Clinical Burden of People with Symptomatic and Exacerbating COPD While on Triple Inhaled Therapy †
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Source and Study Populations
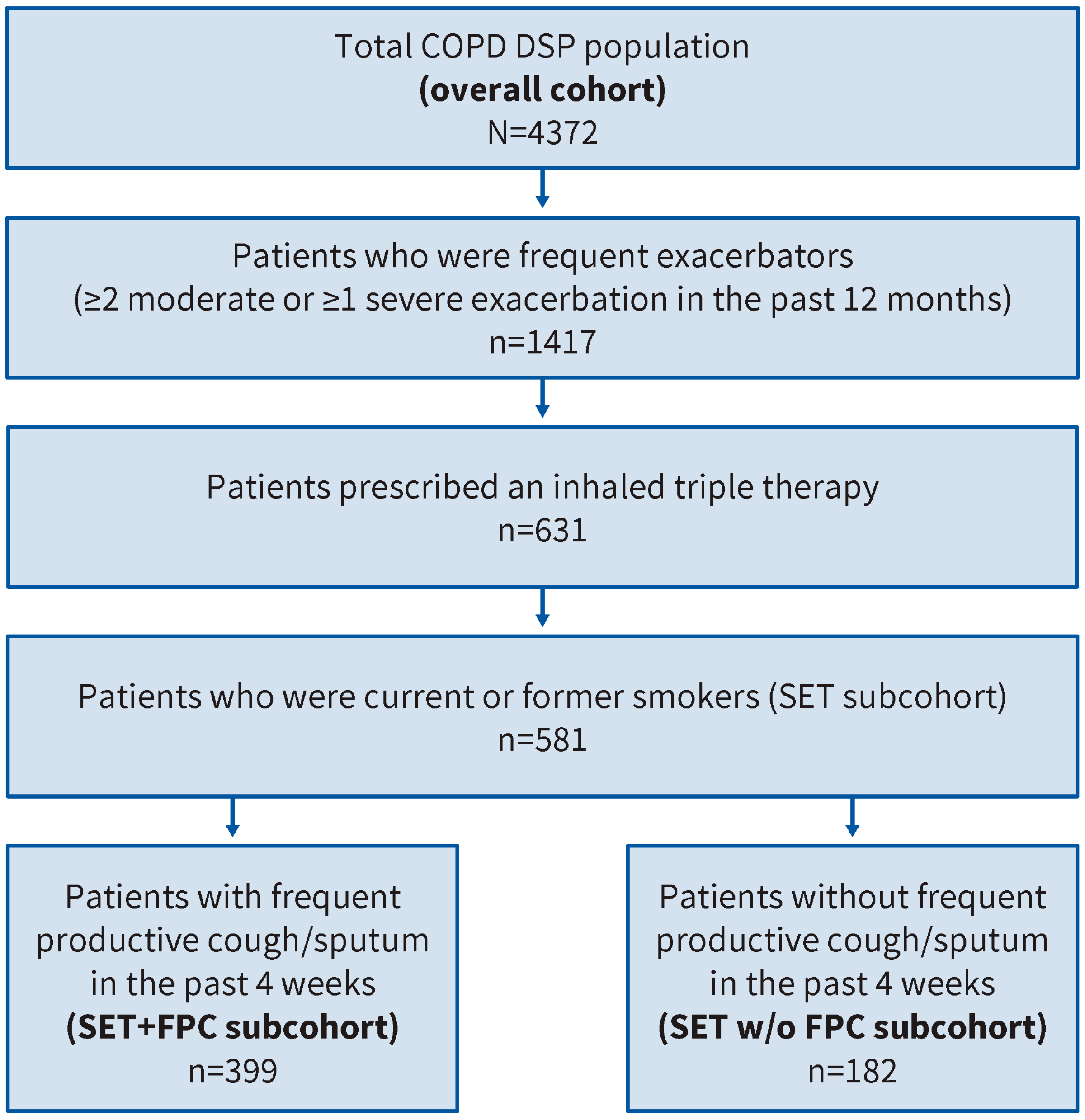
2.2.1. Overall Cohort
2.2.2. Study Populations
2.3. Ethics
2.4. Measures
2.5. Data Analysis
3. Results
3.1. Sociodemographic and Medical Conditions
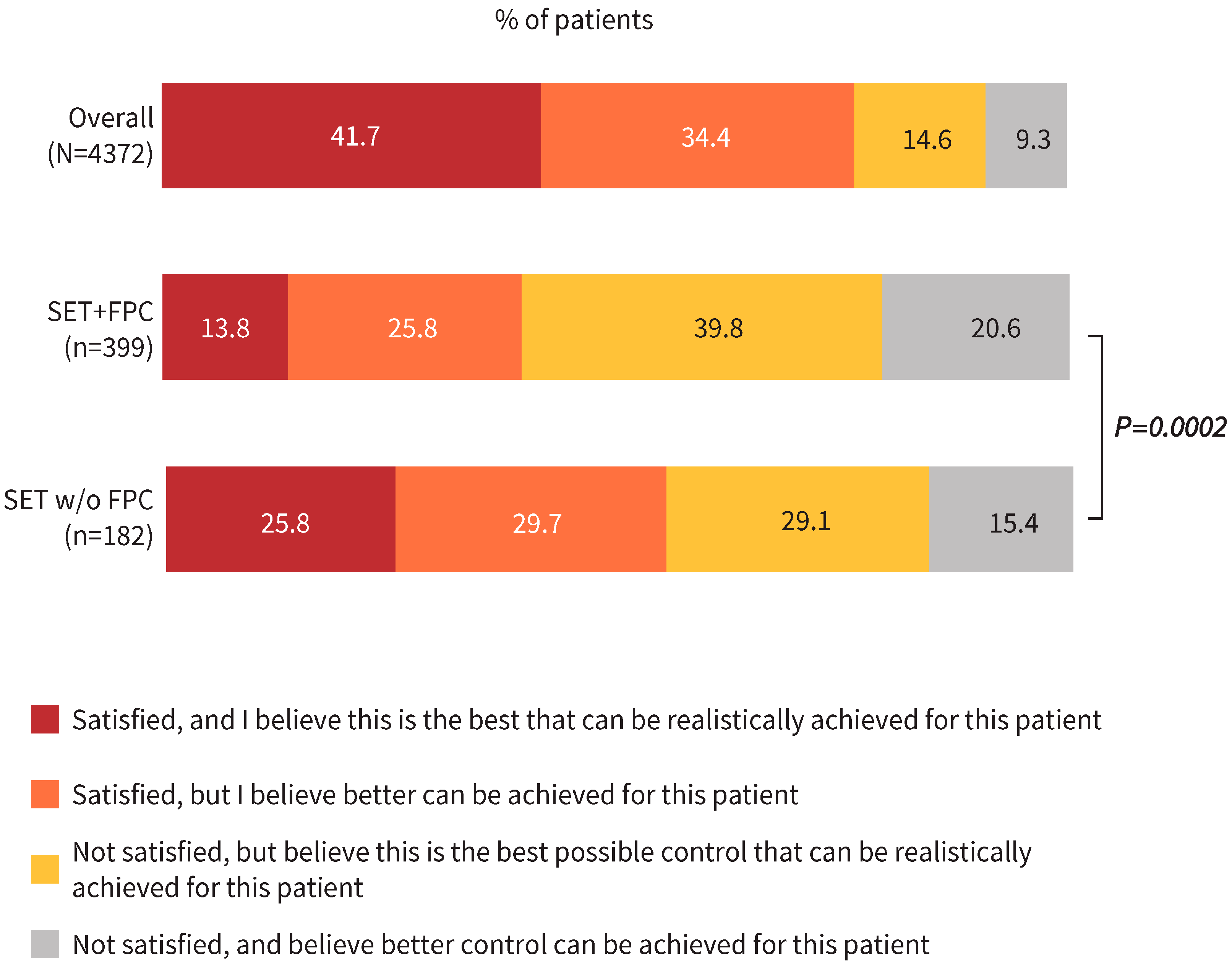
3.2. Physicians’ Satisfaction with COPD Control
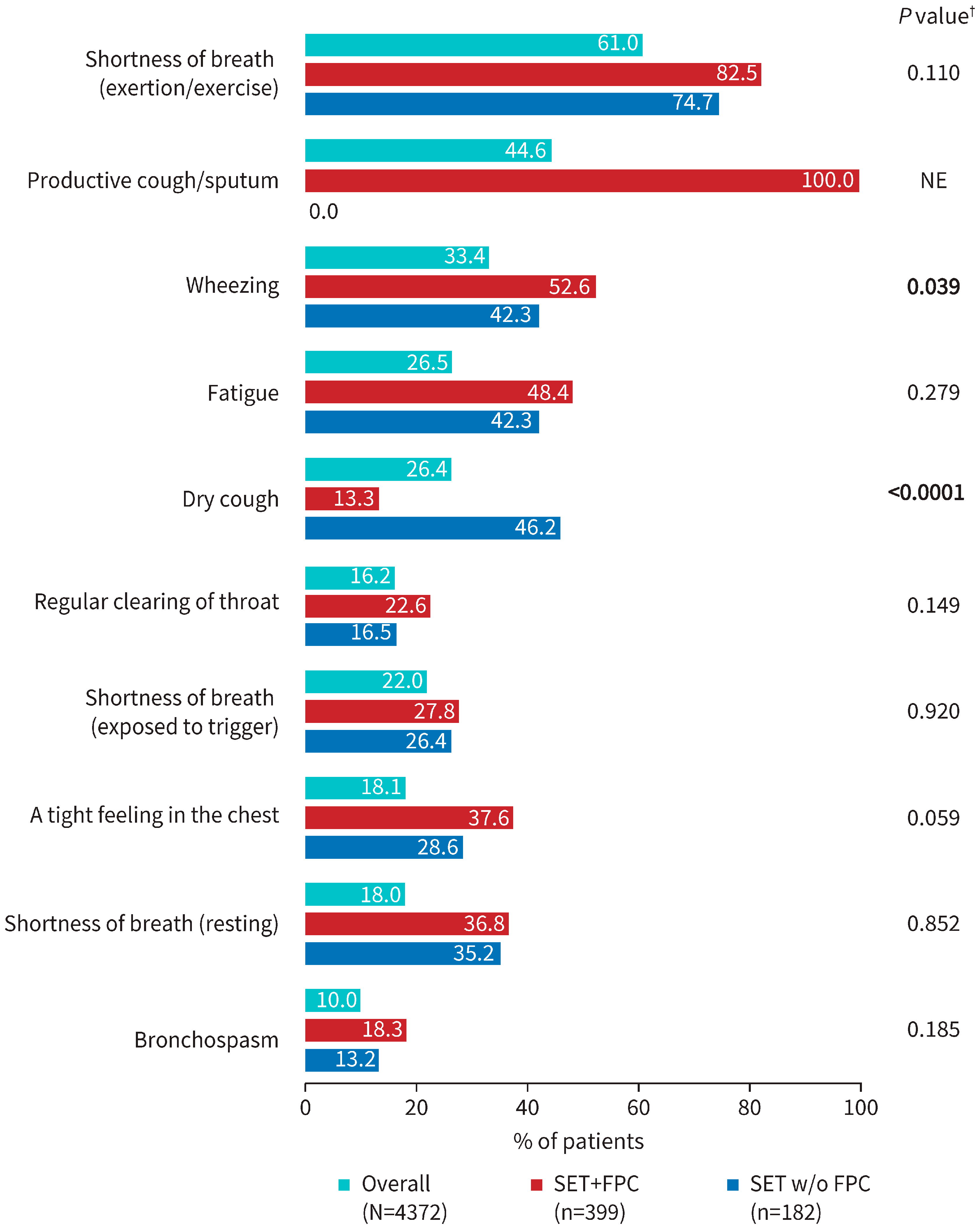
3.3. Physician-Reported Severity of Patient COPD Symptoms
3.4. Patient-Reported Outcome Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BD | Bronchodilator |
| BEC | Blood eosinophil count |
| CAT | COPD Assessment Test |
| COPD | Chronic obstructive pulmonary disease |
| DSP | Disease Specific Programme |
| EQ-VAS | European Quality of Life Visual Analogue Scale |
| FEV1 | Forced expiratory volume in 1 s |
| FPC | Frequent productive cough |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| HRQOL | Health-related quality of life |
| mMRC | Modified Medical Research Council |
| NE | Not evaluable |
| Q | Quartile |
| RCT | Randomized controlled trials |
| SET | Current or former smokers with frequent/severe exacerbations while on triple inhaled therapy |
References
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes de Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and nomenclature of chronic obstructive pulmonary disease: Time for its revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, M.; Feenstra, T.L.; Boland, M.; Briggs, A.H.; Borg, S.; Jansson, S.A.; Risebrough, N.A.; Slejko, J.F.; Rutten-van Mölken, M.P. Prediction models for exacerbations in different COPD patient populations: Comparing results of five large data sources. Int. J. Chron. Obstruct Pulmon Dis. 2017, 12, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Skolnik, N.; Hansen, G.J.; Anzueto, A.; Donaldson, G.C.; Dransfield, M.T.; Varghese, P. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur. J. Intern. Med. 2020, 73, 1–6. [Google Scholar] [CrossRef]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef]
- Krishnan, J.K.; Criner, G.J.; Lashari, B.H.; Martinez, F.J.; Kim, V.; Lindoulsi, A.; Khokhlovich, E.; Altman, P.; Karcher, H.; Schoenberger, M. Disease onset and burden in patients with chronic bronchitis and COPD: A Real-World Evidence Study. Chronic Obstr. Pulm. Dis. 2025, 12, 127–136. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yoon, H.K.; Lee, S.Y.; Kim, J.W.; Choi, H.S.; Kim, Y.-I.; Jung, K.-S.; Yoo, K.H.; Kim, W.J.; Rhee, C.K. Comparison of clinical characteristics between chronic bronchitis and non-chronic bronchitis in patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 2022, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report). Available online: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf (accessed on 28 May 2024).
- Igboekwe, E.; Verma, S.; Paczkowski, R. Real-world disease burden and healthcare resource utilization among patients with COPD and asthma using triple therapy (FF/UMEC/VI) in the United States. Int. J. Chron. Obstruct Pulmon Dis. 2024, 19, 281–296. [Google Scholar] [CrossRef]
- Chen, S.; Small, M.; Lindner, L.; Xu, X. Symptomatic burden of COPD for patients receiving dual or triple therapy. Int. J. Chron. Obstruct Pulmon Dis. 2018, 13, 1365–1376. [Google Scholar] [CrossRef]
- Rabe, K.F.; Rennard, S.; Martinez, F.J.; Celli, B.R.; Singh, D.; Papi, A.; Bafadhel, M.; Heble, J.; Radwan, A.; Soler, X.; et al. Targeting type 2 inflammation and epithelial alarmins in chronic obstructive pulmonary disease: A biologics outlook. Am. J. Respir. Crit. Care Med. 2023, 208, 395–405. [Google Scholar] [CrossRef]
- Kersul, A.L.; Cosio, B.G. Biologics in COPD. Open Respir. Arch. 2024, 6, 100306. [Google Scholar] [CrossRef]
- Plichta, J.; Kuna, P.; Panek, M. Biologic drugs in the treatment of chronic inflammatory pulmonary diseases: Recent developments and future perspectives. Front. Immunol. 2023, 14, 1207641. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; Patel, N.; et al. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N. Engl. J. Med. 2024, 390, 2274–2283. [Google Scholar] [CrossRef]
- Varricchi, G.; Poto, R. Towards precision medicine in COPD: Targeting type 2 cytokines and alarmins. Eur. J. Intern. Med. 2024, 125, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Benford, M.; Harris, N.; Karavali, M.; Piercy, J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes—A means to understand. Curr. Med. Res. Opin. 2008, 24, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Higgins, V.; de Courcy, J.; Doslikova, K.; Davis, V.A.; Karavali, M.; Piercy, J. Real-world evidence generation from patients, their caregivers and physicians supporting clinical, regulatory and guideline decisions: An update on Disease Specific Programmes. Curr. Med. Res. Opin. 2023, 39, 1707–1715. [Google Scholar] [CrossRef]
- Babineaux, S.M.; Curtis, B.; Holbrook, T.; Milligan, G.; Piercy, J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: The Disease Specific Programme. BMJ Open 2016, 6, e010352. [Google Scholar] [CrossRef]
- Higgins, V.; Piercy, J.; Roughley, A.; Milligan, G.; Leith, A.; Siddall, J.; Benford, M. Trends in medication use in patients with type 2 diabetes mellitus: A long-term view of real-world treatment between 2000 and 2015. Diabetes Metab. Syndr. Obes. 2016, 9, 371–380. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef]
- Singh, D.; Guller, P.; Reid, F.; Doffman, S.; Seppälä, U.; Psallidas, I.; Moate, R.; Smith, R.; Kiraga, J.; Jimenez, E.; et al. S92 FRONTIER-4: A phase 2a study to investigate tozorakimab (anti-IL-33 mAb) in COPD. Thorax 2024, 79, A68. [Google Scholar] [CrossRef]
- Dotan, Y.; So, J.Y.; Kim, V. Chronic Bronchitis: Where are we now? Chronic Obstr. Pulm. Dis. 2019, 6, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Rapsomaniki, E.; Janson, C.; Keen, C.; Make, B.J.; Burgel, P.-R.; Tomaszewski, E.L.; Müllerová, H.; Reddel, H.K.; Benhabib, G.; et al. Frequent productive cough: Symptom burden and future exacerbation risk among patients with asthma and/or COPD in the NOVELTY study. Respir. Med. 2022, 200, 106921. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Adamek, L.; Nadeau, G.; Banik, N. Comparisons of health status scores with MRC grades in COPD: Implications for the GOLD 2011 classification. Eur. Respir. J. 2013, 42, 647–654. [Google Scholar] [CrossRef]
- Janssens, W.; Nordon, C.; Coak, E.; Pennant, T.; Bell, G.; Mullerova, H.; Draffan, L.; Alves, J.A.; Fagerås, M.; De Soyza, A. Characteristics of exacerbating COPD patients with productive cough and on inhaled triple therapy, by smoking status: A real-world multi-country study. Eur. Respir. J. 2024, 64, PA1288. [Google Scholar] [CrossRef]
- De Soyza, A.; Nordon, C.; Fagerås, M.; Bell, G.; Mullerova, H.; Pennant, T.; Draffan, L.; Alves, J.A.; Coak, E.; Janssens, W. Perception of COPD control by physicians managing symptomatic exacerbating patients receiving inhaled triple therapy: A real-world multi-country 2022 survey. Eur. Respir. J. 2024, 64, PA1289. [Google Scholar] [CrossRef]
- van Eerd, E.A.; van der Meer, R.M.; van Schayck, O.C.; Kotz, D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2016, 2016, CD010744. [Google Scholar] [CrossRef]
- Landt, E.M.; Çolak, Y.; Nordestgaard, B.G.; Lange, P.; Dahl, M. Chronic cough associated with COPD exacerbation, pneumonia and death in the general population. ERJ Open Res. 2024, 10, 00697–02023. [Google Scholar] [CrossRef]
- Miravitlles, M.; Ribera, A. Understanding the impact of symptoms on the burden of COPD. Respir. Res. 2017, 18, 67. [Google Scholar] [CrossRef]
- Mettler, S.K.; Nath, H.P.; Grumley, S.; Orejas, J.L.; Dolliver, W.R.; Nardelli, P.; Yen, A.C.; Kligerman, S.J.; Jacobs, K.; Manapragada, P.P.; et al. Silent airway mucus plugs in COPD and clinical implications. Chest 2024, 166, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Brusselle, G.; Dal Negro, R.W.; Ferrer, M.; Kardos, P.; Levy, M.L.; Perez, T.; Soler Cataluna, J.J.; van der Molen, T.; Adamek, L.; et al. Properties of the COPD assessment test in a cross-sectional European study. Eur. Respir. J. 2011, 38, 29–35. [Google Scholar] [CrossRef] [PubMed]
- EUROQOL 2024. EUROQOL: Population Norms. Available online: https://euroqol.org/information-and-support/resources/population-norms/ (accessed on 2 July 2024).
- Chen, S.; Miravitlles, M.; Rhee, C.K.; Pavord, I.D.; Jones, R.; Carter, V.; Emmanuel, B.; Alacqua, M.; Price, D.B. Patients with chronic obstructive pulmonary disease and evidence of eosinophilic inflammation experience exacerbations despite receiving maximal inhaled maintenance therapy. Int. J. Chron. Obstruct Pulmon Dis. 2022, 17, 2187–2200. [Google Scholar] [CrossRef]
| Overall N = 4372 | SET+FPC n = 399 | SET w/o FPC n = 182 | p Value * | |
|---|---|---|---|---|
| Mean (SD) age, years | 64.7 (10.9) | 69.1 (9.2) | 67.2 (9.3) | 0.019 |
| Male, n (%) | 2965 (67.8) | 288 (72.2) | 124 (68.1) | 0.375 |
| Smoking status, n (%) | 0.713 | |||
| Current smoker | 1343 (30.7) | 150 (37.6) | 72 (39.6) | |
| Former smoker | 2510 (57.4) | 249 (62.4) | 110 (60.4) | |
| Never smoked | 441 (10.1) | n/a † | n/a † | |
| Unknown | 78 (1.8) | n/a † | n/a † | |
| Cardiovascular and metabolic diseases, n (%) | ||||
| Hypertension | 2148 (49.1) | 256 (64.2) | 103 (56.6) | 0.097 |
| Elevated cholesterol/hyperlipidemia | 987 (22.6) | 114 (28.6) | 45 (24.7) | 0.367 |
| Coronary artery disease | 393 (9.0) | 51 (12.8) | 25 (13.7) | 0.791 |
| Cardiac arrhythmias | 355 (8.1) | 79 (19.8) | 17 (9.3) | 0.002 |
| Congestive heart failure | 206 (4.7) | 37 (9.3) | 16 (8.8) | 1.000 |
| Cerebrovascular disease | 174 (4.0) | 32 (8.0) | 10 (5.5) | 0.305 |
| Angina pectoris | 158 (3.6) | 26 (6.5) | 15 (8.2) | 0.486 |
| Myocardial infarction | 148 (3.4) | 25 (6.3) | 14 (7.7) | 0.592 |
| Diabetes without chronic complication | 601 (13.7) | 78 (19.5) | 33 (18.1) | 0.734 |
| Diabetes with chronic complication | 143 (3.3) | 24 (6.0) | 5 (2.7) | 0.104 |
| Asthma codiagnosis, n (%) | 123 (2.8) | 13 (3.3) | 5 (2.7) | 1.000 |
| Oxygen therapy use, n (%) | 0.7703 | |||
| Ambulatory | 404 (9.2) | 57 (14.3) | 22 (12.1) | |
| Long-term | 317 (7.3) | 83 (20.8) | 37 (20.3) | |
| None | 3651 (83.5) | 259 (64.9) | 123 (67.6) | |
| Most recent BEC, cells/μL ‡ | ||||
| Patients with missing data | n = 3793 | n = 342 | n = 148 | |
| Patients included | n = 579 | n = 57 | n = 34 | |
| Mean (SD) | 337.7 (594.2) | 277.5 (250.1) | 306.2 (664.4) | 0.770 |
| Median (Q1, Q3) | 184.0 (120.0, 300.0) | 200.0 (124.0, 300.0) | 193.5 (104.5, 254.5) | |
| <150, n (%) | 184 (31.8) | 16 (28.1) | 13 (38.2) | 0.242 |
| 150–299, n (%) | 238 (41.1) | 24 (42.1) | 14 (41.2) | |
| ≥300, n (%) | 157 (27.1) | 17 (29.8) | 7 (20.6) | |
| Most recent post-BD FEV1 (% predicted) ‡ | ||||
| Patients with missing data | n = 2535 | n = 212 | n = 96 | |
| Patients included | n = 1837 | n = 187 | n = 86 | |
| Mean (SD) | 65.4 (17.9) | 50.2 (16.5) | 48.9 (15.7) | 0.517 |
| Median (Q1, Q3) | 68.0 (55.0, 79.0) | 49.0 (38.0, 62.0) | 49.5 (38.0, 61.0) | |
| Degree of airflow obstruction (GOLD stage), n (%) | 0.984 | |||
| ≥80% (GOLD 1 [mild]) | 454 (24.7) | 13 (7.0) | 2 (2.3) | |
| 50–79% (GOLD 2 [moderate]) | 1024 (55.7) | 73 (39.0) | 41 (47.7) | |
| 30–49% (GOLD 3 [severe]) | 282 (15.4) | 83 (44.4) | 33 (38.4) | |
| <30% (GOLD 4 [very severe]) | 77 (4.2) | 18 (9.6) | 10 (11.6) | |
| Pattern of exacerbations in the last 12 months | 0.010 | |||
| None or mild, n (%) | 2281 (52.2) | n/a † | n/a † | |
| 1 moderate, n (%) | 674 (15.4) | n/a † | n/a † | |
| ≥2 moderate (no severe), n (%) | 421 (9.6) | 128 (32.1) | 38 (20.9) | |
| ≥1 severe (±moderate), n (%) | 996 (22.8) | 271 (67.9) | 144 (79.1) | |
| Mean (SD) number of exacerbations | 1.1 (1.4) | 2.5 (1.4) | 2.3 (1.3) | 0.073 |
| Breathlessness (dyspnea scale), n (%) ¶ | 0.004 | |||
| 1181 (27.0) | 14 (3.5) | 11 (6.0) | |
| 1610 (36.8) | 73 (18.3) | 55 (30.2) | |
| 959 (21.9) | 142 (35.6) | 58 (31.9) | |
| 464 (10.6) | 134 (33.6) | 35 (19.2) | |
| 158 (3.6) | 36 (9.0) | 23 (12.6) | |
| Patients with a breathlessness score grade ≥ 2 | 1581 (36.2) | 312 (78.2) | 116 (63.7) | 0.0004 |
| FPC in the last 4 weeks, n (%) | 1948 (44.6) | 399 (100.0) § | n/a † | |
| Prescription of triple inhaled therapy, n (%) | 1205 (27.6) | 399 (100.0) § | 182 (100.0) § | |
| Duration of current treatment, weeks # | ||||
| Mean (SD) | 69.7 (112.3) | 54.8 (72.1) | 55.2 (62.2) | 0.957 |
| Median (Q1, Q3) | 48.0 (12.0, 80.0) | 36.0 (12.0, 54.0) | 42.5 (17.8, 71.2) |
| Overall N = 4372 | SET+FPC n = 399 | SET w/o FPC n = 182 | p Value * | |
|---|---|---|---|---|
| CAT total score † | ||||
| Patients with missing data | n = 2497 | n = 271 | n = 113 | |
| Patients included | n = 1875 | n = 128 | n = 69 | |
| Mean score (SD) | 17.9 (8.6) | 25.0 (7.6) | 22.8 (8.8) | 0.076 |
| Median (Q1, Q3) | 18.0 (11.0, 24.0) | 26.0 (21.0, 30.0) | 24.0 (16.0, 30.0) | |
| CAT cough domain score †,‡ | ||||
| Patients with missing data | n = 2535 | n = 272 | n = 115 | |
| Patients included | n = 1837 | n = 127 | n = 67 | |
| <2, n (%) | 395 (21.5) | 7 (5.5) | 4 (6.0) | 1.000 |
| ≥2, n (%) | 1442 (78.5) | 120 (94.5) | 63 (94.0) | |
| CAT phlegm/sputum domain score †,¶ | ||||
| Patients with missing data | n = 2535 | n = 272 | n = 115 | |
| Patients included | n = 1837 | n = 127 | n = 67 | |
| <2, n (%) | 524 (28.5) | 13 (10.2) | 14 (20.9) | 0.051 |
| ≥2, n (%) | 1313 (71.5) | 114 (89.8) | 53 (79.1) | |
| EQ-VAS † | ||||
| Patients with missing data | n = 2510 | n = 271 | n = 113 | |
| Patients included | n = 1862 | n = 128 | n = 69 | |
| Mean (SD) score | 67.2 (17.7) | 52.9 (17.6) | 57.6 (19.6) | 0.084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Soyza, A.; Nordon, C.; Coak, E.; Pennant, T.; Mullerova, H.; Fageras, M.; Alves, J.A.; Janssens, W. Clinical Burden of People with Symptomatic and Exacerbating COPD While on Triple Inhaled Therapy. J. Clin. Med. 2025, 14, 6488. https://doi.org/10.3390/jcm14186488
De Soyza A, Nordon C, Coak E, Pennant T, Mullerova H, Fageras M, Alves JA, Janssens W. Clinical Burden of People with Symptomatic and Exacerbating COPD While on Triple Inhaled Therapy. Journal of Clinical Medicine. 2025; 14(18):6488. https://doi.org/10.3390/jcm14186488
Chicago/Turabian StyleDe Soyza, Anthony, Clementine Nordon, Emily Coak, Tia Pennant, Hana Mullerova, Malin Fageras, João Andre Alves, and Wim Janssens. 2025. "Clinical Burden of People with Symptomatic and Exacerbating COPD While on Triple Inhaled Therapy" Journal of Clinical Medicine 14, no. 18: 6488. https://doi.org/10.3390/jcm14186488
APA StyleDe Soyza, A., Nordon, C., Coak, E., Pennant, T., Mullerova, H., Fageras, M., Alves, J. A., & Janssens, W. (2025). Clinical Burden of People with Symptomatic and Exacerbating COPD While on Triple Inhaled Therapy. Journal of Clinical Medicine, 14(18), 6488. https://doi.org/10.3390/jcm14186488







