Differential Effects of Corneal Biomechanics on Superficial and Deep Vessel Density and Their Association with Central Visual Function in Glaucoma Patients with Myopia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, A.P.; Garway-Heath, D.F.; Poostchi, A.; Wong, T.; Chan, K.C.; Sachdev, N. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3262–3268. [Google Scholar] [CrossRef]
- Congdon, N.G.; Broman, A.T.; Bandeen-Roche, K.; Grover, D.; Quigley, H.A. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am. J. Ophthalmol. 2006, 141, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Meira-Freitas, D.; Lisboa, R.; Kuang, T.M.; Zangwill, L.M.; Weinreb, R.N. Corneal hysteresis as a risk factor for glaucoma progression: A prospective longitudinal study. Ophthalmology 2013, 120, 1533–1540. [Google Scholar] [CrossRef]
- Mangouritsas, G.; Morphis, G.; Mourtzoukos, S.; Feretis, E. Association between corneal hysteresis and central corneal thickness in glaucomatous and non-glaucomatous eyes. Acta Ophthalmol. 2009, 87, 901–905. [Google Scholar] [CrossRef]
- Mansouri, K.; Leite, M.T.; Weinreb, R.N.; Tafreshi, A.; Zangwill, L.M.; Medeiros, F.A. Association between corneal biomechanical properties and glaucoma severity. Am. J. Ophthalmol. 2012, 153, 419–427.e411. [Google Scholar] [CrossRef]
- Sigal, I.A.; Yang, H.; Roberts, M.D.; Grimm, J.L.; Burgoyne, C.F.; Demirel, S.; Downs, J.C. IOP-induced lamina cribrosa deformation and scleral canal expansion: Independent or related? Investig. Ophthalmol. Vis. Sci. 2011, 52, 9023–9032. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, H.L.; Yang, H.J.; Park, C.K. Characteristics of corneal biomechanical responses detected by a non-contact scheimpflug-based tonometer in eyes with glaucoma. Acta Ophthalmol. 2017, 95, e556–e563. [Google Scholar] [CrossRef]
- Catania, F.; Morenghi, E.; Rosetta, P.; Paolo, V.; Vinciguerra, R. Corneal Biomechanics Assessment with Ultra High Speed Scheimpflug Camera in Primary Open Angle Glaucoma Compared with Healthy Subjects: A meta-analysis of the Literature. Curr. Eye Res. 2023, 48, 161–171. [Google Scholar] [CrossRef]
- Wang, W.; Du, S.; Zhang, X. Corneal Deformation Response in Patients With Primary Open-Angle Glaucoma and in Healthy Subjects Analyzed by Corvis ST. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5557–5565. [Google Scholar] [CrossRef]
- Jung, Y.; Chun, H.; Moon, J.I. Corneal deflection amplitude and visual field progression in primary open-angle glaucoma. PLoS ONE 2019, 14, e0220655. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Y.; Bao, C.; Zhu, Y.; Pan, Y. The role of corneal biomechanics in visual field progression of primary open-angle glaucoma with ocular normotension or hypertension: A prospective longitude study. Front. Bioeng. Biotechnol. 2023, 11, 1174419. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ye, Y.; Chen, Z.; Xu, J.; Yang, Y.; Fan, Y.; Liu, P.; Chong, I.T.; Yu, K.; Lam, D.C.C.; et al. Corneal Stiffness and Modulus of Normal-Tension Glaucoma in Chinese. Am. J. Ophthalmol. 2022, 242, 131–138. [Google Scholar] [CrossRef]
- Wu, N.; Chen, Y.; Sun, X. Association Between Ocular Biomechanics Measured With Corvis ST and Glaucoma Severity in Patients With Untreated Primary Open Angle Glaucoma. Transl. Vis. Sci. Technol. 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ramm, L.; Herber, R.; Lorenz, G.; Jasper, C.S.; Pillunat, L.E.; Pillunat, K.R. Evaluation of corneal biomechanical properties using the ocular response analyzer and the dynamic Scheimpflug-Analyzer Corvis ST in high pressure and normal pressure open-angle glaucoma patients. PLoS ONE 2023, 18, e0281017. [Google Scholar] [CrossRef]
- Jung, Y.; Park, H.-Y.L.; Oh, S.; Park, C.K. Corneal biomechanical responses detected using corvis st in primary open angle glaucoma and normal tension glaucoma. Medicine 2020, 99, e19126. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, Z.S.; Deshmukh, S.; Dixit, S.; Sreenivasaiah, S.; Shroff, S.; Devi, S.; Webers, C.A.B.; Rao, H.L. A comparison of the corneal biomechanics in pseudoexfoliation glaucoma, primary open-angle glaucoma and healthy controls using Corvis ST. PLoS ONE 2020, 15, e0241296. [Google Scholar] [CrossRef]
- Pradhan, Z.S.; Deshmukh, S.; Dixit, S.; Gudetti, P.; Devi, S.; Webers, C.A.B.; Rao, H.L. A comparison of the corneal biomechanics in pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and healthy controls using Corvis® Scheimpflug Technology. Indian J. Ophthalmol. 2020, 68, 787–792. [Google Scholar] [CrossRef]
- Elagamy, A.; Alnasser, H.K.; Alghamdi, W.S.; Berika, M.; Aldisi, D. Relationship of Corneal Biomechanics Measured by Corvis ST and Optic Nerve Head Parameters in Healthy Saudi Females. Clin. Ophthalmol. 2024, 18, 2851–2863. [Google Scholar] [CrossRef]
- Aoki, S.; Kiuchi, Y.; Tokumo, K.; Fujino, Y.; Matsuura, M.; Murata, H.; Nakakura, S.; Asaoka, R. Association between optic nerve head morphology in open-angle glaucoma and corneal biomechanical parameters measured with Corvis ST. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 629–637. [Google Scholar] [CrossRef]
- Jung, Y.; Park, H.L.; Park, C.K. Relationship between corneal deformation amplitude and optic nerve head structure in primary open-angle glaucoma. Medicine 2019, 98, e17223. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Cao, K.; Zhang, Y.; Xie, Y.; Pang, R.; Shi, Y.; Wang, H.; Wang, N. Relationship between corneal stiffness parameters and lamina cribrosa curvature in normal tension glaucoma. Eur. J. Ophthalmol. 2021, 31, 3049–3056. [Google Scholar] [CrossRef]
- Jung, Y.; Park, H.Y.; Park, C.K. Association between Corneal Deformation Amplitude and Posterior Pole Profiles in Primary Open-Angle Glaucoma. Ophthalmology 2016, 123, 959–964. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jung, K.I.; Park, H.L.; Park, C.K. Three-Dimensional Evaluation of Posterior Pole and Optic Nerve Head in Myopes with Glaucoma. Sci. Rep. 2017, 7, 18001. [Google Scholar] [CrossRef]
- Park, H.-Y.L.; Lee, K.; Park, C.K. Optic Disc Torsion Direction Predicts the Location of Glaucomatous Damage in Normal-Tension Glaucoma Patients with Myopia. Ophthalmology 2012, 119, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, J.R.; Kook, M.S. Optic Disc Torsion Presenting as Unilateral Glaucomatous-Appearing Visual Field Defect in Young Myopic Korean Eyes. Ophthalmology 2014, 121, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Park, H.-Y.L.; Shin, H.-Y.; Park, C.K. Optic Disc Tilt Direction Determines the Location of Initial Glaucomatous Damage. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4991–4998. [Google Scholar] [CrossRef]
- Kim, Y.C.; Koo, Y.H.; Jung, K.I.; Park, C.K. Impact of Posterior Sclera on Glaucoma Progression in Treated Myopic Normal-Tension Glaucoma Using Reconstructed Optical Coherence Tomographic Images. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2198–2207. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Shimada, N.; Yasuzumi, K.; Hayashi, K.; Yoshida, T.; Kojima, A.; Moriyama, M.; Tokoro, T. Long-term Development of Significant Visual Field Defects in Highly Myopic Eyes. Am. J. Ophthalmol. 2011, 152, 256–265.e251. [Google Scholar] [CrossRef]
- Kimura, Y.; Hangai, M.; Morooka, S.; Takayama, K.; Nakano, N.; Nukada, M.; Ikeda, H.O.; Akagi, T.; Yoshimura, N. Retinal nerve fiber layer defects in highly myopic eyes with early glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6472–6478. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Sugisaki, K.; Araie, M.; Murata, H.; Kanamori, A.; Inoue, T.; Ishikawa, S.; Yoshikawa, K.; Maeda, H.; Yamada, Y.; et al. Relationship between Vision-Related Quality of Life and Central 10° of the Binocular Integrated Visual Field in Advanced Glaucoma. Sci. Rep. 2019, 9, 14990. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Gracitelli, C.P.; Boer, E.R.; Weinreb, R.N.; Zangwill, L.M.; Rosen, P.N. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015, 122, 293–301. [Google Scholar] [CrossRef]
- Roberti, G.; Manni, G.; Riva, I.; Holló, G.; Quaranta, L.; Agnifili, L.; Figus, M.; Giammaria, S.; Rastelli, D.; Oddone, F. Detection of central visual field defects in early glaucomatous eyes: Comparison of Humphrey and Octopus perimetry. PLoS ONE 2017, 12, e0186793. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.E.; Kim, S.A.; Shin, D.Y.; Park, C.K.; Park, H.L. Ocular and Hemodynamic Factors Contributing to the Central Visual Function in Glaucoma Patients With Myopia. Investig. Ophthalmol. Vis. Sci. 2022, 63, 26. [Google Scholar] [CrossRef]
- Park, H.L.; Jeon, S.J.; Park, C.K. Features of the Choroidal Microvasculature in Peripapillary Atrophy Are Associated With Visual Field Damage in Myopic Patients. Am. J. Ophthalmol. 2018, 192, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, T.W.; Kim, J.A.; Kim, J.A. Central Visual Field Damage and Parapapillary Choroidal Microvasculature Dropout in Primary Open-Angle Glaucoma. Ophthalmology 2018, 125, 588–596. [Google Scholar] [CrossRef]
- Park, S.C.; De Moraes, C.G.; Teng, C.C.W.; Tello, C.; Liebmann, J.M.; Ritch, R. Initial Parafoveal Versus Peripheral Scotomas in Glaucoma: Risk Factors and Visual Field Characteristics. Ophthalmology 2011, 118, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Park, C.K.; Park, H.L. Factors Affecting Visual Acuity and Central Visual Function in Glaucoma Patients with Myopia. Am. J. Ophthalmol. 2023, 253, 106–118. [Google Scholar] [CrossRef]
- Jeon, S.J.; Park, H.L.; Kim, Y.C.; Kim, E.K.; Park, C.K. Association of Scleral Deformation Around the Optic Nerve Head With Central Visual Function in Normal-Tension Glaucoma and Myopia. Am. J. Ophthalmol. 2020, 217, 287–296. [Google Scholar] [CrossRef]
- Sung, M.S.; Heo, H.; Ji, Y.S.; Park, S.W. Predicting the risk of parafoveal scotoma in myopic normal tension glaucoma: Role of optic disc tilt and rotation. Eye 2017, 31, 1051–1059. [Google Scholar] [CrossRef]
- Choi, J.A.; Park, H.Y.L.; Park, C.K. Difference in the posterior pole profiles associated with the initial location of visual field defect in early-stage normal tension glaucoma. Acta Ophthalmol. 2015, 93, e94–e99. [Google Scholar] [CrossRef]
- Jonas, J.B.; Xu, L. Histological changes of high axial myopia. Eye 2014, 28, 113–117. [Google Scholar] [CrossRef]
- Harper, A.R.; Summers, J.A. The dynamic sclera: Extracellular matrix remodeling in normal ocular growth and myopia development. Exp. Eye Res. 2015, 133, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.; Fullwood, N. Corneal and scleral collagens—A microscopist’s perspective. Micron 2001, 32, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Ohno-Matsui, K.; Panda-Jonas, S. Myopia: Anatomic Changes and Consequences for Its Etiology. Asia Pac. J. Ophthalmol. 2019, 8, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Jeon, S.J.; Kim, E.K.; Jung, K.I.; Park, H.Y.L.; Park, C.K. Association between peripapillary scleral deformation and choroidal microvascular circulation in glaucoma. Sci. Rep. 2019, 9, 18503. [Google Scholar] [CrossRef]
- Choi, J.A.; Kim, J.-S.; Park, H.-Y.L.; Park, H.; Park, C.K. The Foveal Position Relative to the Optic Disc and the Retinal Nerve Fiber Layer Thickness Profile in Myopia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1419–1426. [Google Scholar] [CrossRef]
- Ashrafkhorasani, M.; Besharati, S.; Mohammadzadeh, V.; Zou, J.; Figueroa, J.; Mohammadi, M.; Nouri-Mahdavi, K. Enhancing Detection of Glaucoma Progression: Utility of 24-2 Visual Field Central Points vs. 10-2 Visual Fields. Ophthalmol. Glaucoma 2025, 8, 117–125. [Google Scholar] [CrossRef]
- He, J.; Chen, Q.; Yin, Y.; Zhou, H.; Fan, Y.; Zhu, J.; Zou, H.; Xu, X. Association between retinal microvasculature and optic disc alterations in high myopia. Eye 2019, 33, 1494–1503. [Google Scholar] [CrossRef]
- Yang, D.; Cao, D.; Zhang, L.; Yang, C.; Lan, J.; Zhang, Y.; Zeng, J. Macular and peripapillary vessel density in myopic eyes of young Chinese adults. Clin. Exp. Optom. 2020, 103, 830–837. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Zhang, R.; Yu, Y.; Xiao, X.; Wu, C. Evaluation of retinal vascular density and related factors in youth myopia without maculopathy using OCTA. Sci. Rep. 2021, 11, 15361. [Google Scholar] [CrossRef]
- Na, K.I.; Lee, W.J.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Evaluation of Optic Nerve Head and Peripapillary Choroidal Vasculature Using Swept-source Optical Coherence Tomography Angiography. J. Glaucoma 2017, 26, 665–668. [Google Scholar] [CrossRef]
- Kamalipour, A.; Moghimi, S.; Eslani, M.; Nishida, T.; Mohammadzadeh, V.; Micheletti, E.; Girkin, C.A.; Fazio, M.A.; Liebmann, J.M.; Zangwill, L.M.; et al. A Prospective Longitudinal Study to Investigate Corneal Hysteresis as a Risk Factor of Central Visual Field Progression in Glaucoma. Am. J. Ophthalmol. 2022, 240, 159–169. [Google Scholar] [CrossRef]
- Chuangsuwanich, T.; Hung, P.T.; Wang, X.; Liang, L.H.; Schmetterer, L.; Boote, C.; Girard, M.J.A. Morphometric, Hemodynamic, and Biomechanical Factors Influencing Blood Flow and Oxygen Concentration in the Human Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhou, J.B. Scleral remodeling in myopia development. Int. J. Ophthalmol. 2022, 15, 510–514. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Jobling, A.I.; Gentle, A. Biomechanics of the Sclera in Myopia: Extracellular and Cellular Factors. Optom. Vision. Sci. 2009, 86, E23–E30. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ji, J.; Chen, X.; Zhang, J.; Wen, X.; Liu, L. Retinal glia in myopia: Current understanding and future directions. Front. Cell Dev. Biol. 2024, 12, 1512988. [Google Scholar] [CrossRef]
- Chuangsuwanich, T.; Tun, T.A.; Braeu, F.A.; Yeoh, C.H.Y.; Chong, R.S.; Wang, X.; Aung, T.; Hoang, Q.V.; Girard, M.J.A. How Myopia and Glaucoma Influence the Biomechanical Susceptibility of the Optic Nerve Head. Investig. Ophthalmol. Vis. Sci. 2023, 64, 12. [Google Scholar] [CrossRef]

| Parameters | High A1 Deflection Amplitude (n = 21) | Low A1 Deflection Amplitude (n = 21) | p-Value |
|---|---|---|---|
| Age, years | 57.2 ± 14.5 | 48.7 ± 9.8 | 0.033 |
| Intraocular pressure, mmHg | 16.1 ± 4.2 | 14.4 ± 2.4 | 0.102 |
| Axial length, mm | 26.5 ± 1.3 | 26.4 ± 1.1 | 0.813 |
| Central corneal thickness | 546.9 ± 32.0 | 536.8 ± 29.7 | 0.296 |
| Disc–foveal angle, degree | 7.1 ± 2.3 | 7.0 ± 4.8 | 0.943 |
| Disc torsion, degree | 18.0 ± 9.1 | 16.0 ± 10.1 | 0.510 |
| PPA area | 9953.1 ± 6686.6 | 9580.3 ± 6202.8 | 0.852 |
| Peripapillary VD | |||
| Superficial temporal VD | 38.4 ± 10.5 | 40.6 ± 10.0 | 0.482 |
| Superficial nasal VD | 37.9 ± 11.0 | 42.8 ± 11.2 | 0.155 |
| Deep VD | 43.9 ± 12.9 | 36.2 ± 10.7 | 0.042 |
| Superficial macular VD | 31.1 ± 4.2 | 32.9 ± 3.5 | 0.137 |
| Deep macular VD | 34.9 ± 2.5 | 34.4 ± 2.1 | 0.440 |
| Central 12-point MD sum in SITA 24-2, dB | −2.9 ± 4.2 | −2.6 ± 3.4 | 0.762 |
| MD in SITA 24-2, dB | −5.1 ± 5.0 | −4.3 ± 4.9 | 0.633 |
| PSD in SITA 24-2, dB | 5.8 ± 4.0 | 4.7 ± 4.5 | 0.419 |
| RNFL thickness | 76.57 ± 11.9 | 77.43 ± 10.7 | 0.807 |
| A1 Deformation Amp. [mm] | A1 Deflection Amp. [mm] | Whole-Eye Movement Max [mm] | ||
|---|---|---|---|---|
| Peripapillary VD | ||||
| Superficial temporal VD | r | −0.31 | −0.09 | −0.45 |
| p | 0.05 | 0.58 | <0.01 | |
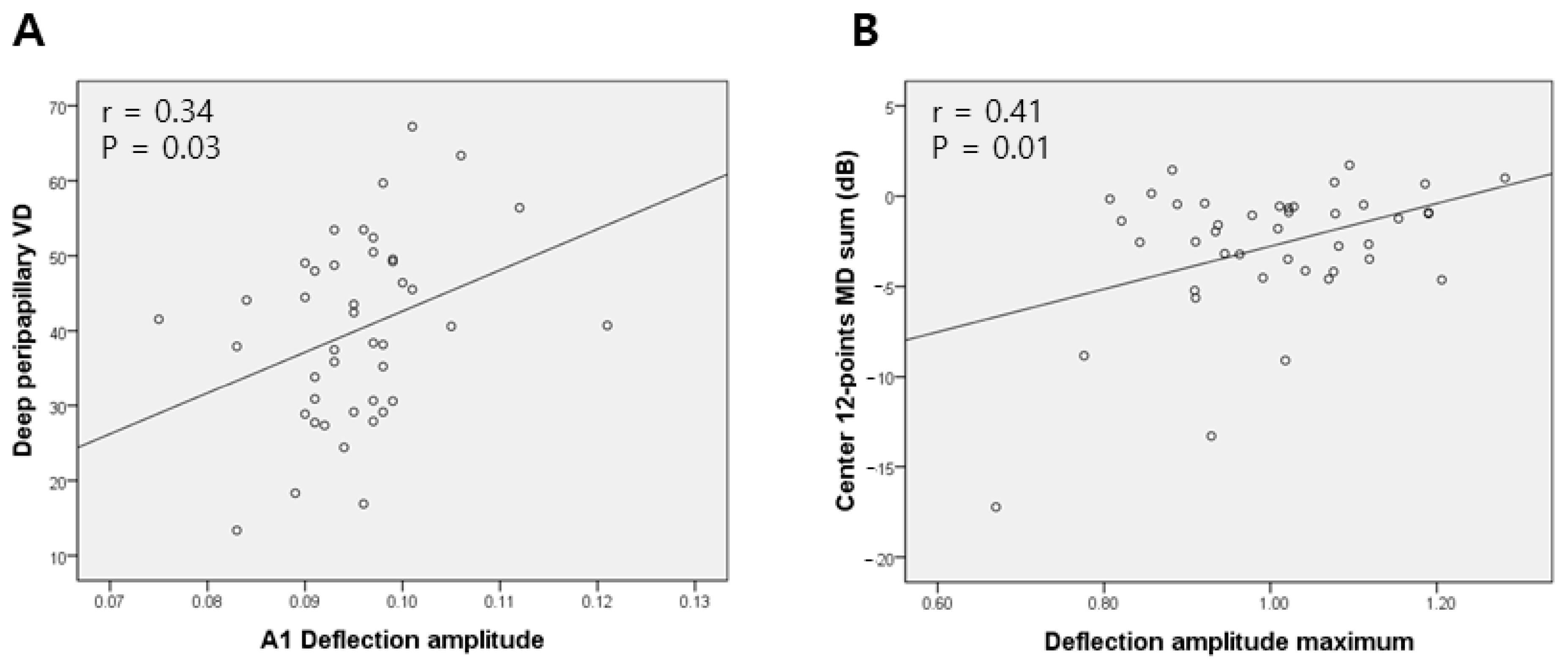
| Deep VD | r | 0.08 | 0.34 | 0.09 |
| p | 0.63 | 0.03 | 0.57 |
| Univariate | Multivariate | Collinearity Statistics | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | Tolerance | VIF | |
| Age | −0.351 (−0.575–−0.126) | 0.003 | −0.208 (−0.426–0.011) | 0.062 | 0.89 | 1.12 |
| CCT | 0.038 (−0.066–0.143) | 0.462 | ||||
| IOP | −0.487 (−1.414–0.440) | 0.295 | ||||
| AXL | 1.129 (−1.594–3.853) | 0.407 | ||||
| MD | 0.707 (0.088–1.326) | 0.026 | 0.665 (0.073–1.258) | 0.029 | 0.81 | 1.24 |
| RNFL thickness | 0.397 (0.134–0.660) | 0.004 | 0.125 (−0.161–0.410) | 0.383 | 0.66 | 1.53 |
| A1 DA [mm] | −221.830 (−442.544–−1.117) | 0.049 | −32.058 (−242.386–178.269) | 0.759 | 0.80 | 1.25 |
| Whole-eye movement max [mm] | −62.970 (−103.244–−22.696) | 0.003 | −42.340 (−76.404–−8.276) | 0.016 | 0.95 | 1.05 |
| Univariate | Multivariate | Collinearity Statistics | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | Tolerance | VIF | |
| Age | −0.090 (−0.392–0.211) | 0.549 | −0.220 (−0.522–0.082) | 0.148 | 0.90 | 1.12 |
| CCT | 0.010 (−0.117–0.138) | 0.869 | ||||
| IOP | 0.230 (−0.904–1.363) | 0.684 | −0.312 (−1.463–0.838) | 0.586 | 0.87 | 1.15 |
| AXL | −0.509 (−3.827–2.808) | 0.758 | −1.647 (−4.887–1.593) | 0.310 | 0.94 | 1.07 |
| A1 DA [mm] | 547.019 (62.377–1.0 × 103) | 0.028 | 745.458 (190.572–1.3 × 103) | 0.01 | 0.77 | 1.30 |
| Univariate | Multivariate | Collinearity Statistics | ||||
|---|---|---|---|---|---|---|
| Exp(B) (95% CI) | p-Value | Exp(B) (95% CI) | p-Value | Tolerance | VIF | |
| Age | 1.024 (0.974–1.077) | 0.349 | ||||
| CCT | 0.983 (0.961–1.004) | 0.110 | ||||
| IOP | 1.013 (0.841–1.220) | 0.893 | 1.013 (0.803–1.277) | 0.916 | 0.94 | 1.07 |
| AXL | 0.649 (0.369–1.139) | 0.132 | 0.743 (0.381–1.449) | 0.383 | 0.79 | 1.27 |
| Peripapillary VD | ||||||
| Superficial temporal VD | 0.907 (0.838–0.982) | 0.017 | 0.924 (0.849–1.006) | 0.069 | 0.75 | 1.33 |
| Whole-eye movement max [mm] | 1.8 × 105 (4.1 × 100–7.9 × 109) | 0.026 | 1.1 × 103 (0.003–3.9 × 108) | 0.280 | 0.62 | 1.60 |
| Univariate | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | VIF | B (95% CI) | p-Value | VIF | |
| Age | 0.051 (−0.039–0.141) | 0.262 | ||||||
| CCT | 0.003 (−0.035–0.042) | 0.863 | ||||||
| IOP | −0.357 (−0.682–−0.032) | 0.032 | 0.053 (−0.424–0.531) | 0.822 | 2.96 | −0.017 (−0.487–0.453) | 0.942 | 2.78 |
| AXL | 0.107 (−0.899–1.113) | 0.831 | −0.379 (−1.368–0.610) | 0.442 | 1.49 | 0.014 (−0.857–0.885) | 0.974 | 1.12 |
| Disc–foveal angle, degree | −0.346 (−0.647–−0.045) | 0.025 | −0.401 (−0.665–−0.138) | 0.004 | 1.04 | −0.411 (−0.679–−0.143) | 0.004 | 1.04 |
| Peripapillary VD | ||||||||
| Superficial temporal VD | 0.115 (0.004–0.227) | 0.042 | 0.101 (0.003–0.198) | 0.043 | 1.06 | 0.123 (0.019–0.226) | 0.022 | 1.17 |
| HC deformation amp. [mm] | 10.443 (1.577–19.308) | 0.022 | 12.442 (−0.303–25.187) | 0.055 | 2.70 | |||
| HC deflection amp. [mm] | 12.181 (3.703–20.660) | 0.006 | 15.055 (1.694–28.415) | 0.028 | 3.16 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohn, K.; Jung, Y.; Park, H.-Y.L. Differential Effects of Corneal Biomechanics on Superficial and Deep Vessel Density and Their Association with Central Visual Function in Glaucoma Patients with Myopia. J. Clin. Med. 2025, 14, 6515. https://doi.org/10.3390/jcm14186515
Ohn K, Jung Y, Park H-YL. Differential Effects of Corneal Biomechanics on Superficial and Deep Vessel Density and Their Association with Central Visual Function in Glaucoma Patients with Myopia. Journal of Clinical Medicine. 2025; 14(18):6515. https://doi.org/10.3390/jcm14186515
Chicago/Turabian StyleOhn, Kyoung, Younhea Jung, and Hae-Young Lopilly Park. 2025. "Differential Effects of Corneal Biomechanics on Superficial and Deep Vessel Density and Their Association with Central Visual Function in Glaucoma Patients with Myopia" Journal of Clinical Medicine 14, no. 18: 6515. https://doi.org/10.3390/jcm14186515
APA StyleOhn, K., Jung, Y., & Park, H.-Y. L. (2025). Differential Effects of Corneal Biomechanics on Superficial and Deep Vessel Density and Their Association with Central Visual Function in Glaucoma Patients with Myopia. Journal of Clinical Medicine, 14(18), 6515. https://doi.org/10.3390/jcm14186515






