Impact of CARD14 rs34367357 Mutation, Nutrition Status, and Seasonality on the Response to Biologic Therapy in Psoriasis—A Retrospective Observational Single-Center Study
Abstract
1. Introduction
2. Aims
3. Material and Methods
3.1. Study Design and Population
3.2. Clinical Assessment
3.3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BSA | Body Surface Area |
| CI | Confidence Interval |
| DLQI | Dermatology Life Quality Index |
| MANOVA | Multivariate Analysis of Variance |
| NHF | (Polish) National Health Fund |
| PASI | Psoriasis Area and Severity Index |
| QoL | Quality of Life |
| SD | Standard Deviation |
| SMPC | Summary of Product Characteristics |
| UV | Ultraviolet |
References
- Armstrong, A.W.; Blauvelt, A.; Callis Duffin, K.; Huang, Y.H.; Savage, L.J.; Guo, L.; Merola, J.F. Psoriasis. Nat. Rev. Dis. Primers 2025, 11, 45. [Google Scholar] [CrossRef]
- Eggers, K.R.; Møllegaard, K.M.; Gregersen, L.; Overgaard, S.H.; Hikmat, Z.; Ellingsen, T.; Kjeldsen, J.; Pedersen, A.K.; Petersen, S.R.; Jawhara, M.; et al. Impact of Obesity on Treatment Response in Patients With Chronic Inflammatory Disease Receiving Biologic Therapy: Secondary Analysis of the Prospective Multicentre BELIEVE Cohort Study. Scand. J. Immunol. 2025, 101, e70035. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Román-Galeano, N.M.; Verde, L.; Annunziata, G.; Marchetti, M.; Matos, A.; Campuzano-Donoso, M.; Reytor-González, C.; Muscogiuri, G.; Barrea, L.; et al. Targeting Cytokine Dysregulation in Psoriasis: The Role of Dietary Interventions in Modulating the Immune Response. Int. J. Mol. Sci. 2025, 26, 2895. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Liu, L.; Wang, C.; Sun, X.; Zhou, Y.; Hong, S.; Cai, X.; Xu, W.; Li, X. Global prevalence of obesity in patients with psoriasis: An analysis in the past two decades. Autoimmun. Rev. 2024, 23, 103577. [Google Scholar] [CrossRef]
- Lu, L.; Xu, Y.; Shi, M.; Liu, A. Psychosocial interventions for psoriasis: A Bayesian network meta-analysis. J. Dermatol. Treat. 2025, 36, 2427321. [Google Scholar] [CrossRef]
- Monis, M.; Mathur, P.; Ritu; Mishra, A.K.; Rani, L. Comprehensive Insights into Psoriasis: Pathophysiology, An Advanced Exploration of Current Landscape and Future Prospects in “Therapeutic Strategies”. Recent Adv. Inflamm. Allergy Drug Discov. 2025, 19, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Chakith, M.R.S.; Pradeep, S.; Gangadhar, M.; Maheshwari, N.C.; Pasha, S.; Kollur, S.P.; Shivamallu, C.; Allur Mallanna, S. Advancements in understanding and treating psoriasis: A comprehensive review of pathophysiology, diagnosis, and therapeutic approaches. PeerJ 2025, 13, e19325. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Allela, O.Q.B.; Abdul Kareem, R.; Sanghvi, G.; PadmaPriya, G.; Thakur, R.; Kumari, M.; Gupta, S.; Khaitov, K.; Sameer, H.N.; et al. Psoriasis: Immunological and genetic blueprints driving pathogenesis and potential for personalized therapies. Iran. J. Basic Med. Sci. 2025, 28, 680–690. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Li, W.; Sun, Y.; Liu, H. Switching biologic agent in patients with psoriasis: A systematic review and meta-analysis. J. Dermatol. Treat. 2025, 36, 2521082. [Google Scholar] [CrossRef] [PubMed]
- Cassalia, F.; Lunardon, A.; Frattin, G.; Danese, A.; Caroppo, F.; Fortina, A.B. How Hormonal Balance Changes Lives in Women with Psoriasis. J. Clin. Med. 2025, 14, 582. [Google Scholar] [CrossRef]
- Dattola, A.; Bernardini, N.; Caldarola, G.; Coppola, R.; De Simone, C.; Giordano, D.; Giunta, A.; Moretta, G.; Pagnanelli, G.; Panasiti, V.; et al. Effectiveness of Ixekizumab in Elderly Patients for the Treatment of Moderate-to-Severe Psoriasis: Results From a Multicenter, Retrospective Real-Life Study in the Lazio Region. Dermatol. Pract. Concept. 2024, 14, e2024166. [Google Scholar] [CrossRef] [PubMed]
- Eder, L.; Mylvaganam, S.; Pardo Pardo, J.; Petkovic, J.; Strand, V.; Mease, P.; Colaco, K. Sex-related differences in patient characteristics, and efficacy and safety of advanced therapies in randomised clinical trials in psoriatic arthritis: A systematic literature review and meta-analysis. Lancet Rheumatol. 2023, 5, e716–e727. [Google Scholar] [CrossRef] [PubMed]
- Belli, R.; Dattolo, A.; Sampogna, F.; Gubinelli, E.; Lulli, D.; Moretta, G.; Scala, E.; Sanna, L.; Megna, M.; Cannizzaro, M.V.; et al. Leptin: A gender and obesity-related marker predictive of metabolic comorbidities and therapeutic response to anti-IL-23 biologic drugs in psoriatic patients. Front. Immunol. 2025, 16, 1607312. [Google Scholar] [CrossRef]
- Costantino, M.; Conti, V.; Corbi, G.; Giudice, V.; Caro, F.; Filippelli, A. Marked Reduction of Oxidant Species after Sulfureous Crenotherapy in Females with Joint Diseases and Psoriasis: A Retrospective Real-Life Study. J. Clin. Med. 2023, 12, 5731. [Google Scholar] [CrossRef]
- Blicharz, L.; Czuwara, J.; Rudnicka, L.; Torrelo, A. Autoinflammatory Keratinization Diseases-The Concept, Pathophysiology, and Clinical Implications. Clin. Rev. Allergy Immunol. 2023, 65, 377–402. [Google Scholar] [CrossRef]
- Suleman, S.; Chhabra, G.; Raza, R.; Hamid, A.; Qureshi, J.A.; Ahmad, N. Association of CARD14 Single-Nucleotide Polymorphisms with Psoriasis. Int. J. Mol. Sci. 2022, 23, 9336. [Google Scholar] [CrossRef]
- Potestio, L.; Martora, F.; Lauletta, G.; Vallone, Y.; Battista, T.; Megna, M. The Role of Interleukin 23/17 Axis in Psoriasis Management: A Comprehensive Review of Clinical Trials. Clin. Cosmet. Investig. Dermatol. 2024, 17, 829–842. [Google Scholar] [CrossRef]
- Akiyama, M. Autoinflammatory Keratinization Diseases (AiKDs): Expansion of Disorders to Be Included. Front Immunol. 2020, 11, 280. [Google Scholar] [CrossRef]
- Roszkiewicz, M.; Dopytalska, K.; Szymańska, E.; Jakimiuk, A.; Walecka, I. Environmental risk factors and epigenetic alternations in psoriasis. Ann. Agric. Environ. Med. 2020, 27, 335–342. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Skibińska, M.; Ciążyńska, M.; Noweta, M.; Czerwińska, A.; Krzyścin, J.; Narbutt, J.; Lesiak, A. Psoriasis and Seasonality: Exploring the Genetic and Epigenetic Interactions. Int. J. Mol. Sci. 2024, 25, 11670. [Google Scholar] [CrossRef]
- Jensen, K.K.; Serup, J.; Alsing, K.K. Psoriasis and seasonal variation: A systematic review on reports from Northern and Central Europe-Little overall variation but distinctive subsets with improvement in summer or wintertime. Skin Res. Technol. 2022, 28, 180–186. [Google Scholar] [CrossRef]
- Purzycka-Bohdan, D.; Kisielnicka, A.; Zabłotna, M.; Nedoszytko, B.; Nowicki, R.J.; Reich, A.; Samotij, D.; Szczęch, J.; Krasowska, D.; Bartosińska, J.; et al. Chronic Plaque Psoriasis in Poland: Disease Severity, Prevalence of Comorbidities, and Quality of Life. J. Clin. Med. 2022, 11, 1254. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Noweta, M.; Narbutt, J.; Owczarek, W.; Ciążyńska, M.; Czerwińska, A.; Krzyścin, J.; Lesiak, A.; Skibińska, M. Does the effectiveness of biological medications in the treatment for psoriasis depend on the moment of starting therapy? A preliminary study. Postepy Dermatol. Allergol. 2024, 41, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Nigro, A.; Taylor, A.C.; Saal, R.; Ormaza Vera, A.; Enos, C. The Effects of Cardiometabolic Comorbidities on Biologic Treatment for Psoriasis with Respect to PASI Scores: A Qualitative Systematic Review. Psoriasis 2024, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prema, S.S.; Shanmugamprema, D. Systemic Psoriasis: From Molecular Mechanisms to Global Management Strategies. Clin. Rev. Allergy Immunol. 2025, 68, 79. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.R.; Nascimento, A.; Marque, C.; Miot, H.A. Seasonality of the hospitalizations at a dermatologic ward (2007–2017). An. Bras. Dermatol. 2018, 93, 755–758. [Google Scholar] [CrossRef]
- Ferguson, F.J.; Lada, G.; Hunter, H.J.A.; Bundy, C.; Henry, A.L.; Griffiths, C.E.M.; Kleyn, C.E. Diurnal and seasonal variation in psoriasis symptoms. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e45–e47. [Google Scholar] [CrossRef]
- Mrowietz, U.; Dieckmann, T.; Gerdes, S.; Szymczak, S.; von Spreckelsen, R.; Körber, A. ActiPso: Definition of activity types for psoriatic disease: A novel marker for an advanced disease classification. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2027–2033. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Q.; Luo, Y.; Lu, W.; Jin, L.; Chen, M.; Zhu, W.; Kuang, Y. Seasonal Variation of Psoriasis and Its Impact in the Therapeutic Management: A Retrospective Study on Chinese Patients. Clin. Cosmet. Investig. Dermatol. 2021, 14, 459–465. [Google Scholar] [CrossRef]
- Camilion, J.V.; Khanna, S.; Anasseri, S.; Laney, C.; Mayrovitz, H.N. Physiological, Pathological, and Circadian Factors Impacting Skin Hydration. Cureus 2022, 14, e27666. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Wang, T. Gut microbiota and skin pathologies: Mechanism of the gut-skin axis in atopic dermatitis and psoriasis. Int. Immunopharmacol. 2024, 141, 112658. [Google Scholar] [CrossRef]
- Pachauri, A.; Sharma, S. Unravelling the gut-skin axis: The role of gut microbiota in pathogenesis and management of psoriasis. Inflammopharmacology 2025, 33, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bi, H.; Lin, K.; Chen, Y.; Xian, H.; Li, Y.; Xie, H.; Zheng, G.; Wang, P.; Chen, Y.; et al. The Skin-Brain Axis in Psoriasis and Depression: Roles of Inflammation, Hormones, Neuroendocrine Pathways, Neuropeptides, and the Microbiome. Psoriasis 2025, 15, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, J.Y.; Cheong, S.; Kang, J.H. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: A nationwide population-based cohort study. Obes. Res. Clin. Pract. 2023, 17, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Farías, M.M.; Serrano, V.; de la Cruz, C. Psoriasis and obesity: A review and practical recommendations. Actas Dermo-Sifiliográficas 2011, 102, 505–509. [Google Scholar] [CrossRef]
- Bassi, M.; Singh, S. Impact of Obesity on Response to Biologic Therapies in Patients with Inflammatory Bowel Diseases. BioDrugs 2022, 36, 197–203. [Google Scholar] [CrossRef]
- Jordan, C.T.; Cao, L.; Roberson, E.D.; Pierson, K.C.; Yang, C.F.; Joyce, C.E.; Ryan, C.; Duan, S.; Helms, C.A.; Liu, Y.; et al. PSORS2 is due to mutations in CARD14. Am. J. Hum. Genet. 2012, 90, 784–795. [Google Scholar] [CrossRef]
- Mellett, M.; Meier, B.; Mohanan, D.; Schairer, R.; Cheng, P.; Satoh, T.K.; Kiefer, B.; Ospelt, C.; Nobbe, S.; Thome, M.; et al. CARD14 Gain-of-Function Mutation Alone Is Sufficient to Drive IL-23/IL-17-Mediated Psoriasiform Skin Inflammation In Vivo. J. Investig. Dermatol. 2018, 138, 2010–2023. [Google Scholar] [CrossRef]
- Singh, S.; Pradhan, D.; Puri, P.; Sharma, S.; Jain, A.K. Profiling CARD14 gene expression in Indian Psoriasis patients. Sci. Rep. 2024, 14, 28798. [Google Scholar] [CrossRef]
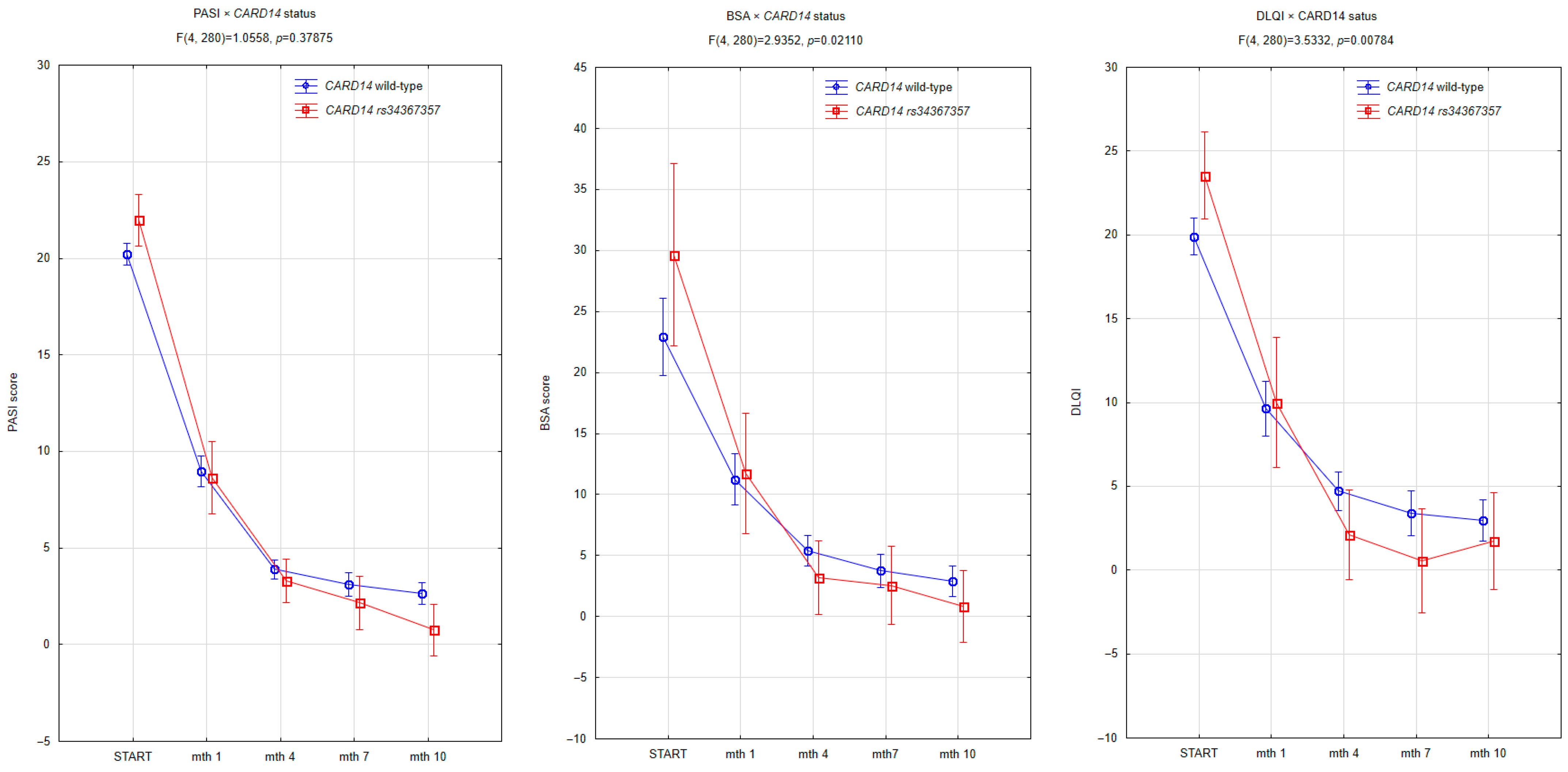




| Variable | No Mutation (Mean ± SD or n) | Mutation (Mean ± SD or n) | p-Value |
|---|---|---|---|
| females | 22 | 6 | 0.4115 |
| males | 39 | 5 | 0.4115 |
| BMI | 27.1 ± 6.3 | 26.7 ± 4.4 | 0.8051 |
| age of psoriasis onset (years) | 22.4 ± 14.9 | 13.9 ± 8.4 | 0.0134 |
| age at treatment start (years) | 35.4 ± 14.7 | 36.7 ± 14.9 | 0.7887 |
| baseline PASI | 20.2 ± 4.4 | 22.0 ± 4.7 | 0.2664 |
| baseline BSA | 22.9 ± 10.9 | 29.6 ± 19.3 | 0.2858 |
| baseline DLQI | 19.9 ± 4.3 | 23.5 ± 4.5 | 0.0265 |
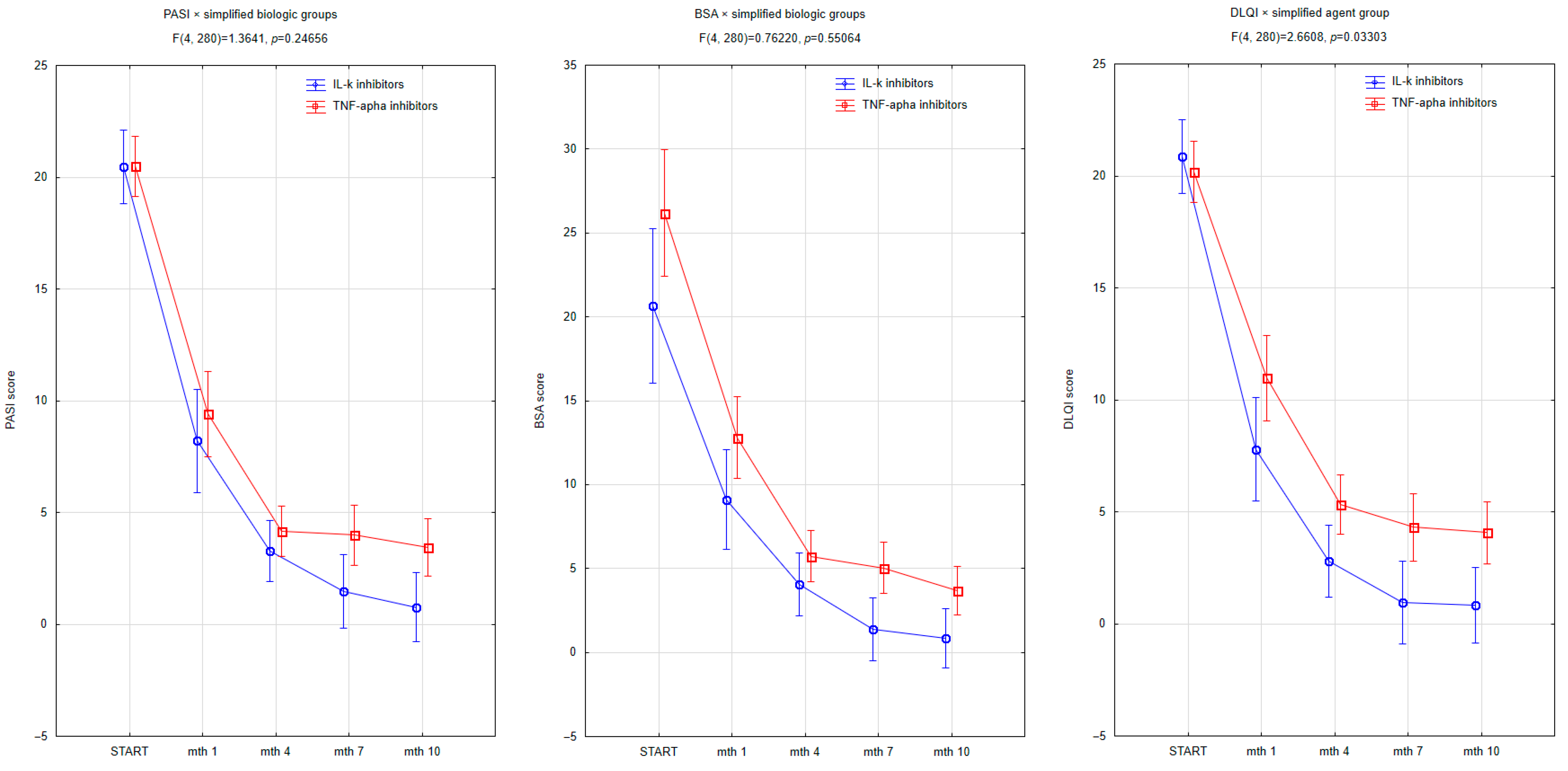
| Biologic agents | |||
| TNF-alpha inhibitors | 36 | 7 | |
| IL-23 inhibitors | 6 | 0 | |
| IL-12/23 inhibitors | 6 | 0 | |
| IL-17 inhibitors | 13 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedźwiedź, M.; Czerwińska, A.; Krzyścin, J.; Dróżdż, I.; Skoczylas, S.; Narbutt, J.; Lesiak, A. Impact of CARD14 rs34367357 Mutation, Nutrition Status, and Seasonality on the Response to Biologic Therapy in Psoriasis—A Retrospective Observational Single-Center Study. J. Clin. Med. 2025, 14, 6458. https://doi.org/10.3390/jcm14186458
Niedźwiedź M, Czerwińska A, Krzyścin J, Dróżdż I, Skoczylas S, Narbutt J, Lesiak A. Impact of CARD14 rs34367357 Mutation, Nutrition Status, and Seasonality on the Response to Biologic Therapy in Psoriasis—A Retrospective Observational Single-Center Study. Journal of Clinical Medicine. 2025; 14(18):6458. https://doi.org/10.3390/jcm14186458
Chicago/Turabian StyleNiedźwiedź, Michał, Agnieszka Czerwińska, Janusz Krzyścin, Izabela Dróżdż, Sebastian Skoczylas, Joanna Narbutt, and Aleksandra Lesiak. 2025. "Impact of CARD14 rs34367357 Mutation, Nutrition Status, and Seasonality on the Response to Biologic Therapy in Psoriasis—A Retrospective Observational Single-Center Study" Journal of Clinical Medicine 14, no. 18: 6458. https://doi.org/10.3390/jcm14186458
APA StyleNiedźwiedź, M., Czerwińska, A., Krzyścin, J., Dróżdż, I., Skoczylas, S., Narbutt, J., & Lesiak, A. (2025). Impact of CARD14 rs34367357 Mutation, Nutrition Status, and Seasonality on the Response to Biologic Therapy in Psoriasis—A Retrospective Observational Single-Center Study. Journal of Clinical Medicine, 14(18), 6458. https://doi.org/10.3390/jcm14186458






