A Rare Case of Complete Myxoma Detachment Leading to Abdominal Aortic Occlusion and Secondary Visceral Necrosis: A Case Description and an Analysis of the Literature
Abstract
1. Introduction
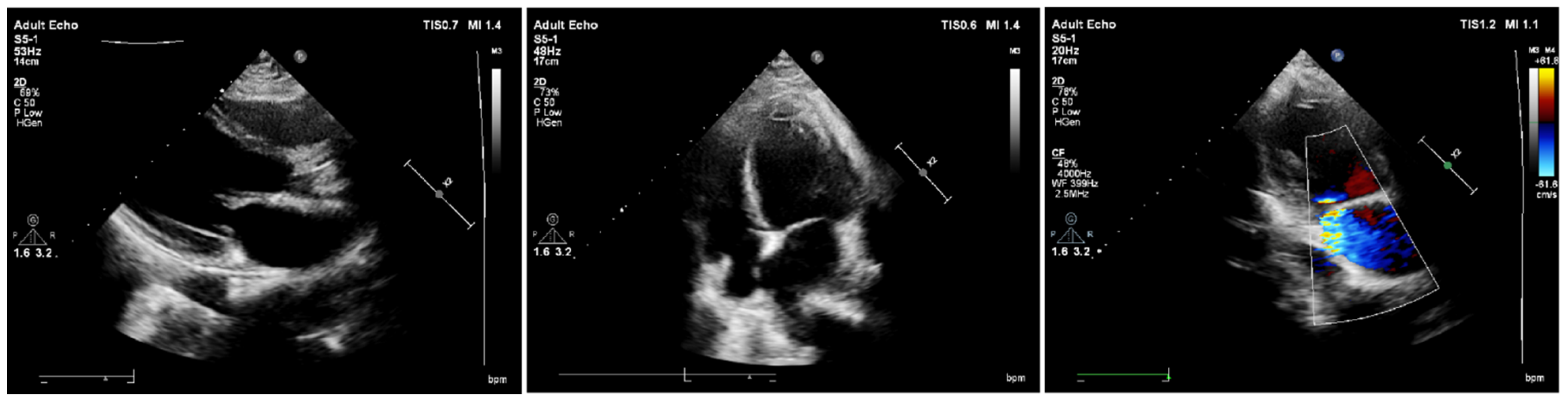
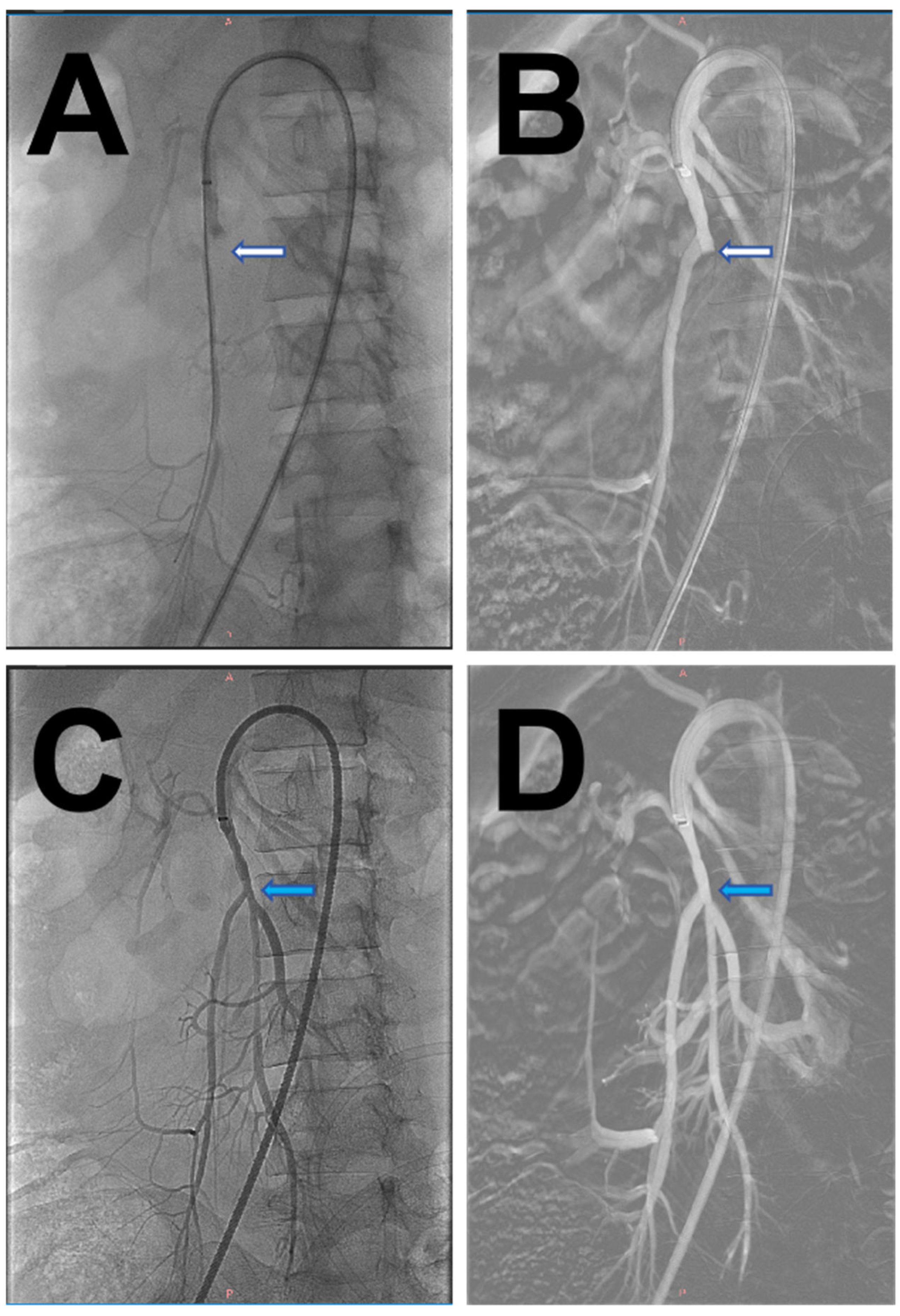
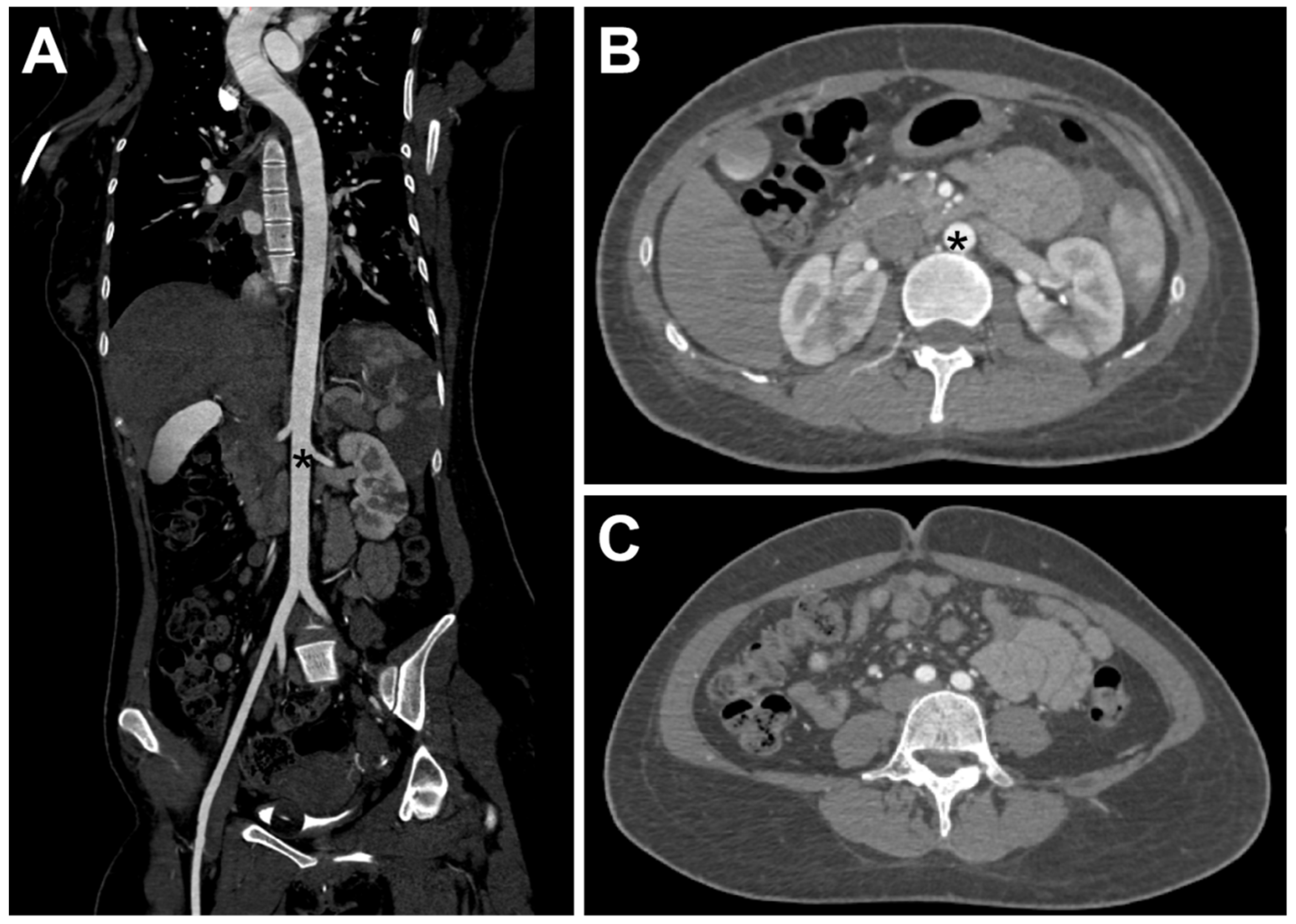
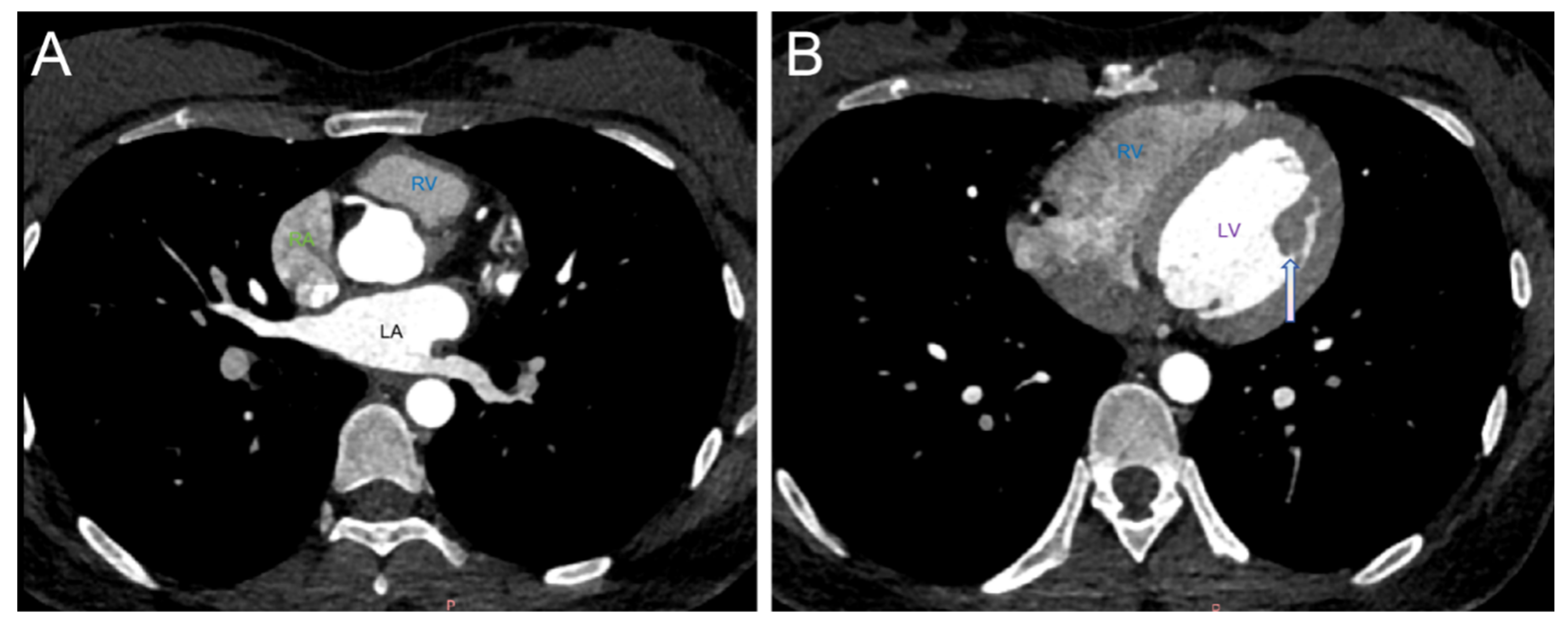
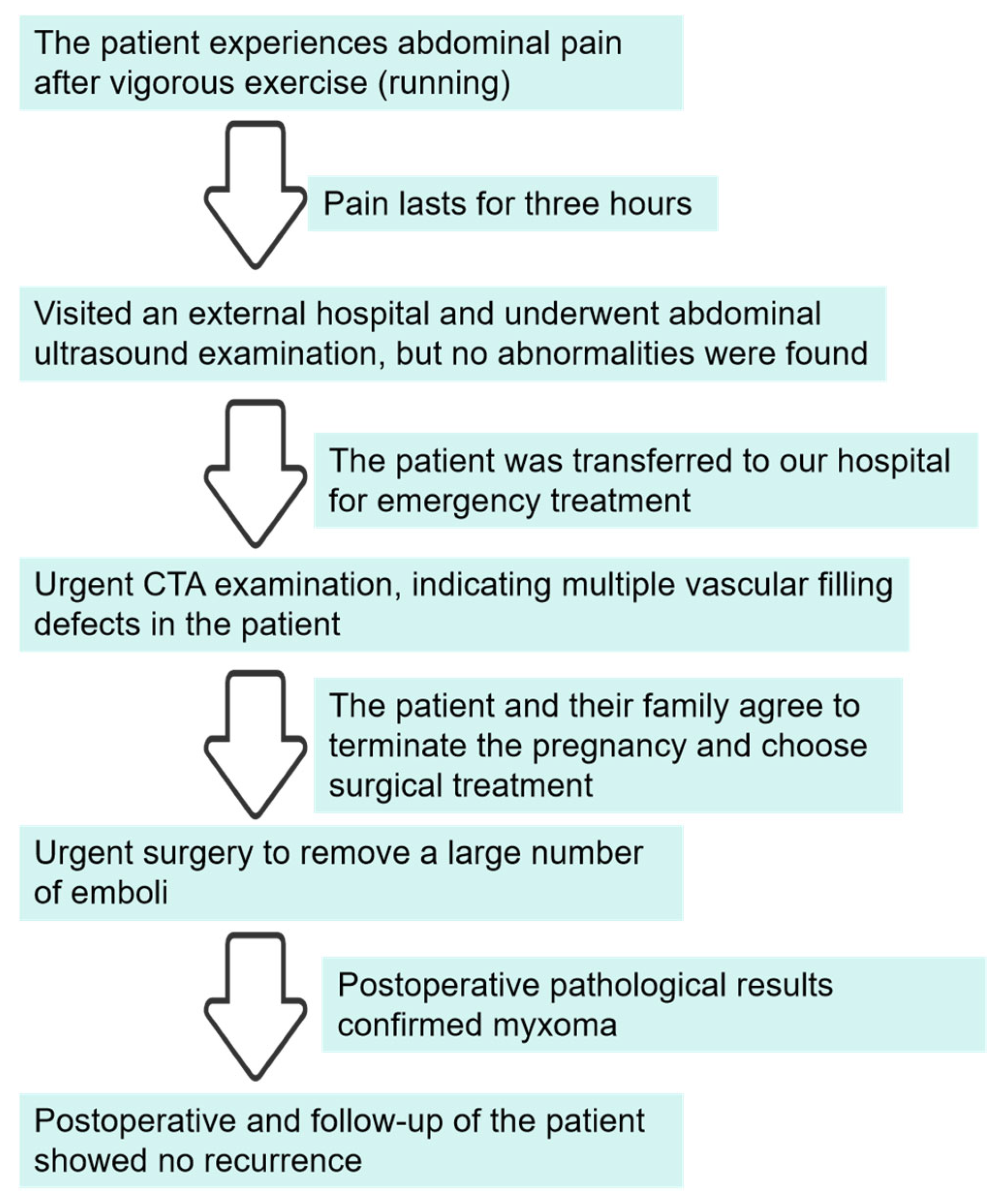
2. Case Report
3. Discussion and Conclusions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naser, N.; Hadziomerovic, N.; Bahram, D.; Kacila, M.; Pandur, S. Giant right at-rial myxoma with symptoms of right heart failure. Med. Arch. 2021, 75, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Desai, R.; Shamim, S.; Gandhi, Z.; Shrivastava, A.; Patel, D.; Uzair Lodhi, M.; Raina, J.; Itare, V.; Mahmood, A.; et al. Aotie valve myxoma-a systematic review of published cases. Int. J. Clin. Pact. 2021, 75, 1456–1461. [Google Scholar] [CrossRef]
- Ma, G.; Wang, D.; He, Y.; Zhang, R.; Zhou, Y.; Ying, K. Pulmonary embolism as the initial manifestation of right atrial myxoma: A case report and review of the literature. Medicine 2019, 98, 18386–18391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, F.; Zhu, F.; Yao, C. A case report of left atrial myxoma-induced acute myocardial infarction and successive stroke. Medicine 2018, 97, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Idir, M.; Oysel, N.; Guibaud, J.P.; Labouyrie, E.; Roudaut, R. Fragmentation of a right atrial myxoma presenting as a pulmonary embolism. J. Am. Soc. Echocardiogr. 2000, 13, 61–63. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, S.W.; Sidhu, P.; Aherne, T.; Luke, D.; Wood, A.E.; Neligan, M.C.; McGovern, E. Atrial myxoma national incidence, diagnosis and surgical management. Ir. J. Med. Sci. 1993, 162, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Kuon, E.; Kreplin, M.; Weiss, W.; Dahm, J.B. The challenge presented by right atrial myxoma. Herz 2004, 29, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Keeling, I.M.; Oberwalder, P.; Anelli-Monti, M.; Schuchlenz, H.; Demel, U.; Tilz, G.P.; Rehak, P.; Rigler, B. Cardiac myxomas:24 years of experience in 49 patients. Eur. J. Cardiothorae Surg. 2002, 22, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Pinede, L.; Duhaut, P.; Loire, R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine 2001, 80, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Nehaj, F.; Sokol, J.; Mokan, M.; Jankovicova, V.; Kovar, F.; Kubaskova, M.; Mizera, S.; Mokan, M. Outcomes of patients with newly diagnosed cardiac myxoma: A retrospective multicentric study. Biomed. Res. Int. 2018, 1, 8320–8325. [Google Scholar] [CrossRef] [PubMed]
- Hom, K.D.; Becich, M.J.; Rhee, R.Y.; Pham, S.M. Left atrial myxoma with embolization presenting as an acute infrarenal aortic occlusion. J. Vasc. Surg. 1997, 26, 341–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shavit, L.; Appelbaum, L.; Grenader, T. Atrial myxoma presenting with total occlusion of the abdominal aorta and multiple peripheral embolism. Eur. J. Intern. Med. 2007, 18, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Neff, C.M.; McCowan, C.L. Complete aortic occlusion caused by cardiac myxoma emboli. Am. J. Emerg. Med. 2008, 26, 110.e1–110.e2. [Google Scholar] [CrossRef] [PubMed]
- Coley, C.; Lee, K.R.; Steiner, M.; Thompson, C.S. Complete embolization of a left atrial myxoma resulting in acute lower extremity ischemia. Tex. Heart Inst. J. 2005, 32, 238–240. [Google Scholar] [PubMed]
- Fang, B.R.; Chang, C.P.; Cheng, C.W.; Yang, N.I.; Shieh, M.C.; Lee, N. Total detachment of cardiac myxoma causing saddle embolization and mimicking aortic dissection. Jpn. Heart J. 2004, 45, 359–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eriksen, U.H.; Baandrup, U.; Jensen, B.S. Total disruption of left atrial myxoma causing a cerebral attack and a saddle embolus in the iliac bifurcation. Int. J. Cardiol. 1992, 35, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Jessiman, I.M.; French, S. Entire left atrial myxoma presenting as a saddle embolus. Thorax 1969, 24, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Engberding, R.; Daniel, W.G.; Erbel, R.; Kasper, W.; Lestuzzi, C.; Curtius, J.M.; Sutherland, G.R.; Lambertz, H.; Von Hehn, A.; Lesbre, J.P.; et al. Diagnosis of heart tumors by transoesophagel echocardiography: A multicentre study in 154 patients. Eur. Heart J. 1993, 14, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Grebenc, M.L.; Rosado-de-Christenson, M.L.; Green, C.E.; Burke, A.P.; Galvin, J.R. Cardiac myxoma: Imaging features in 83 patients. Radiographics 2002, 22, 673–689. [Google Scholar] [CrossRef] [PubMed]


| Feature | ||||
|---|---|---|---|---|
| Age | mean age 55 years (range 22–79 years) | |||
| Sex | males (24.5%) | females (75.5%) | ||
| Primary site | left atrium (87.7%) | mitral valve (6.1%) | right atrium (4.1%) | left and right atria (2.0%) |
| Occlusion site | left ventricle (40.8%) | mitral stenosis (10.2%) | threatened left ventricular outflow tract obstruction(2%) | |
| Clinical symptoms | Cardiac signs appeared (93.9%) | Preoperative embolic events had occurred (26.5%) | ||
| outcomes | patients remained without cardiac symptoms (81.1%) | early mortality rate (2.0%) | late mortality rate (6.1%) | actuarial survival rate (0.74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhang, W.; Yu, J. A Rare Case of Complete Myxoma Detachment Leading to Abdominal Aortic Occlusion and Secondary Visceral Necrosis: A Case Description and an Analysis of the Literature. J. Clin. Med. 2025, 14, 6526. https://doi.org/10.3390/jcm14186526
Hu X, Zhang W, Yu J. A Rare Case of Complete Myxoma Detachment Leading to Abdominal Aortic Occlusion and Secondary Visceral Necrosis: A Case Description and an Analysis of the Literature. Journal of Clinical Medicine. 2025; 14(18):6526. https://doi.org/10.3390/jcm14186526
Chicago/Turabian StyleHu, Xu, Wenzhao Zhang, and Jianqun Yu. 2025. "A Rare Case of Complete Myxoma Detachment Leading to Abdominal Aortic Occlusion and Secondary Visceral Necrosis: A Case Description and an Analysis of the Literature" Journal of Clinical Medicine 14, no. 18: 6526. https://doi.org/10.3390/jcm14186526
APA StyleHu, X., Zhang, W., & Yu, J. (2025). A Rare Case of Complete Myxoma Detachment Leading to Abdominal Aortic Occlusion and Secondary Visceral Necrosis: A Case Description and an Analysis of the Literature. Journal of Clinical Medicine, 14(18), 6526. https://doi.org/10.3390/jcm14186526






