mitochondrial DNA-Mediated TLR9 Activation in Neonatal Hyperoxia-Induced Lung Injury. Biomedicines 2024, 12, 686. [Google Scholar] [CrossRef]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernstrom, J.; Moser, D.; Kaufman, B.A.; et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef]
- Tripathi, A.; Bartosh, A.; Whitehead, C.; Pillai, A. Activation of cell-free mtDNA-TLR9 signaling mediates chronic stress-induced social behavior deficits. Mol. Psychiatry 2023, 28, 3806–3815. [Google Scholar] [CrossRef]
- Lazo, S.; Noren Hooten, N.; Green, J.; Eitan, E.; Mode, N.A.; Liu, Q.R.; Zonderman, A.B.; Ezike, N.; Mattson, M.P.; Ghosh, P.; et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 2021, 20, e13283. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef] [PubMed]
- Gebb, S.A.; Decoux, A.; Waggoner, A.; Wilson, G.L.; Gillespie, M.N. Mitochondrial DNA damage mediates hyperoxic dysmorphogenesis in rat fetal lung explants. Neonatology 2013, 103, 91–97. [Google Scholar] [CrossRef]
- Kim, S.J.; Cheresh, P.; Williams, D.; Cheng, Y.; Ridge, K.; Schumacker, P.T.; Weitzman, S.; Bohr, V.A.; Kamp, D.W. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J. Biol. Chem. 2014, 289, 6165–6176. [Google Scholar] [CrossRef] [PubMed]
- Aloy, A.; Schragl, E.; Neth, H.; Donner, A.; Kluwick, A. Flow pattern of respiratory gases in superimposed high-frequency jet ventilation (SHFJV) with the jet laryngoscope. Der Anaesthesist 1995, 44, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kleeberger, S.R. Mitochondrial biology in airway pathogenesis and the role of NRF2. Arch. Pharmacal Res. 2020, 43, 297–320. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Beyer, A.M.; Durand, M.; Clough, A.V.; Zhu, D.; Norwood Toro, L.; Terashvili, M.; Ebben, J.D.; Hill, R.B.; Audi, S.H.; et al. Hyperoxia Causes Mitochondrial Fragmentation in Pulmonary Endothelial Cells by Increasing Expression of Pro-Fission Proteins. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, X.; Wang, L.; Yu, G. Alveolar cells under mechanical stressed niche: Critical contributors to pulmonary fibrosis. Mol. Med. 2020, 26, 95. [Google Scholar] [CrossRef] [PubMed]

| Variables | Mean ± SD | Range | n (%) |
|---|---|---|---|
| Age (years) | 48.8 ± 16.1 | 22–79 | 30 |
| Height (cm) | 174.2 ± 9.2 | 160–196 | 30 |
| Weight (kg) | 77.4 ± 12.0 | 58–101 | 30 |
| BMI (kg/m2) | 25.5 ± 3.2 | 19.8–31.2 | 30 |
| Male | 18 (60%) | ||
| Female | 12 (40%) | ||
| ASA I | 19 (63%) | ||
| ASA II | 9 (30%) | ||
| ASA III | 2 (7%) | ||
| Type of surgery: | |||
| Vocal cord surgery | 14 (47%) | ||
| Laser microsurgery | 11 (37%) | ||
| Subglottic surgery | 3 (10%) | ||
| Panendoscopy | 1 (3%) | ||
| Supraglottic surgery | 1 (3%) |
| Variable | Before Jet Ventilation—T0 | After Jet Ventilation—T1 | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
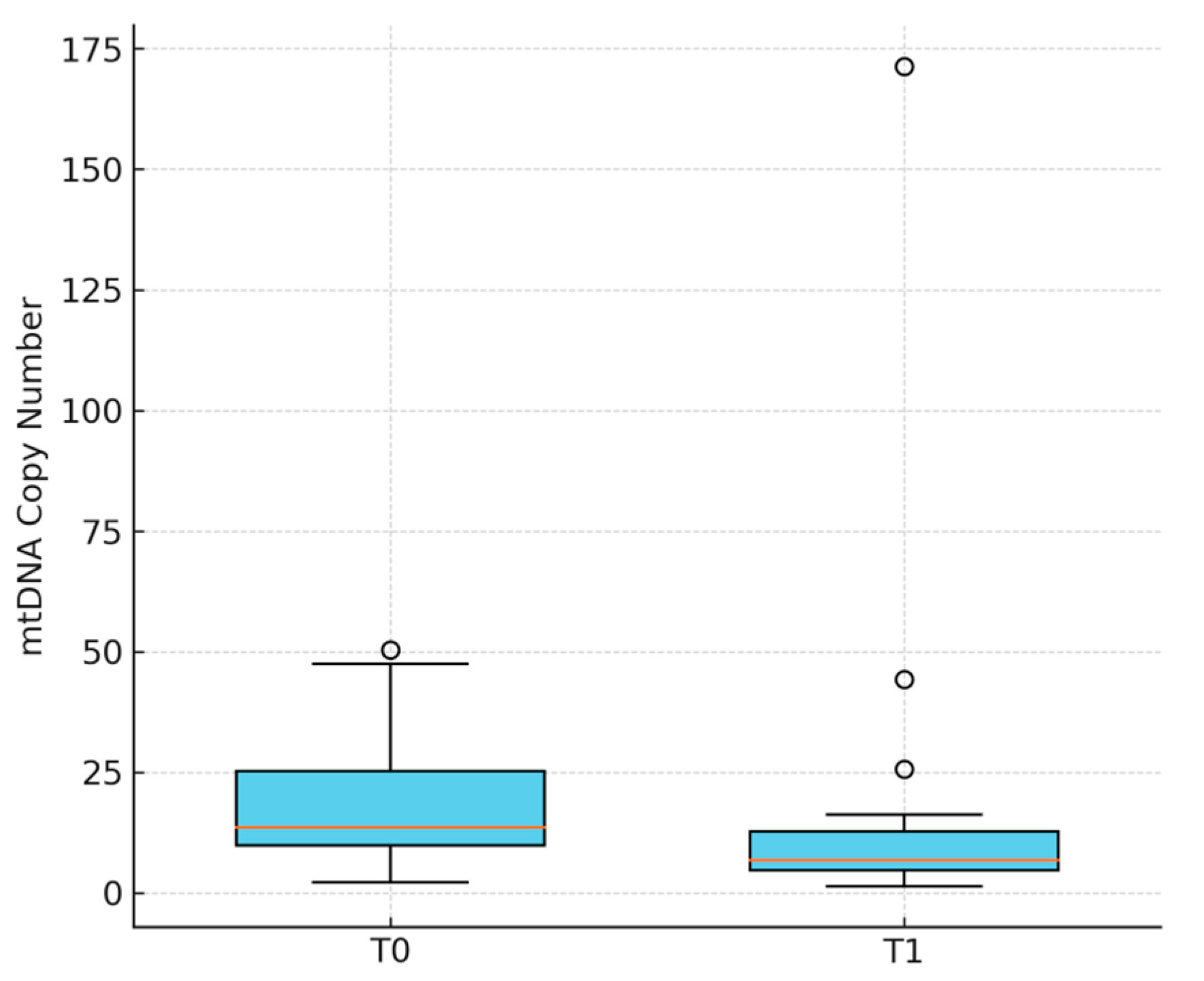
| Circulating mtDNA (copies/µL) a | 18.0 ± 12.6 | 14.6 ± 30.8 | −3.4 (−16.4–9.6) | 0.009 |
| PaO2 (mmHg) a | 123.5 ± 80.5 | 222.4 ± 86.9 | +98.9 (53.5–144.3) | 0.000 |
| SaO2 (%) | 96.8 ± 2.7 | 99.2 ± 1.2 | 2.4 (1.4–3.4) | 0.000 |
| PaCO2 (mmHg) | 40.6 ± 5.7 | 39.2 ± 5.6 | −1.4 (−4.1–1.3) | 0.328 |
| Hb (g/dL) | 13.3 ± 1.3 | 12.3 ± 1.3 | −1.0 (−1.2–−0.8) | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windpassinger, M.; Prusak, M.; Ruetzler, K.; Plattner, O.; Stanisz, I.; Haider, P. Cell-Free Circulating Mitochondrial DNA Levels Following High-Frequency Jet Ventilation—A Post Hoc Analysis. J. Clin. Med. 2025, 14, 6528. https://doi.org/10.3390/jcm14186528
Windpassinger M, Prusak M, Ruetzler K, Plattner O, Stanisz I, Haider P. Cell-Free Circulating Mitochondrial DNA Levels Following High-Frequency Jet Ventilation—A Post Hoc Analysis. Journal of Clinical Medicine. 2025; 14(18):6528. https://doi.org/10.3390/jcm14186528
Chicago/Turabian StyleWindpassinger, Marita, Michal Prusak, Kurt Ruetzler, Olga Plattner, Isabella Stanisz, and Patrick Haider. 2025. "Cell-Free Circulating Mitochondrial DNA Levels Following High-Frequency Jet Ventilation—A Post Hoc Analysis" Journal of Clinical Medicine 14, no. 18: 6528. https://doi.org/10.3390/jcm14186528
APA StyleWindpassinger, M., Prusak, M., Ruetzler, K., Plattner, O., Stanisz, I., & Haider, P. (2025). Cell-Free Circulating Mitochondrial DNA Levels Following High-Frequency Jet Ventilation—A Post Hoc Analysis. Journal of Clinical Medicine, 14(18), 6528. https://doi.org/10.3390/jcm14186528






