Respiratory Muscle Strength in Rheumatoid Arthritis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Collection and Patient Characteristics
2.3. Respiratory Muscle Strength Assessment
2.4. General Muscle Strength Assessment
2.5. Pulmonary Function Tests, Diffusion Capacity of the Lungs, and Exercise Testing with Standardized 6-Minute Walk Test
2.6. RA Activity Assessment
2.7. Statistical Analysis
3. Results
| RA Patients (n = 36) | Controls (n = 36) | |
| Female (n [%]) | 26 [72%] | 26 [72%] |
| BMI (kg/m2—mean ± SD) | 26.0 ± 4.1 | 25.0 ± 4.0 |
| Body length (m—mean ± SD) | 1.7 ± 0.1 | 1.7 ± 0.09 |
| Body weight (kg—mean ± SD) | 73 ± 13 | 74 ± 13 |
| Age (years—mean ± SD) | 48 ± 15 | 48 ± 14 |
| * Smoking status (n [%]) | ||
| Never | 12 (33) | 29 (81) |
| Active | 10 (28) | 4 (11) |
| Quit | 14 (39) | 3 (8) |
| DAS28—all patients (mean ± SD) | 2.3 ± 1.2 |
| DAS28 high disease activity > 5.1 (n) | 2 |
| DAS28 moderate disease activity > 3.2 to <5.1 (n) | 4 |
| DAS28 low disease activity < 3.2 to >2.6 (n) | 5 |
| DAS28 Remission < 2.6 (n) | 25 |
| Rheumatoid factor /RF (IU/mL—mean ± SD) | 48 ± 66 |
| Creatine Kinase/CK (IU/L—mean ± SD) | 82 ± 39 |
| C-reactive Protein/CRP < 0.4 mg/dL (n) | 33 |
| C-reactive Protein/CRP > 0.4 < 0 1.6 mg/dL (n) | 3 |
| Hemoglobin/Hb (g/dL—mean ± SD) | 14.6 ± 1.3 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Microsoft Word—Musculoskeletal Health in Europe Report v5.0—EU_eumusc.net_Report_final.pdf. Available online: https://www.eular.org/web/static/lib/pdfjs/web/viewer.html?file=https://www.eular.org/document/download/440/73b14c75-5759-4d5f-98b8-3a694e233a70/440 (accessed on 30 July 2024).
- Roubenoff, R.; Roubenoff, R.A.; Cannon, J.G.; Kehayias, J.J.; Zhuang, H.; Dawson-Hughes, B.; Dinarello, C.A.; Rosenberg, I.H. Rheumatoid cachexia: Cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J. Clin. Investig. 1994, 93, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.C.E.; Fernandes, K.Z.; Lora, P.S.; Filippin, L.I.; Xavier, R.M. Prevalence of rheumatoid cachexia in rheumatoid arthritis: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Farrow, M.; Biglands, J.; Tanner, S.; Hensor, E.M.A.; Buch, M.H.; Emery, P.; Tan, A.L. Muscle deterioration due to rheumatoid arthritis: Assessment by quantitative MRI and strength testing. Rheumatology 2021, 60, 1216–1225. [Google Scholar] [CrossRef]
- Li, T.-H.; Chang, Y.-S.; Liu, C.-W.; Su, C.-F.; Tsai, H.-C.; Tsao, Y.-P.; Liao, H.-T.; Chen, M.-H.; Chuang, C.-C.; Yang, Y.-Y.; et al. The prevalence and risk factors of sarcopenia in rheumatoid arthritis patients: A systematic review and meta-regression analysis. Semin. Arthritis Rheum. 2021, 51, 236–245. [Google Scholar] [CrossRef]
- Bennett, J.L.; Pratt, A.G.; Dodds, R.; Sayer, A.A.; Isaacs, J.D. Rheumatoid sarcopenia: Loss of skeletal muscle strength and mass in rheumatoid arthritis. Nat. Rev. Rheumatol. 2023, 19, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid arthritis-associated lung disease. Eur. Respir. Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef]
- Singh, T.D.; Wijdicks, E.F.M. Neuromuscular Respiratory Failure. Neurol. Clin. 2021, 39, 333–353. [Google Scholar] [CrossRef]
- Kabitz, H.-J.; Walterspacher, S.; Walker, D.; Windisch, W. Inspiratory muscle strength in chronic obstructive pulmonary disease depending on disease severity. Clin. Sci. (Lond) 2007, 113, 243–249. [Google Scholar] [CrossRef]
- Kabitz, H.-J.; Lang, F.; Walterspacher, S.; Sorichter, S.; Müller-Quernheim, J.; Windisch, W. Impact of Impaired Inspiratory Muscle Strength on Dyspnea and Walking Capacity in Sarcoidosis. Chest 2006, 130, 1496–1502. [Google Scholar] [CrossRef]
- Schreiber, T.; Windisch, W. Respiratory muscle involvement in sarcoidosis. Expert. Rev. Respir. Med. 2018, 12, 545–548. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Kabitz, H.-J.; Walterspacher, S.; Mellies, U.; Criée, C.P.; Windisch, W. Empfehlungen der Deutschen Atemwegsliga zur Messung der Atemmuskelfunktion. Pneumologie 2014, 68, 307–314. [Google Scholar] [CrossRef]
- Criée, C.P.; Smith, H.J.; Preisser, A.M.; Bösch, D.; Butt, U.; Borst, M.M.; Hämäläinen, N.; Husemann, K.; Jörres, R.A.; Kardos, P.; et al. Aktuelle Empfehlungen zur Lungenfunktionsdiagnostik: Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin (DGP), Deutsche Atemwegsliga (DAL), Deutsche Lungenstiftung (DLS) sowie Deutsche Gesellschaft für Arbeitsmedizin und Umweltmedizin. Atemwegs Lungenkrankh. 2024, 50, 111–184. [Google Scholar] [CrossRef]
- Dustri Online Services. Available online: https://www.dustri.com/nc/books-in-german/category/pneumologie/b/empfehlungen-zur-ganzkoumlrperplethysmographie-bodyplethysmographie.html (accessed on 19 October 2024).
- ATS Statement. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Siegel, J.N.; Zhen, B.-G. Use of the American College of Rheumatology N (ACR-N) index of improvement in rheumatoid arthritis: Argument in favor. Arthritis Rheum. 2005, 52, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef] [PubMed]
- Sferra da Silva, G.; de Almeida Lourenço, M.; de Assis, M.R. Hand strength in patients with RA correlates strongly with function but not with activity of disease. Adv. Rheumatol. 2018, 58, 20. [Google Scholar] [CrossRef] [PubMed]
- Basakci Calik, B.; Gur Kabul, E.; Bozcuk, S.; Bayindir Akbas, A.N.; Çobankara, V. POS1463-HPR Prediction of pulmonary problems in individuals with rheumatoid arthritis-related interstitial lung disease using workstation analysis. Ann. Rheum. Dis. 2025, 84, 1466. [Google Scholar] [CrossRef]
- Žura, N.; Vukorepa, M.; Jurak, I.; Perić, P.; Botonjić, J.; Matijević, A.; Mitrović, H.K.; Žerjavić, N.L.; Durmiš, K.K.; Kalebota, N.; et al. Decrease in handgrip strength in rheumatoid arthritis (RA): Is there a sex-related difference? Rheumatol. Int. 2021, 41, 1795–1802. [Google Scholar] [CrossRef]
- Windisch, W.; Hennings, E.; Sorichter, S.; Hamm, H.; Criée, C.P. Peak or plateau maximal inspiratory mouth pressure: Which is best? Eur. Respir. J. 2004, 23, 708–713. [Google Scholar] [CrossRef]
- Walterspacher, S.; Schlager, D.; Walker, D.J.; Müller-Quernheim, J.; Windisch, W.; Kabitz, H.-J. Respiratory muscle function in interstitial lung disease. Eur. Respir. J. 2013, 42, 211–219. [Google Scholar] [CrossRef] [PubMed]
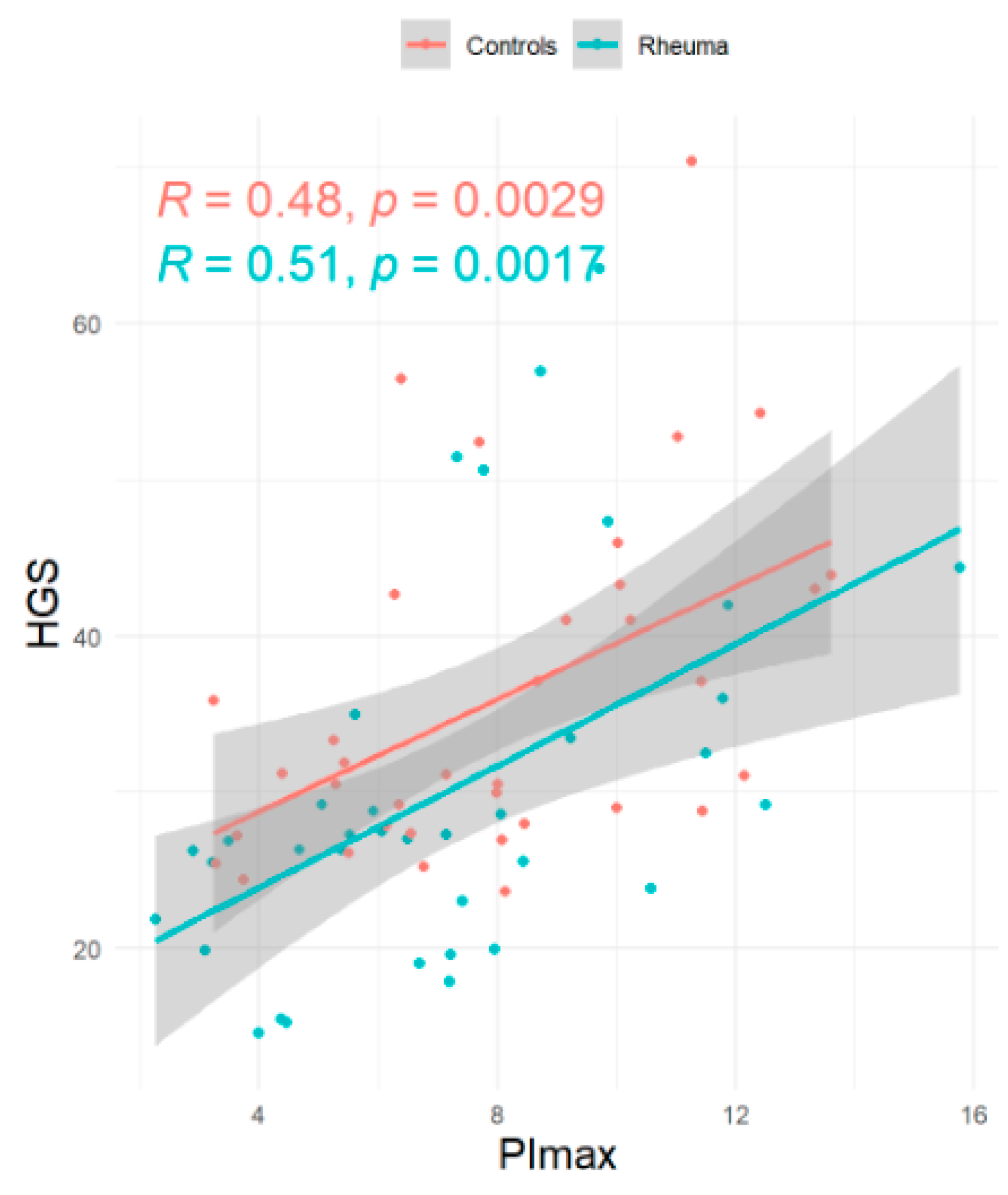
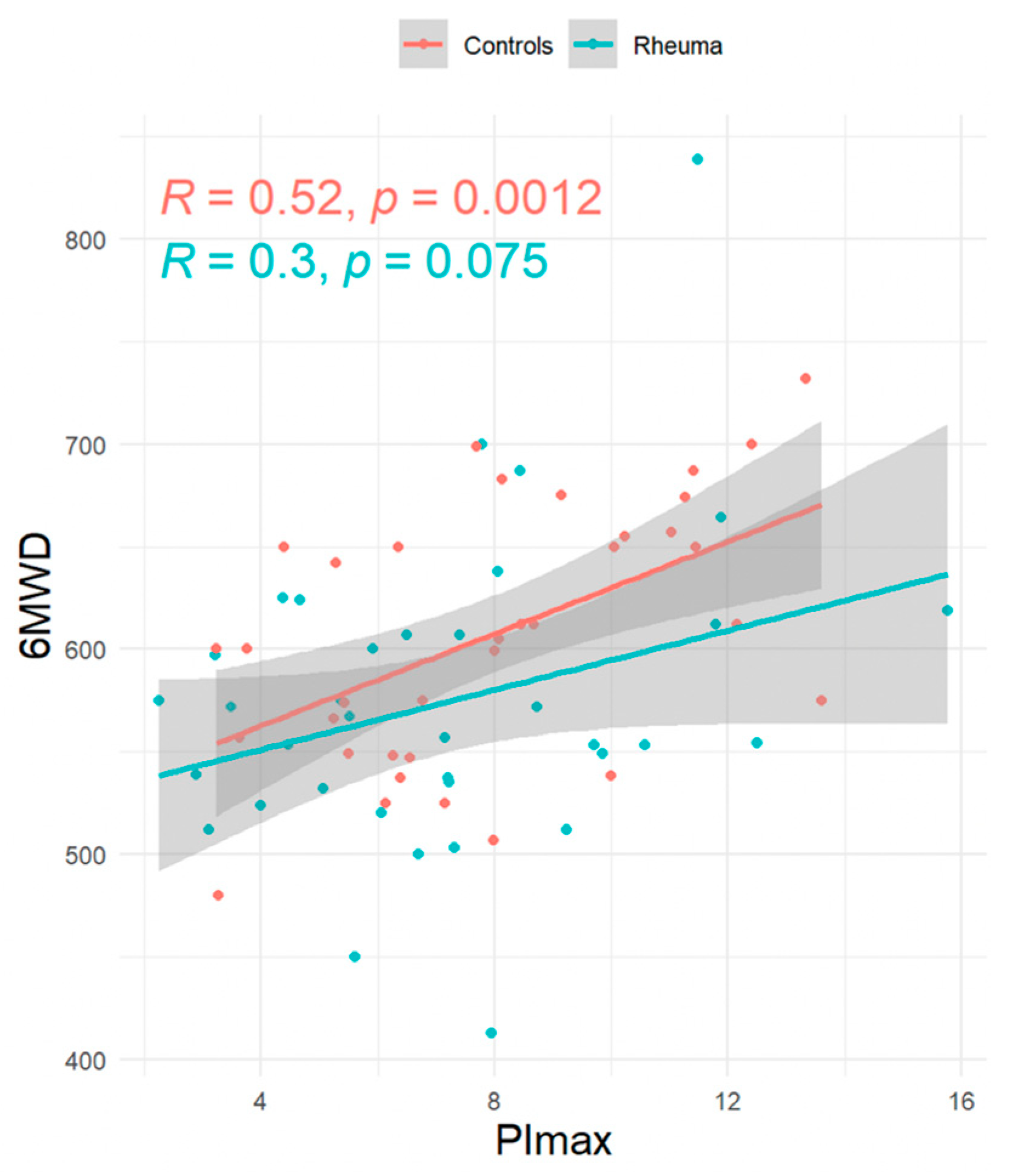
| RA Patients (n = 36) | Controls (n = 36) | Pairwise Comparison Mean Difference (n = 36) | Pairwise Comparison 95% Confidence Intervall | |
| PImax (kPa—mean ± SD) | 7.2 ± 3.1 | 8.0 ± 2.9 | −0.81 ± 3 | [−1.84; 0.21] |
| PEmax (kPa—mean ± SD) | 9.4 ± 4.6 | 11.4 ± 5.3 | −2 ± 6.7 | [−4.23; 0.28] |
| SnPNa (kPa—mean ± SD) | 7.9 ± 2.5 | 8.7 ± 3.0 | −0.86 ± 2.9 | [−1.84; 0.13] |
| P0.1 (kPa—mean ± SD) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.17 | [−0.06; 0.05] |
| HGS (kg—mean ± SD) | 30.1 ± 11.9 | 36.2 ± 10.8 | −5.8 ± 11.0 | [−9.40; 2.28] |
| 6MWD (m—mean ± SD) | 574.3 ± 74.9 | 607 ± 63 | −32 ± 89 | [−62.62; −1.66] |
| SpO2 before 6MWT (%—mean ± SD) | 96 ± 2.7 | 96 ± 1.3 | 0.03 ± 2.8 | [−0.92; 0.97] |
| SpO2 after 6MWD (%—mean ± SD) | 96 ± 1.2 | 96 ± 1.5 | 0.18 ± 1.7 | [−0.41; 0.78] |
| FEV1 (L—mean ± SD) | 3.0 ± 0.7 | 3.1 ± 0.9 | −0.11 ± 0.68 | [−0.34; 0.12] |
| RV (L—mean ± SD) | 2.0 ± 0.6 | 2.0 ± 0.8 | −0.15 ± 0.73 | [−0.39; 0.1] |
| Tiffeneau Index (%—mean ± SD) | 77 ± 9 | 75 ± 9 | 1.7 ± 11.0 | [−2.06; 5.56] |
| FVCex (L—mean ± SD) | 3.9 ± 1.0 | 4.1 ± 1.1 | −0.22 ± 0.68 | [−0.45; 0.01] |
| TLC (L—mean ± SD) | 5.4 ± 1.3 | 6.1 ± 1.3 | −0.17 ± 1.7 | [−0.74; 0.39] |
| TLco (%predicted) | 75.9 ± 13.5 | 79.5 ± 14 | −3.6 ± 20 | [−10.35; 3.12] |
| Kco (% predicted) | 83.2 ± 13.3 | 85.1 ± 14.1 | −1.9 ± 20 | [−8.57; 4.85] |
| Estimate | 2.5% | 97.5% | p (>[t]) | |
|---|---|---|---|---|
| Primary endpoint | ||||
| PImax (kPa) | −0.89 | −2.11 | 0.31 | 0.14 |
| Secondary endpoints | ||||
| HGS (kg) | −5.97 | −9.42 | −2.50 | 0.001 |
| PEmax (kPa) | −2.00 | −4.17 | 0.15 | 0.07 |
| P0.1 (kPa) | −0.009 | −0.06 | 0.04 | 0.99 |
| SnPNa (kPa) | −0.90 | −2.05 | 0.24 | 0.11 |
| 6MWD (m) | −32.31 | −65.13 | 0.70 | 0.05 |
| FEV1 (L) | −0.10 | −0.36 | 0.15 | 0.42 |
| FVCex (L) | −0.20 | −0.50 | 0.09 | 0.16 |
| TLC (L) | −0.11 | −0.67 | 0.44 | 0.68 |
| RV (L) | 0.15 | −0.22 | 0.53 | 0.42 |
| TLco (% predicted) | −0.62 | −1.12 | −0.05 | 0.03 |
| Kco (% predicted) | −0.01 | −0.11 | 0.08 | 0.76 |
| OR | 2.5% CI | 97.5% CI | p-Value | |
| Primary endpoint | ||||
| PImax (kPA) | 1.7 | 0.65 | 4.77 | 0.14 |
| Secondary endpoint | ||||
| PEmax (kPA) | 2.2 | 0.77 | 7.21 | 0.14 |
| SnPNa (kPA) | 1.74 | 0.65 | 4.77 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, M.; Zimmermann, M.; Thomas, L.; Strunk, J.; Kroppen, D.; Majorski, D.S.; Stanzel, S.B.; Wollsching-Strobel, M.; Schulz, M.; Windisch, W.; et al. Respiratory Muscle Strength in Rheumatoid Arthritis. J. Clin. Med. 2025, 14, 6455. https://doi.org/10.3390/jcm14186455
Berger M, Zimmermann M, Thomas L, Strunk J, Kroppen D, Majorski DS, Stanzel SB, Wollsching-Strobel M, Schulz M, Windisch W, et al. Respiratory Muscle Strength in Rheumatoid Arthritis. Journal of Clinical Medicine. 2025; 14(18):6455. https://doi.org/10.3390/jcm14186455
Chicago/Turabian StyleBerger, Melanie, Maximilian Zimmermann, Leon Thomas, Johannes Strunk, Doreen Kroppen, Daniel Sebastian Majorski, Sarah Bettina Stanzel, Maximilian Wollsching-Strobel, Maxi Schulz, Wolfram Windisch, and et al. 2025. "Respiratory Muscle Strength in Rheumatoid Arthritis" Journal of Clinical Medicine 14, no. 18: 6455. https://doi.org/10.3390/jcm14186455
APA StyleBerger, M., Zimmermann, M., Thomas, L., Strunk, J., Kroppen, D., Majorski, D. S., Stanzel, S. B., Wollsching-Strobel, M., Schulz, M., Windisch, W., & Schumacher, F. (2025). Respiratory Muscle Strength in Rheumatoid Arthritis. Journal of Clinical Medicine, 14(18), 6455. https://doi.org/10.3390/jcm14186455







