Biomechanical Analysis of Fixation Strength in Unstable Intertrochanteric Femoral Fracture Models Based on the Caput–Collum–Diaphyseal Angle of Cephalomedullary Nails and Position of Lag Screws
Abstract
1. Introduction
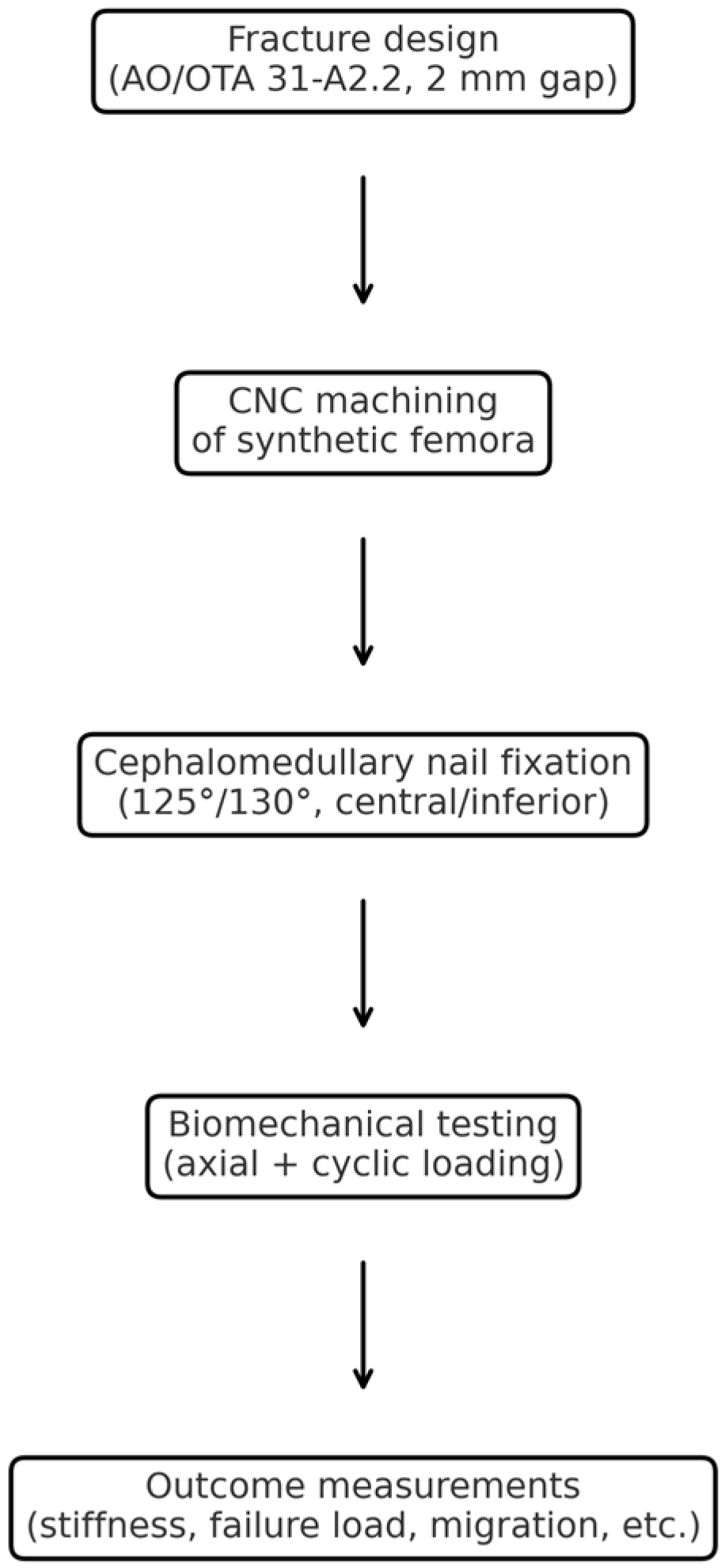
2. Materials and Methods
2.1. Specimen Preparation
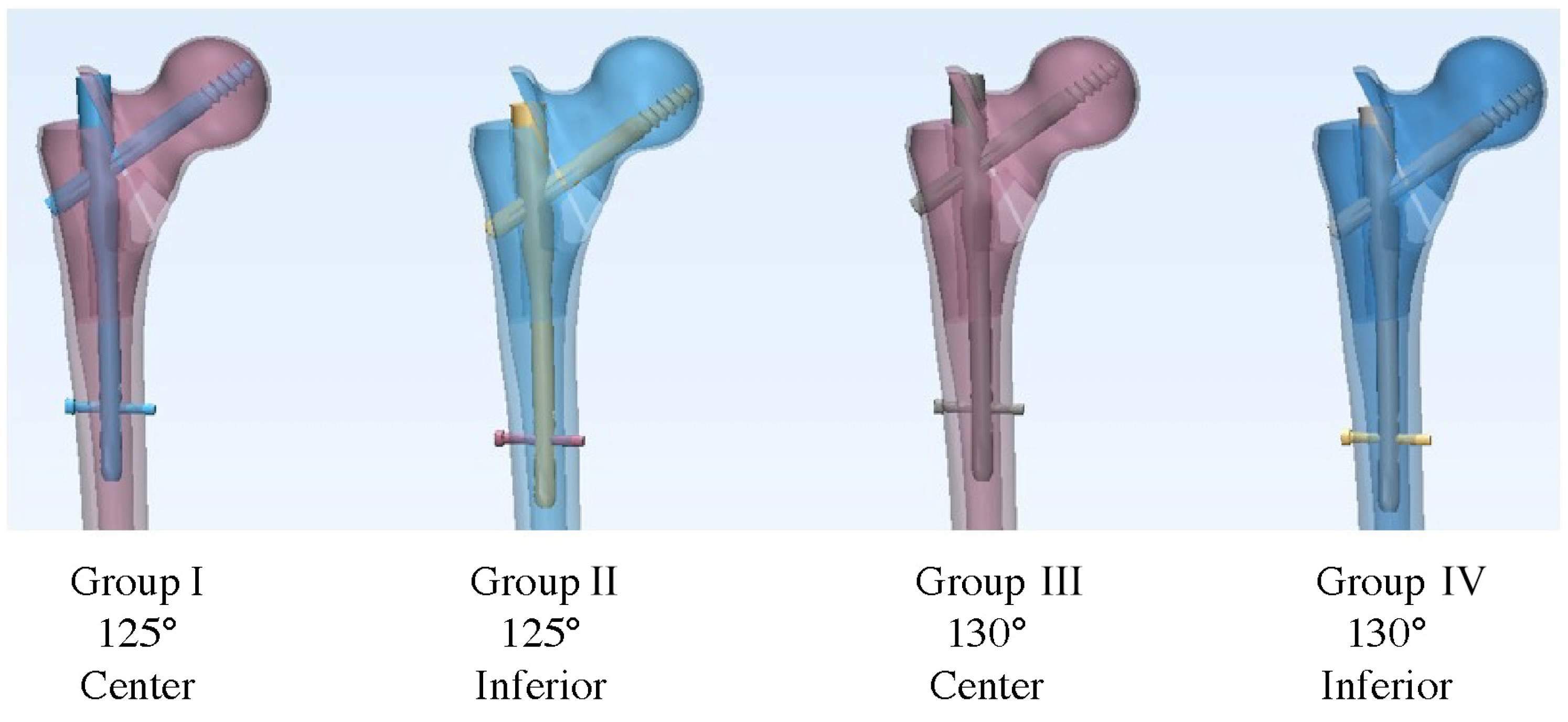
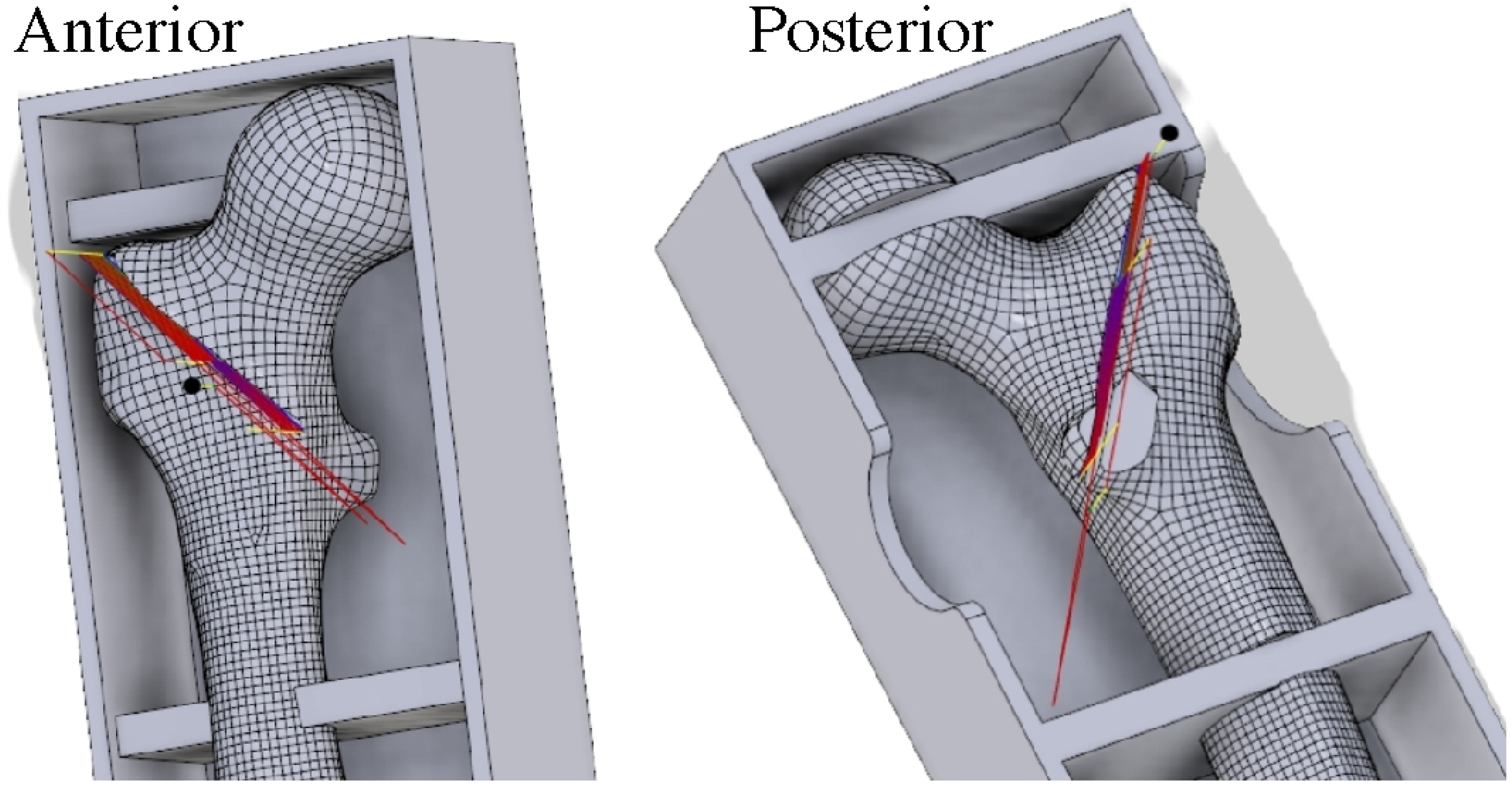
2.2. Fixation Device Insertion
2.3. Fracture Model Creation
2.4. Biomechanical Testing
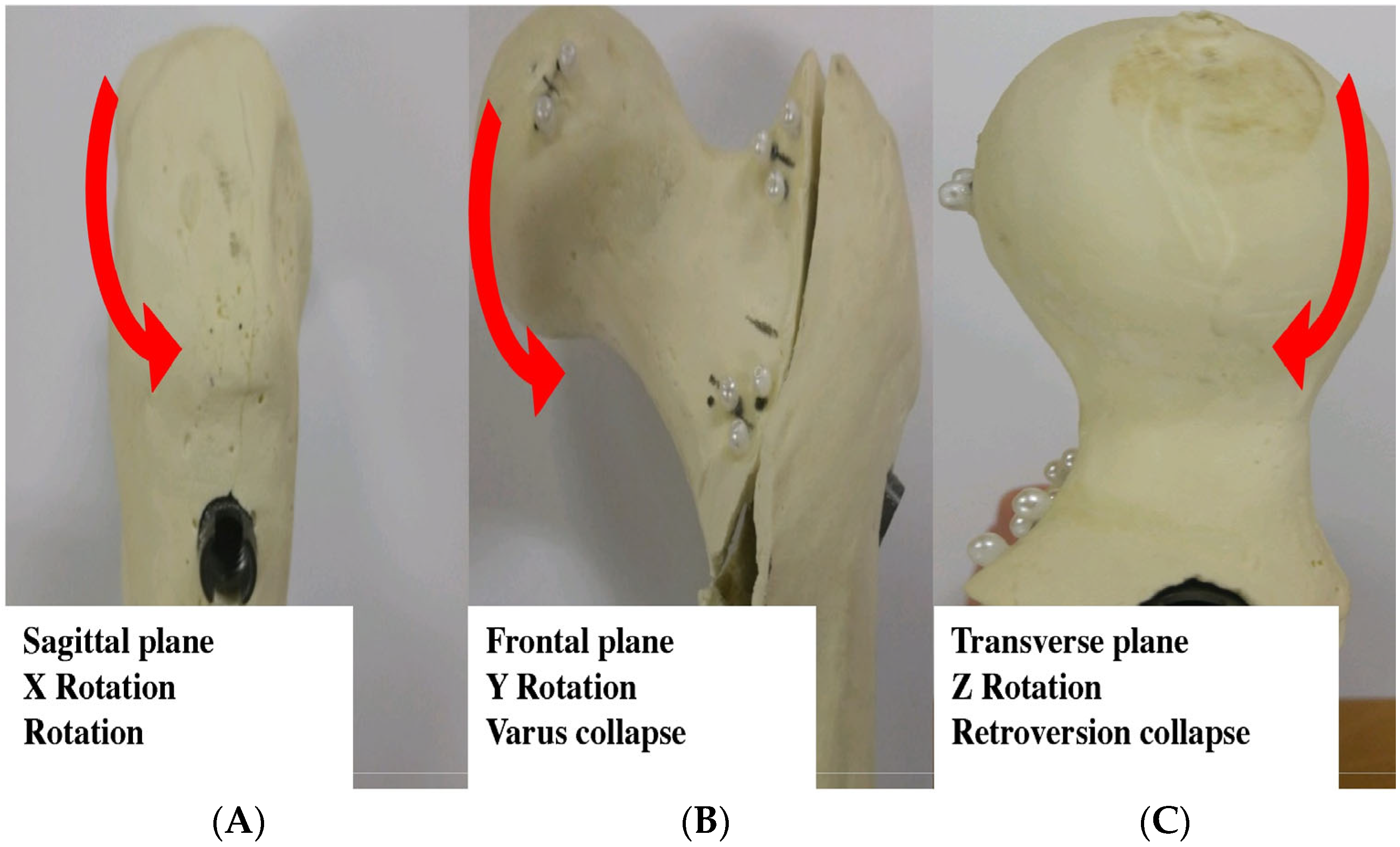
2.5. Measurement of 3D Fragment Rotation
2.6. Measurement of Lag Screw Migration
2.7. Statistical Analysis
3. Results
3.1. Three-Dimensional Femoral Head Rotational Deformation
3.2. Lag Screw Migration
3.3. Construct Stiffness and Failure Load
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAD | Tip–apex distance |
| AP | Anteroposterior |
| CCD | Caput–collum–diaphyseal |
| Cal-TAD | Calcar-referenced TAD |
| 3D | Three-dimensional |
| CNC | Computer numerical control |
References
- Cooper, C.; Campion, G.; Melton, L.J., 3rd. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Dhanwal, D.K.; Dennison, E.M.; Harvey, N.C.; Cooper, C. Epidemiology of hip fracture: Worldwide geographic variation. Indian J. Orthop. 2011, 45, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Johansen, A. Hip fracture. BMJ 2006, 333, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. Hip fracture epidemiological trends, outcomes, and risk factors, 1970–2009. Int. J. Gen. Med. 2010, 3, 1–17. [Google Scholar] [CrossRef]
- Bukata, S.V.; Digiovanni, B.F.; Friedman, S.M.; Hoyen, H.; Kates, A.; Kates, S.L.; Mears, S.C.; Mendelson, D.A.; Serna, F.H., Jr.; Sieber, F.E.; et al. A guide to improving the care of patients with fragility fractures. Geriatr. Orthop. Surg. Rehabil. 2011, 2, 5–37. [Google Scholar] [CrossRef]
- Simmermacher, R.K.; Bosch, A.M.; Van der Werken, C. The AO/ASIF-proximal femoral nail (PFN): A new device for the treatment of unstable proximal femoral fractures. Injury 1999, 30, 327–332. [Google Scholar] [CrossRef]
- Warren, J.A.; Sundaram, K.; Hampton, R.; McLaughlin, J.; Patterson, B.; Higuera, C.A.; Piuzzi, N.S. Cephalomedullary nailing versus sliding hip screws for Intertrochanteric and basicervical hip fractures: A propensity-matched study of short-term outcomes in over 17,000 patients. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 243–250. [Google Scholar] [CrossRef]
- Haidukewych, G.J. Intertrochanteric fractures: Ten tips to improve results. Instr. Course Lect. 2010, 59, 503–509. [Google Scholar]
- Baumgaertner, M.R.; Curtin, S.L.; Lindskog, D.M.; Keggi, J.M. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J. Bone Jt. Surg. Am. 1995, 77, 1058–1064. [Google Scholar] [CrossRef]
- Kashigar, A.; Vincent, A.; Gunton, M.J.; Backstein, D.; Safir, O.; Kuzyk, P.R. Predictors of failure for cephalomedullary nailing of proximal femoral fractures. Bone Jt. J. 2014, 96-B, 1029–1034. [Google Scholar] [CrossRef]
- Bojan, A.J.; Beimel, C.; Taglang, G.; Collin, D.; Ekholm, C.; Jonsson, A. Critical factors in cut-out complication after Gamma Nail treatment of proximal femoral fractures. BMC Musculoskelet. Disord. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, B.H.; Latz, D.; Schiffner, E.; Jungbluth, P.; Windolf, J.; Grassmann, J. CCD angle & hip fractures—Predictor of fracture symmetry? J. Orthop. 2021, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Jung, C.H.; Shen, Q.H.; Cha, Y.H.; Park, C.H.; Yoo, J.I.; Song, H.K.; Jeon, Y.; Won, Y.Y. Mechanical effect of different implant caput-collum-diaphyseal angles on the fracture surface after fixation of an unstable intertrochanteric fracture: A finite element analysis. Asian J. Surg. 2019, 42, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Tang, L.; Ye, Q.; Huang, W.; Li, J. Are computer numerical control (CNC)-manufactured patient-specific metal templates available for posterior thoracic pedicle screw insertion? Feasibility and accuracy evaluation. Eur. Spine J. 2017, 26, 2927–2933. [Google Scholar] [CrossRef]
- Owaydhah, W.H.; Alobaidy, M.A.; Alraddadi, A.S.; Soames, R.W. Three-dimensional analysis of the proximal humeral and glenoid geometry using MicroScribe 3D digitizer. Surg. Radiol. Anat. 2017, 39, 767–772. [Google Scholar] [CrossRef]
- Moldovan, F.; Gligor, A.; Bataga, T. Structured Integration and Alignment Algorithm: A Tool for Personalized Surgical Treatment of Tibial Plateau Fractures. J. Pers. Med. 2021, 11, 190. [Google Scholar] [CrossRef]
- Heiner, A.D. Structural properties of fourth-generation composite femurs and tibias. J. Biomech. 2008, 41, 3282–3284. [Google Scholar] [CrossRef]
- Ling, L.; Qu, Z.; Zhou, K. Effect of Fracture Reduction with Different Medial Cortical Support on Stability After Cephalomedullary Nail Fixation of Unstable Pertrochanteric Fractures: A Biomechanical Analysis. Indian J. Orthop. 2022, 56, 34–40. [Google Scholar] [CrossRef]
- Walmsley, D.; Nicayenzi, B.; Kuzyk, P.R.; Machin, A.; Bougherara, H.; Schemitsch, E.H.; Zdero, R. Biomechanical analysis of the cephalomedullary nail versus the trochanteric stabilizing plate for unstable intertrochanteric femur fractures. Proc. Inst. Mech. Eng. H. 2016, 230, 1133–1140. [Google Scholar] [CrossRef]
- Kwak, D.K.; Kim, W.H.; Lee, S.J.; Rhyu, S.H.; Jang, C.Y.; Yoo, J.H. Biomechanical Comparison of Three Different Intramedullary Nails for Fixation of Unstable Basicervical Intertrochanteric Fractures of the Proximal Femur: Experimental Studies. Biomed. Res. Int. 2018, 2018, 7618079. [Google Scholar] [CrossRef]
- Pastor, T.; Zderic, I.; Gehweiler, D.; Gardner, M.J.; Stoffel, K.; Richards, G.; Knobe, M.; Gueorguiev, B. Biomechanical analysis of recently released cephalomedullary nails for trochanteric femoral fracture fixation in a human cadaveric model. Arch. Orthop. Trauma. Surg. 2022, 142, 3787–3796. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.; Panagopoulos, A.; Pasiou, E.; Kourkoulis, S.K.; Diamantakos, I.; Anastopoulos, G.; Tserpes, K.; Tatani, I.; Lakoumentas, J.; Megas, P. Optimizing fixation methods for stable and unstable intertrochanteric hip fractures treated with sliding hip screw or cephalomedullary nailing: A comparative biomechanical and finite element analysis study. Injury 2022, 53, 4072–4085. [Google Scholar] [CrossRef]
- Paxton, N.C.; Wilkinson, B.G.; Fitzpatrick, D.; Owen, E.C.; Luposchainsky, S.; Dalton, P.D. Technical improvements in preparing 3D printed anatomical models for comminuted fracture preoperative planning. 3D Print. Med. 2023, 9, 25. [Google Scholar] [CrossRef]
- Law, G.W.; Wong, Y.R.; Yew, A.K.; Choh, A.C.T.; Koh, J.S.B.; Howe, T.S. Medial migration in cephalomedullary nail fixation of pertrochanteric hip fractures: A biomechanical analysis using a novel bidirectional cyclic loading model. Bone Jt. Res. 2019, 8, 313–322. [Google Scholar] [CrossRef]
- Gruneweller, N.; Leunig, J.; Zderic, I.; Gueorguiev, B.; Colcuc, C.; Wahnert, D.; Vordemvenne, T. Lumbopelvic Stabilization with Two Methods of Triangular Osteosynthesis: A Biomechanical Study. J. Clin. Med. 2024, 13, 4744. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-C.; Oh, C.-W.; Oh, J.-K. Biomechanical comparison of proximal interlocking screw constructs in different subtrochanteric fracture models. J. Orthop. Sci. 2021, 26, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, U.; Herfat, S.; Herzog, M.; Viscogliosi, P.; Pekmezci, M. Fatigue Failure in Extra-Articular Proximal Tibia Fractures: Locking Intramedullary Nail Versus Double Locking Plates-A Biomechanical Study. J. Orthop. Trauma 2017, 31, e49–e54. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Kim, Y.H.; Inoue, N.; Chao, E.Y. Kinematic simulation of fracture reduction and bone deformity correction under unilateral external fixation. J. Biomech. 2002, 35, 1047–1058. [Google Scholar] [CrossRef]
- Watanabe, Y.; Minami, G.; Takeshita, H.; Fujii, T.; Takai, S.; Hirasawa, Y. Migration of the lag screw within the femoral head: A comparison of the intramedullary hip screw and the Gamma Asia-Pacific nail. J. Orthop. Trauma 2002, 16, 104–107. [Google Scholar] [CrossRef]
- Fitzpatrick, D.C.; Sheerin, D.V.; Wolf, B.R.; Wuest, T.K. A randomized, prospective study comparing intertrochanteric hip fracture fixation with the dynamic hip screw and the dynamic helical hip system in a community practice. Iowa Orthop. J. 2011, 31, 166–172. [Google Scholar]
- Mahar, A.; Sink, E.; Faro, F.; Oka, R.; Newton, P.O. Differences in biomechanical stability of femur fracture fixation when using titanium nails of increasing diameter. J. Child. Orthop. 2007, 1, 211–215. [Google Scholar] [CrossRef]
- Kuzyk, P.R.; Zdero, R.; Shah, S.; Olsen, M.; Waddell, J.P.; Schemitsch, E.H. Femoral head lag screw position for cephalomedullary nails: A biomechanical analysis. J. Orthop. Trauma 2012, 26, 414–421. [Google Scholar] [CrossRef]
- Coviello, M.; Abate, A.; Maccagnano, G.; Ippolito, F.; Nappi, V.; Abbaticchio, A.M.; Caiaffa, E.; Caiaffa, V. Tip-apex distance as a risk factor for cut-out in cephalic double-screw nailing of intertrochanteric femur fractures: A retrospective study. Bone Jt. Open 2024, 5, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Garabano, G.; Juri, A.; Perez Alamino, L.; Rodriguez, J.A.; Pesciallo, C.A. Predicting cut-out in intertrochanteric fractures fixed with cephalomedullary nails: The role of tip-to-apex distance referenced to calcar (calTAD)––A retrospective analysis of 158 cases. Eur. J. Orthop. Surg. Traumatol. 2024, 35, 24. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Perren, S.M.; Gueorguiev, B.; Richards, R.G.; Hofmann, G.O.; Fernandez dell’Oca, A.; Hontzsch, D.; Windolf, M. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury 2014, 45 (Suppl. 1), S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Corradi, N.; Caldaria, A.; Bottin, D.; Lo Re, D.; Lorusso, V.; Morotti, C.; Valpiani, G.; Massari, L. New tip-apex distance and calcar-referenced tip-apex distance cut-offs may be the best predictors for cut-out risk after intramedullary fixation of proximal femur fractures. Sci. Rep. 2022, 12, 357. [Google Scholar] [CrossRef]
- Huang, J.-W.; Gao, X.-S.; Yang, Y.-F. Risk factors for cut-outs in geriatric intertrochanteric fractures with cephalomedullary nailing after obtaining acceptable reduction: A case–control study. BMC Musculoskelet. Disord. 2022, 23, 354. [Google Scholar] [CrossRef]
- Mahaisavariya, C.; Jitprapaikulsarn, S.; Mahaisavariya, B.; Chantarapanich, N. Torsional stability of fixation methods in basicervical femoral neck fractures: A biomechanical study. J. Orthop. Surg. Res. 2024, 19, 371. [Google Scholar] [CrossRef]
- Sadowski, C.; Lübbeke, A.; Saudan, M.; Riand, N.; Stern, R.; Hoffmeyer, P. Treatment of reverse oblique and transverse intertrochanteric fractures with use of an intramedullary nail or a 95 screw-plate: A prospective, randomized study. JBJS 2002, 84, 372–381. [Google Scholar] [CrossRef]
- Buyukdogan, K.; Caglar, O.; Isik, S.; Tokgozoglu, M.; Atilla, B. Risk factors for cut-out of double lag screw fixation in proximal femoral fractures. Injury 2017, 48, 414–418. [Google Scholar] [CrossRef]
- Zirngibl, B.; Biber, R.; Bail, H.J. How to prevent cut-out and cut-through in biaxial proximal femoral nails: Is there anything beyond lag screw positioning and tip–apex distance? Int. Orthop. 2013, 37, 1363–1368. [Google Scholar] [CrossRef][Green Version]
- Andruszkow, H.; Frink, M.; Frömke, C.; Matityahu, A.; Zeckey, C.; Mommsen, P.; Suntardjo, S.; Krettek, C.; Hildebrand, F. Tip apex distance, hip screw placement, and neck shaft angle as potential risk factors for cut-out failure of hip screws after surgical treatment of intertrochanteric fractures. Int. Orthop. 2012, 36, 2347–2354. [Google Scholar] [CrossRef]




| Length | 337 mm |
| Neck angle | 135° |
| Anteversion | 15° |
| Head diameter | 48 mm |
| Canal diameter | 10 mm |
| Material | Cortical low density Soft cancellous bone |
| Nail length | 180 mm |
| Nail diameter | 10 mm |
| CCD angle | 125°/130° |
| Lateral bending | 4° |
| Anteversion | 15° |
| Lag screw length | 100 mm |
| Lag screw diameter | 10.5 mm |
| Distal locking screw diameter | 5 mm |
| Distal locking screw length | 40 mm |
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | p-Value |
|---|---|---|---|---|---|
| Stiffness (N/mm) | 170.9 ± 3.9 | 188.8 ± 15.1 | 173.2 ± 2.7 | 183.0 ± 7.8 | 0.038 |
| Failure load (N) | 1312.0 ± 169.7 | 1350.4 ± 97.1 | 1316.5 ± 56.3 | 1327.3 ± 220.7 | 0.047 |
| Lag screw sliding (mm) | 0.64 ± 0.10 | 0.54 ± 0.11 | 0.62 ± 0.11 | 0.56 ± 0.07 | 0.003 |
| Varus collapse (degree) | 1.94 ± 0.41 | 2.25 ± 0.27 | 1.94 ± 0.25 | 1.97 ± 0.44 | 0.013 |
| Rotation (degree) | 1.42 ± 0.19 | 1.72 ± 0.09 | 1.33 ± 0.17 | 1.66 ± 0.18 | 0.025 |
| Retroversion (degree) | 0.51 ± 0.12 | 0.58 ± 0.21 | 0.49 ± 0.15 | 0.58 ± 0.14 | 0.280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.-C.; Lee, S.-J.; Song, H.K. Biomechanical Analysis of Fixation Strength in Unstable Intertrochanteric Femoral Fracture Models Based on the Caput–Collum–Diaphyseal Angle of Cephalomedullary Nails and Position of Lag Screws. J. Clin. Med. 2025, 14, 6495. https://doi.org/10.3390/jcm14186495
Yoon Y-C, Lee S-J, Song HK. Biomechanical Analysis of Fixation Strength in Unstable Intertrochanteric Femoral Fracture Models Based on the Caput–Collum–Diaphyseal Angle of Cephalomedullary Nails and Position of Lag Screws. Journal of Clinical Medicine. 2025; 14(18):6495. https://doi.org/10.3390/jcm14186495
Chicago/Turabian StyleYoon, Yong-Cheol, Sung-Jae Lee, and Hyung Keun Song. 2025. "Biomechanical Analysis of Fixation Strength in Unstable Intertrochanteric Femoral Fracture Models Based on the Caput–Collum–Diaphyseal Angle of Cephalomedullary Nails and Position of Lag Screws" Journal of Clinical Medicine 14, no. 18: 6495. https://doi.org/10.3390/jcm14186495
APA StyleYoon, Y.-C., Lee, S.-J., & Song, H. K. (2025). Biomechanical Analysis of Fixation Strength in Unstable Intertrochanteric Femoral Fracture Models Based on the Caput–Collum–Diaphyseal Angle of Cephalomedullary Nails and Position of Lag Screws. Journal of Clinical Medicine, 14(18), 6495. https://doi.org/10.3390/jcm14186495





