Clinical Efficacy of Extended Transforaminal Endoscopic Lumbar Foraminotomy Compared with the Conventional Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
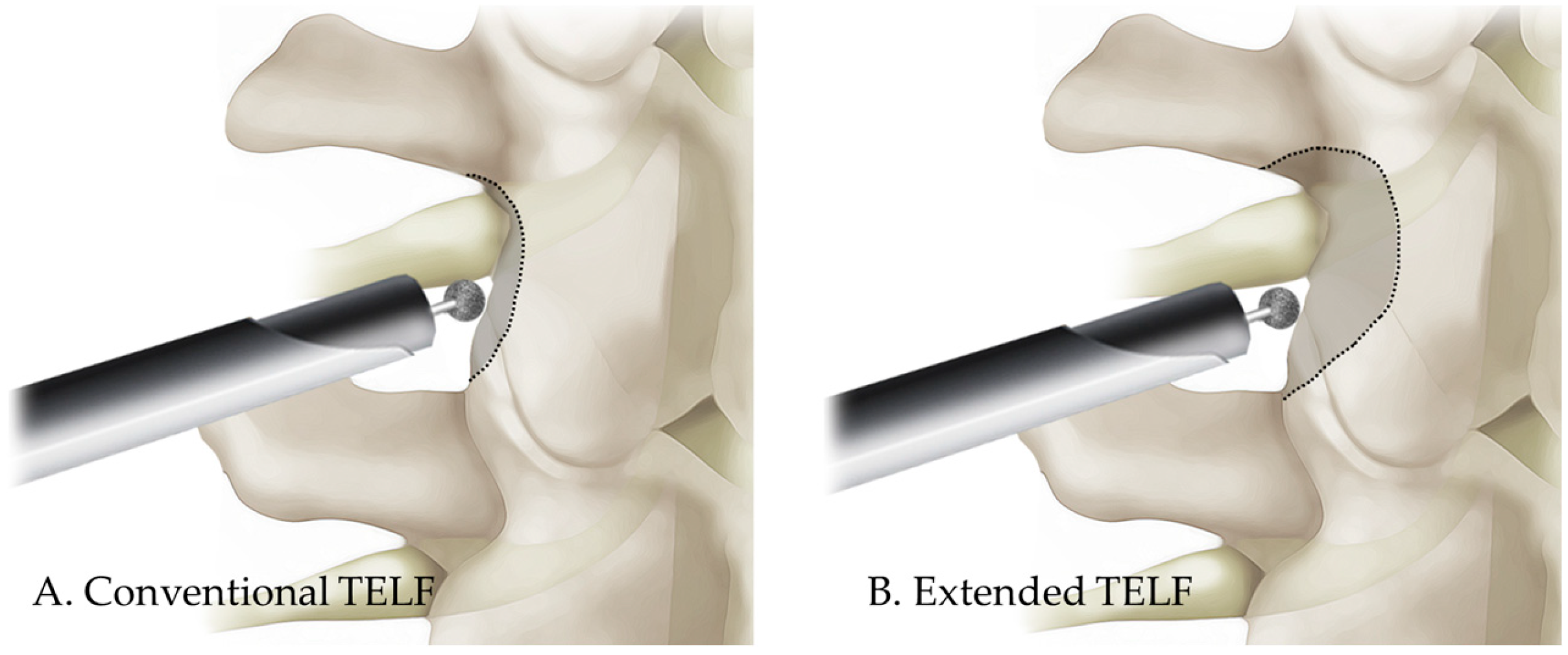
2.2. Surgical Techniques
2.3. Clinical Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Demographic and Operative Data
3.2. Clinical Outcomes
3.3. Complications and Reoperations
4. Discussion
4.1. Clinical Outcome Data
4.2. Conventional TELF and Its Problems
4.3. Extended TELF: A Solution with Strengths and Challenges
4.4. Technical Pearls for Success
4.5. Future Perspectives
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ENR | Exiting nerve root |
| LF | Ligamentum flavum |
| LFS | Lumbar foraminal stenosis |
| ODI | Oswestry disability index |
| TELF | Transforaminal endoscopic lumbar foraminotomy |
| VAS | Visual analog scale |
References
- Knight, M.T.; Vajda, A.; Jakab, G.V.; Awan, S. Endoscopic laser foraminoplasty on the lumbar spine—Early experience. Minim. Invasive Neurosurg. 1998, 41, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Jenis, L.G.; An, H.S. Spine update. Lumbar foraminal stenosis. Spine 2000, 25, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.; Connaughton, P.N. Unilateral facetectomy approach for lateral lumbar disc herniation. J. Neurosurg. 1991, 74, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Kunogi, J.; Hasue, M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine 1991, 16, 1312–1320. [Google Scholar] [CrossRef]
- Chang, S.B.; Lee, S.H.; Ahn, Y.; Kim, J.M. Risk factor for unsatisfactory outcome after lumbar foraminal and far lateral microdecompression. Spine 2006, 31, 1163–1167. [Google Scholar] [CrossRef]
- Lapp, M.A.; Bridwell, K.H.; Lenke, L.G.; Daniel Riew, K.; Linville, D.A.; Eck, K.R.; Ungacta, F.F. Long-term complications in adult spinal deformity patients having combined surgery a comparison of primary to revision patients. Spine 2001, 26, 973–983. [Google Scholar] [CrossRef]
- Zheng, F.; Cammisa, F.P., Jr.; Sandhu, H.S.; Girardi, F.P.; Khan, S.N. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine 2002, 27, 818–824. [Google Scholar] [CrossRef]
- Basques, B.A.; Ibe, I.; Samuel, A.M.; Lukasiewicz, A.M.; Webb, M.L.; Bohl, D.D.; Grauer, J.N. Predicting postoperative morbidity and readmission for revision posterior lumbar fusion. Clin. Spine Surg. 2017, 30, E770–E775. [Google Scholar] [CrossRef]
- Miscusi, M.; Trungu, S.; Ricciardi, L.; Forcato, S.; Piazza, A.; Ramieri, A.; Raco, A. Stand-alone oblique lumbar interbody fusion (OLIF) for the treatment of adjacent segment disease (ASD) after previous posterior lumbar fusion: Clinical and radiological outcomes and comparison with posterior revision surgery. J. Clin. Med. 2023, 12, 2985. [Google Scholar] [CrossRef]
- Mayer, H.M.; Brock, M. Percutaneous endoscopic discectomy: Surgical technique and preliminary results compared to microsurgical discectomy. J. Neurosurg. 1993, 78, 216–225. [Google Scholar] [CrossRef]
- Hermantin, F.U.; Peters, T.; Quartararo, L.; Kambin, P. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J. Bone Jt. Surg. Am. 1999, 81, 958–965. [Google Scholar] [CrossRef]
- Hoogland, T.; Schubert, M.; Miklitz, B.; Ramirez, A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: A prospective randomized study in 280 consecutive cases. Spine 2006, 31, E890–E897. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: A prospective, randomized, controlled study. Spine 2008, 33, 931–939. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Recurrent lumbar disc herniation after conventional discectomy: A prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J. Spinal Disord. Tech. 2009, 22, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; DA Rocha, I.D.; Cristante, A.F.; Marcon, R.M.; Coutinho, T.P.; Torelli, A.G.; Petersen, P.A.; Letaif, O.B.; DE Barros Filho, T.E.P. Percutaneous endoscopic lumbar discectomy versus microdiscectomy for the treatment of lumbar disc herniation: Pain, disability, and complication rate—A randomized clinical trial. Int. J. Spine Surg. 2020, 14, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.N.A.; Subramanian, A.S.; Scott, C.E.H. A randomised controlled trial of transforaminal endoscopic discectomy vs. microdiscectomy. Eur. Spine J. 2017, 26, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Nellensteijn, J.; Ostelo, R.; Bartels, R.; Peul, W.; van Royen, B.; van Tulder, M. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: A systematic review of the literature. Eur. Spine J. 2010, 19, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Zhu, Y.; Tu, G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur. Spine J. 2016, 25, 134–143. [Google Scholar] [CrossRef]
- Li, X.C.; Zhong, C.F.; Deng, G.B.; Liang, R.W.; Huang, C.M. Full-Endoscopic procedures versus traditional discectomy surgery for discectomy: A systematic review and meta-analysis of current global clinical trials. Pain Physician 2016, 19, 103–118. [Google Scholar] [CrossRef]
- Ruan, W.; Feng, F.; Liu, Z.; Xie, J.; Cai, L.; Ping, A. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: A meta-analysis. Int. J. Surg. 2016, 31, 86–92. [Google Scholar] [CrossRef]
- Ding, W.; Yin, J.; Yan, T.; Nong, L.; Xu, N. Meta-analysis of percutaneous transforaminal endoscopic discectomy vs. fenestration discectomy in the treatment of lumbar disc herniation. Orthopade 2018, 47, 574–584. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.; Liu, J.; Yu, B.; Guo, W.; Li, Y.; Liu, Y.; Ruan, W.; Ning, G.; Feng, S. Transforaminal endoscopic discectomy versus conventional microdiscectomy for lumbar discherniation: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2018, 13, 169. [Google Scholar] [CrossRef]
- Barber, S.M.; Nakhla, J.; Konakondla, S.; Fridley, J.S.; Oyelese, A.A.; Gokaslan, Z.L.; Telfeian, A.E. Outcomes of endoscopic discectomy compared with open microdiscectomy and tubular microdiscectomy for lumbar disc herniations: A meta-analysis. J. Neurosurg. Spine 2019, 31, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Gadjradj, P.S.; Harhangi, B.S.; Amelink, J.; van Susante, J.; Kamper, S.; van Tulder, M.; Peul, W.C.; Vleggeert-Lankamp, C.; Rubinstein, S.M. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: A systematic review and meta-analysis. Spine 2021, 46, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Yan, Q.; Cong, L. Comparison of endoscopic discectomy versus non-endoscopic discectomy for symptomatic lumbar disc herniation: A systematic review and meta-analysis. Glob. Spine J. 2022, 12, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Park, H.B. Transforaminal endoscopic lumbar foraminotomy for juxta-fusional foraminal stenosis. J. Clin. Med. 2023, 12, 5745. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W.; Yeom, J.S.; Kim, K.J.; Kim, H.J.; Chung, S.K.; Kang, H.S. A practical MRI grading system for lumbar foraminal stenosis. AJR Am. J. Roentgenol. 2010, 194, 1095–1098. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, S.H.; Lee, H.Y.; Lee, H.J.; Chang, S.B.; Chung, S.K.; Kim, H.J. Validation of the Korean version of the oswestry disability index. Spine 2005, 30, E123–E127. [Google Scholar] [CrossRef]
- Yeung, A.T.; Tsou, P.M. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002, 27, 722–731. [Google Scholar] [CrossRef]
- Martin, W.J.; Ashton-James, C.E.; Skorpil, N.E.; Heymans, M.W.; Forouzanfar, T. What constitutes a clinically important pain reduction in patients after third molar surgery? Pain Res. Manag. 2013, 18, 319–322. [Google Scholar] [CrossRef]
- Ng, L.C.; Tafazal, S.; Sell, P. The effect of duration of symptoms on standard outcome measures in the surgical treatment of spinal stenosis. Eur. Spine J. 2007, 16, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Oh, H.K.; Kim, H.; Lee, S.H.; Lee, H.N. Percutaneous endoscopic lumbar foraminotomy: An advanced surgical technique and clinical outcomes. Neurosurgery 2014, 75, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Telfeian, A.E.; Jasper, G.P.; Francisco, G.M. Transforaminal endoscopic treatment of lumbar radiculopathy after instrumented lumbar spine fusion. Pain Physician 2015, 18, 179–184. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, S.H.; Lee, H.Y. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: Floating retraction technique. Minim. Invasive Neurosurg. 2011, 54, 214–218. [Google Scholar] [CrossRef]
- Sugiura, K.; Payne, C.; Tran, N.T.; Leyendecker, J.; Ogunlade, J.; LaVanne, M.; Derman, P.B.; Quon, R.; Telfeian, A.E.; Hofstetter, C.P.; Endoscopic Spine Research Group (ESRG). Transforaminal full-endoscopic surgery for lumbar foraminal pathologies: A comparative clinical effectiveness study. Neurosurgery 2025, 96, S51–S62. [Google Scholar] [CrossRef]
- Kim, K.D.; Wright, N.M. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: Results of a prospective, multicenter, randomized controlled study. Spine 2011, 36, 1906–1912. [Google Scholar] [CrossRef]
- Ahn, Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev. Med. Devices 2014, 11, 605–616. [Google Scholar] [CrossRef]


| Extended TELF | Conventional TELF | Total | p-Value | |
|---|---|---|---|---|
| No. of patients | 64 | 67 | 131 | |
| Sex ratio (M:F) | 24:40 | 30:37 | 54:77 | NS |
| Mean age (years) | 69.11 ± 7.91 | 69.60 ± 10.21 | 69.36 ± 9.13 | NS |
| Mean BMI (kg/m2) | 23.54 ± 3.46 | 24.02 ± 3.57 | 23.77 ± 3.51 | NS |
| Operative level | NS | |||
| L1–L2 | 0 | 0 | 0 | |
| L2–L3 | 2 | 1 | 3 | |
| L3–L4 | 7 | 7 | 14 | |
| L4–L5 | 26 | 27 | 53 | |
| L5–S1 | 29 | 32 | 61 | |
| Operative time (min) | 60.27 ± 15.81 | 54.57 ± 13.71 | 57.35 ± 14.99 | * 0.0291 |
| Extended TELF | Conventional TELF | p-Value | |
|---|---|---|---|
| VAS | |||
| Preop | 8.09 ± 0.75 | 8.19 ± 0.68 | NS |
| Postop 6 weeks | 3.14 ± 1.60 | 3.73 ± 1.68 | * 0.0419 |
| Postop 6 months | 2.80 ± 1.89 | 3.30 ± 1.91 | NS |
| Postop 1 year | 1.94 ± 1.28 | 2.16 ± 1.34 | NS |
| Postop 2 years | 1.98 ± 1.18 | 2.39 ± 1.11 | * 0.0456 |
| ODI (%) | |||
| Preop | 69.90 ± 10.78 | 70.07 ± 10.62 | NS |
| Postop 6 weeks | 27.83 ± 17.47 | 33.95 ± 16.96 | * 0.0435 |
| Postop 6 months | 27.16 ± 17.29 | 28.69 ±16.74 | NS |
| Postop 1 year | 19.12 ± 16.17 | 21.35 ± 16.31 | NS |
| Postop 2 years | 19.14 ± 16.58 | 23.94 ± 15.46 | 0.0887 |
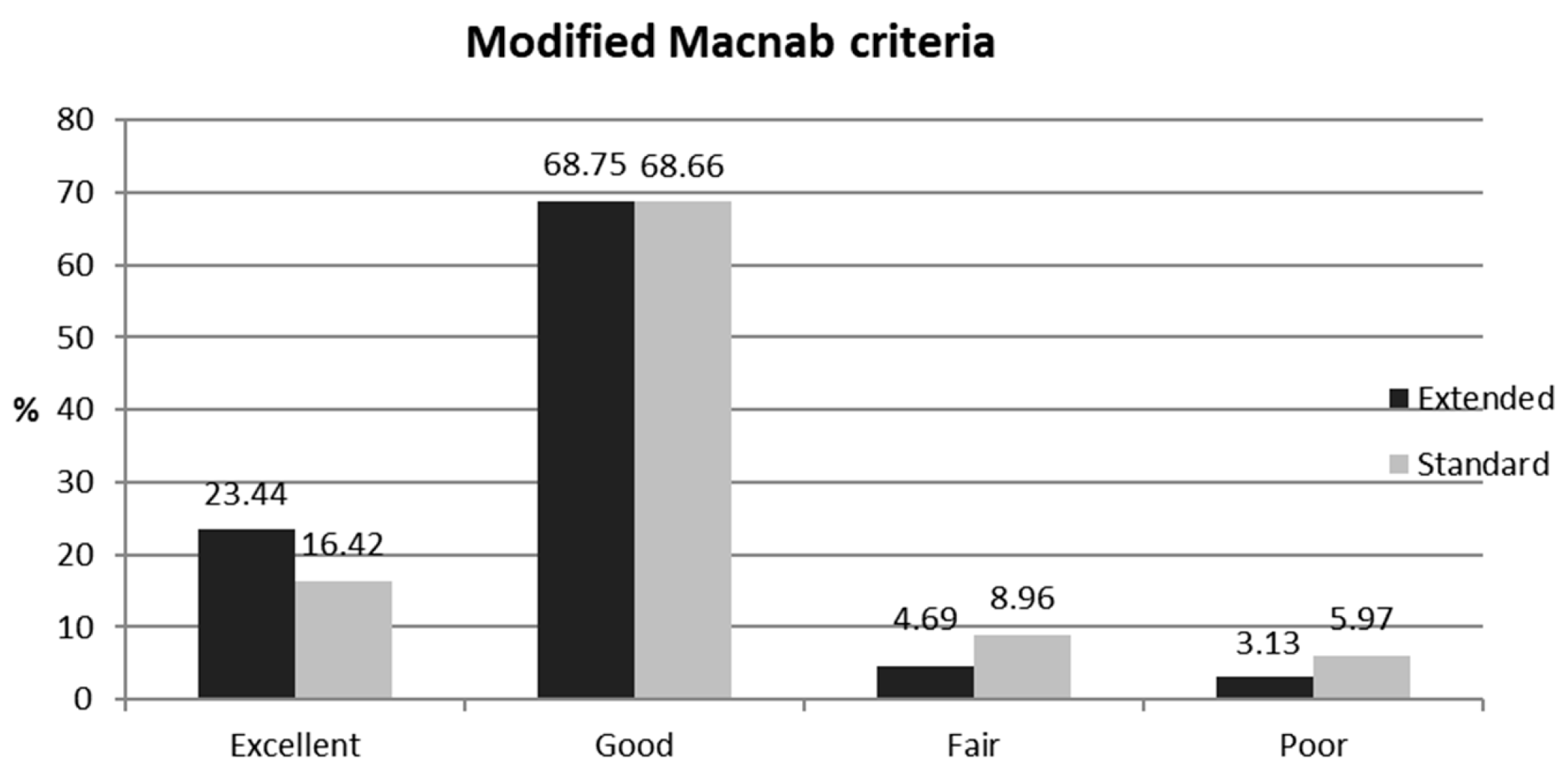
| Modified Macnab (%) | |||
| Excellent | 23.44 | 16.42 | NS |
| Good | 68.75 | 68.66 | NS |
| Fair | 4.69 | 8.96 | NS |
| Poor | 3.13 | 5.97 | NS |
| Dysesthesia | 3 (4.69%) | 5 (7.46%) | NS |
| Dural tear | 0 (0%) | 2 (2.99%) | NS |
| Revision surgery | 1 (1.56%) | 2 (2.99%) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.; Park, H.-B.; Son, S.; Yoo, B.-R. Clinical Efficacy of Extended Transforaminal Endoscopic Lumbar Foraminotomy Compared with the Conventional Technique. J. Clin. Med. 2025, 14, 6446. https://doi.org/10.3390/jcm14186446
Ahn Y, Park H-B, Son S, Yoo B-R. Clinical Efficacy of Extended Transforaminal Endoscopic Lumbar Foraminotomy Compared with the Conventional Technique. Journal of Clinical Medicine. 2025; 14(18):6446. https://doi.org/10.3390/jcm14186446
Chicago/Turabian StyleAhn, Yong, Han-Byeol Park, Seong Son, and Byung-Rhae Yoo. 2025. "Clinical Efficacy of Extended Transforaminal Endoscopic Lumbar Foraminotomy Compared with the Conventional Technique" Journal of Clinical Medicine 14, no. 18: 6446. https://doi.org/10.3390/jcm14186446
APA StyleAhn, Y., Park, H.-B., Son, S., & Yoo, B.-R. (2025). Clinical Efficacy of Extended Transforaminal Endoscopic Lumbar Foraminotomy Compared with the Conventional Technique. Journal of Clinical Medicine, 14(18), 6446. https://doi.org/10.3390/jcm14186446






