Predicting Neonatal Morbidity and Correlations with Maternal and Neonatal Biomarkers in Connection with Fetal Inflammatory Response Syndrome in Premature Births
Abstract
1. Introduction
- Determining the differences between the FIRS and non-FIRS groups for both neonates and the mothers.
- Assessing the characteristics of the placental and umbilical cord tissue from a histologic approach.
- Identifying the prognosis of neonatal conditions based on FIRS and the cord blood IL-6.
2. Materials and Methods
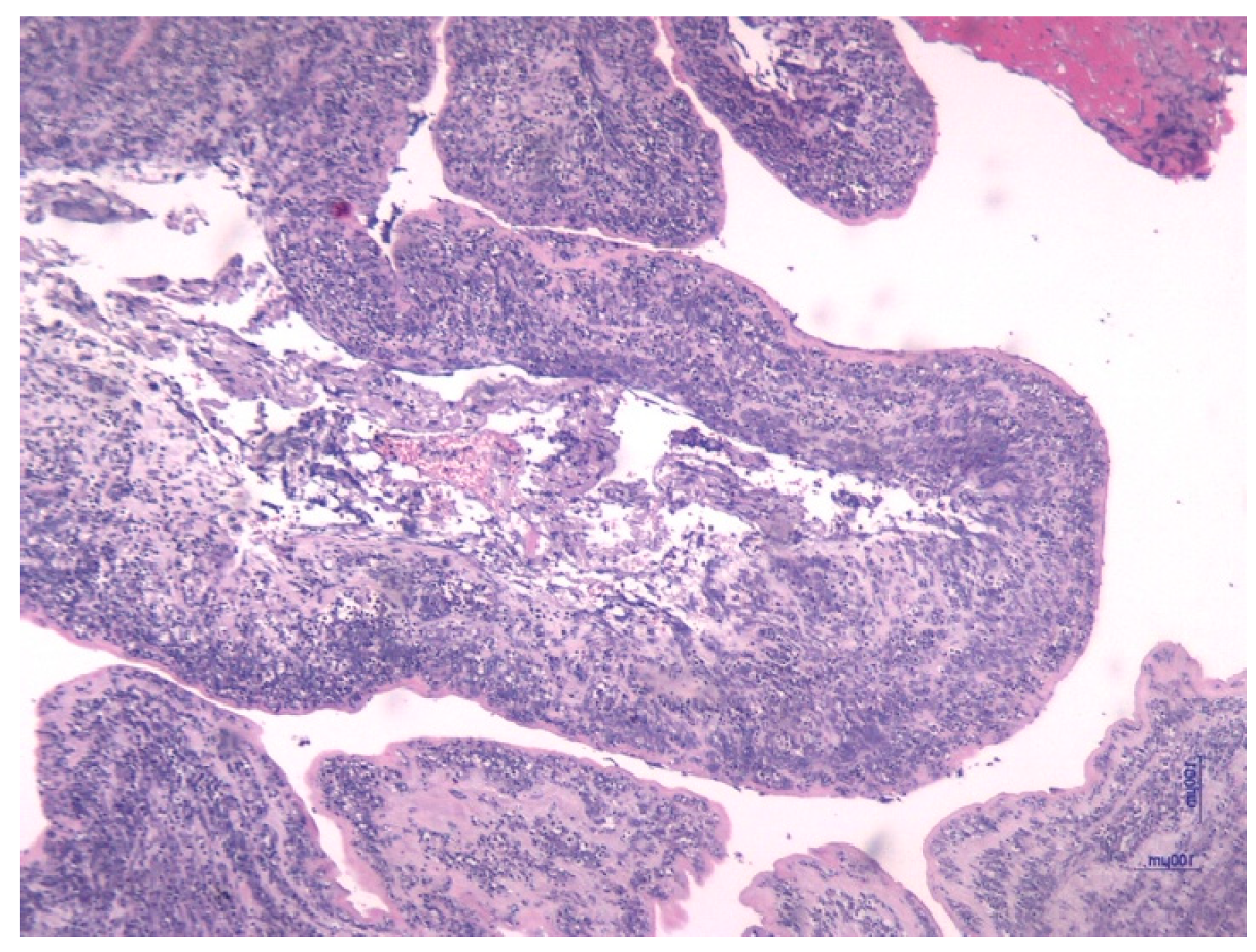
2.1. Study Design and Participants
- ▪
- FIRS group: 75 preterm neonates who met the diagnostic criteria for FIRS;
- ▪
- Non-FIRS group: 50 preterm neonates with no criteria for FIRS.
- -
- Preterm birth (<37 weeks of gestation);
- -
- Umbilical cord blood sampling at birth;
- -
- Signed informed consent from the parents or legal guardians.
- -
- Presence of one or more documented maternal infectious risk factors, such as: clinical chorioamnionitis, premature rupture of membranes (PROM) > 18 h, maternal fever >38 °C during labor, positive maternal group B Streptococcus (GBS) colonization, suspected or confirmed intrauterine infection.
- Major congenital malformations;
- Incomplete clinical and laboratory data;
- Lack of parental informed consent.
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations and Future Directions of Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, Y.; Wintermark, P. Therapeutic interventions for fetal inflammatory response syndrome (FIRS). Semin. Fetal Neonatal Med. 2020, 25, 101112. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Diaz-Primera, R.; Marin-Concha, J.; Para, R.; Lopez, A.M.; Pacora, P.; Gomez-Lopez, N.; Yoon, B.H.; et al. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med. 2020, 25, 101146. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, E.-K.; Shin, S.H.; Choi, Y.-H.; Jung, Y.H.; Kim, S.Y.; Koh, J.W.; Choi, E.K.; Cheon, J.-E.; Kim, H.-S. Factors associated with neurodevelopment in preterm infants with systematic inflammation. BMC Pediatr. 2021, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Dammann, O.; Leviton, A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin. Fetal Neonatal Med. 2006, 11, 363–368. [Google Scholar] [CrossRef]
- Brien, M.E.; Hughes, K.; Girard, S. Prenatal administration of IL-1Ra attenuate the neurodevelopmental impacts following non-pathogenic inflammation during pregnancy. Sci. Rep. 2021, 11, 23404. [Google Scholar] [CrossRef]
- Tessema, B.; Lippmann, N.; Willenberg, A.; Knüpfer, M.; Sack, U.; König, B. The diagnostic performance of interleukin-6 and c-reactive protein for early identification of neonatal sepsis. Diagnostics 2020, 10, 978. [Google Scholar] [CrossRef]
- Eichberger, J.; Resch, B. Reliability of Interleukin-6 Alone and in Combination for Diagnosis of Early Onset Neonatal Sepsis: Systematic Review. Front. Pediatr. 2022, 10, 840778. [Google Scholar] [CrossRef] [PubMed]
- Gude, S.S.; Peddi, N.C.; Vuppalapati, S.; Gopal, S.V.; Ramesh, H.M.; Gude, S.S. Biomarkers of Neonatal Sepsis: From Being Mere Numbers to Becoming Guiding Diagnostics. Cureus 2022, 14, e23215. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Galaz, J.; Xu, Y.; Panaitescu, B.; Slutsky, R.; Motomura, K.; Gill, N.; Para, R.; Pacora, P.; et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am. J. Reprod. Immunol. 2019, 82, e13171. [Google Scholar] [CrossRef]
- Giovannini, E.; Bonasoni, M.P.; Pascali, J.P.; Giorgetti, A.; Pelletti, G.; Gargano, G.; Pelotti, S.; Fais, P. Infection Induced Fetal Inflammatory Response Syndrome (FIRS): State-of- the-Art and Medico-Legal Implications—A Narrative Review. Microorganisms 2023, 11, 1010. [Google Scholar] [CrossRef]
- Pacora, P.; Chaiworapongsa, T.; Maymon, E.; Kim, Y.M.; Gomez, R.; Yoon, B.H.; Ghezzi, F.; Berry, S.M.; Qureshi, F.; Jacques, S.M.; et al. Funisitis and chorionic vasculitis: The histological counterpart of the fetal inflammatory response syndrome. J. Matern. Neonatal Med. 2002, 11, 18–25. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S29–S52. [Google Scholar] [CrossRef] [PubMed]
- Cobo, T.; Kacerovsky, M.; Andrys, C.; Drahosova, M.; Musilova, I.; Hornychova, H.; Jacobsson, B.; Zakar, T. Umbilical Cord Blood IL-6 as Predictor of Early-Onset Neonatal Sepsis in Women with Preterm Prelabour Rupture of Membranes. PLoS ONE 2013, 8, e69341. [Google Scholar] [CrossRef]
- Tann, C.J.; Nakakeeto, M.; A Willey, B.; Sewegaba, M.; Webb, E.L.; Oke, I.; Mutuuza, E.D.; Peebles, D.; Musoke, M.; A Harris, K.; et al. Perinatal risk factors for neonatal encephalopathy: An unmatched case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 103, F250–F256. [Google Scholar] [CrossRef]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef]
- Hofer, N.; Kothari, R.; Morris, N.; Müller, W.; Resch, B. The fetal inflammatory response syndrome is a risk factor for morbidity in preterm neonates. Am. J. Obstet. Gynecol. 2013, 209, 542.e1–542.e11. [Google Scholar] [CrossRef]
- Gotsch, F.; Romero, R.; Kusanovic, J.P.; Mazaki-Tovi, S.; Pineles, B.L.; Erez, O.; Espinoza, J.; Hassan, S.S. The fetal inflammatory response syndrome. Clin. Obstet. Gynecol. 2007, 50, 652–683. [Google Scholar] [CrossRef]
- Mwaniki, M.K.; Atieno, M.; Lawn, J.E.; Newton, C.R.J.C. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012, 379, 445–452. [Google Scholar] [CrossRef]
- Kim, Y.M.; Romero, R.; Chaiworapongsa, T.; Espinoza, J.; Mor, G.; Kim, C.J. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology 2006, 49, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, T.; Zhu, X. Role of maternal–fetal immune tolerance in the establishment and maintenance of pregnancy. Chin. Med J. 2024, 137, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Liew, F.F. Cytokine landscapes of pregnancy: Mapping gestational immune phases. Gynecol. Obstet. Clin. Med. 2024, 4, e000011. [Google Scholar] [CrossRef]
- Narang, K.; Cheek, E.H.; Enninga, E.A.L.; Theiler, R.N. Placental Immune Responses to Viruses: Molecular and Histo-Pathologic Perspectives. Int. J. Mol. Sci. 2021, 22, 2921. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Faye-Petersen, O.M.; Sims, B.; Peralta-Carcelen, M.; Reilly, S.D.; McGwin, G.; Carlo, W.A.; Ambalavanan, N. Histologic Characteristics of the Fetal Inflammatory Response Associated with Neurodevelopmental Impairment and Death in Extremely Preterm Infants. J. Pediatr. 2013, 163, 652–657. [Google Scholar] [CrossRef]
- Romero, R.; Soto, E.; Berry, S.M.; Hassan, S.S.; Kusanovic, J.P.; Yoon, B.H.; Edwin, S.; Mazor, M.; Chaiworapongsa, T. Blood pH and gases in fetuses in preterm labor with and without systemic inflammatory response syndrome. J. Matern. Neonatal Med. 2011, 25, 1160–1170. [Google Scholar] [CrossRef]
- Dulay, A.T.; Buhimschi, I.A.; Zhao, G.; Luo, G.; Abdel-Razeq, S.; Cackovic, M.; Rosenberg, V.A.; Pettker, C.M.; Thung, S.F.; Bahtiyar, M.O.; et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am. J. Obstet. Gynecol. 2008, 198, 426.e1–426.e9. [Google Scholar] [CrossRef]
- Pikora, K.; Krętowska-Grunwald, A.; Krawczuk-Rybak, M.; Sawicka-Żukowska, M. Diagnostic Value and Prognostic Significance of Nucleated Red Blood Cells (NRBCs) in Selected Medical Conditions. Cells 2023, 12, 1817. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Lubach, G.A.; Rahim, O.M.; Degraeuwe, P.; Zimmermann, L.J.; Kramer, B.W.; Villamor, E. Association of Histological and Clinical Chorioamnionitis With Neonatal Sepsis Among Preterm Infants: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Immunol. 2020, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Mally, P.; Xu, J.; Hendricks-Muñoz, K.D. Biomarkers for neonatal sepsis: Recent developments. Res. Rep. Neonatol. 2014, 4, 157–168. [Google Scholar] [CrossRef]
- Yan, R.; Zhou, T. Identification of key biomarkers in neonatal sepsis by integrated bioinformatics analysis and clinical validation. Heliyon 2022, 8, e11634. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, I.; Mace, G.; Sediki, M.; Lirussi, F.; Riethmuller, D.; Lentz, N.; Ramanah, R.; Hoyek, T.; Spagnolo, G.; Laurent, N.; et al. Changes in Maternal Blood Inflammatory Markers As a Predictor Of Chorioamnionitis: A Prospective Multicenter Study. Am. J. Reprod. Immunol. 2014, 73, 79–90. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kusanovic, J.P.; Friel, L.; The, S.H. role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007, 25, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Park, J.S.; Kim, M.; Oh, S.-Y.; Kim, C.J.; Jun, J.K. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am. J. Obstet. Gynecol. 2000, 183, 1124–1129. [Google Scholar] [CrossRef]
- Tang, W.; Mao, J.; Li, K.T.; Walker, J.S.; Chou, R.; Fu, R.; Chen, W.; Darville, T.; Klausner, J.; Tucker, J.D. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: A global systematic review and meta-analysis. Sex. Transm. Infect. 2020, 96, 322–329. [Google Scholar] [CrossRef]
- Daskalakis, G.; Psarris, A.; Koutras, A.; Fasoulakis, Z.; Prokopakis, I.; Varthaliti, A.; Karasmani, C.; Ntounis, T.; Domali, E.; Theodora, M.; et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children 2023, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Nori, W.; Akram, W.; Murshid, R.M.; Jaber, R. The implication of chlamydia and bacterial vaginosis among low-risk pregnant women with preterm birth: A prospective multicentric cohort study. BMC Pregnancy Childbirth 2025, 25, 904. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, L.; Li, H.; Shao, Y. The fetal inflammation response syndrome and adverse neonatal outcomes: A meta-analysis. J. Matern. Neonatal Med. 2019, 34, 3902–3914. [Google Scholar] [CrossRef] [PubMed]
- Ambalavanan, N.; Carlo, W.A.; D’ANgio, C.T.; McDonald, S.A.; Das, A.; Schendel, D.; Thorsen, P.; Higgins, R.D.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 2009, 123, 1132–1141. [Google Scholar] [CrossRef]
- Dessardo, N.S.; Dessardo, S.; Mustać, E.; Banac, S.; Petrović, O.; Peter, B. Chronic lung disease of prematurity and early childhood wheezing: Is foetal inflammatory response syndrome to blame? Early Hum. Dev. 2014, 90, 493–499. [Google Scholar] [CrossRef]
- Nomiyama, M.; Nakagawa, T.; Yamasaki, F.; Hisamoto, N.; Yamashita, N.; Harai, A.; Gondo, K.; Ikeda, M.; Tsuda, S.; Ishimatsu, M.; et al. Contribution of Fetal Inflammatory Response Syndrome (FIRS) with or without Maternal-Fetal Inflammation in The Placenta to Increased Risk of Respiratory and Other Complications in Preterm Neonates. Biomedicines 2023, 11, 611. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zou, N.; Zhang, L.; Wang, W.; Zhang, M.; Wang, C.; Yang, L. Pathogenesis from the microbial-gut-brain axis in white matter injury in preterm infants: A review. Front. Integr. Neurosci. 2023, 17, 1051689. [Google Scholar] [CrossRef] [PubMed]
- Serdar, M.; Walther, K.-A.; Gallert, M.; Kempe, K.; Obst, S.; Labusek, N.; Herrmann, R.; Herz, J.; Felderhoff-Müser, U.; Bendix, I. Prenatal inflammation exacerbates hyperoxia-induced neonatal brain injury. J. Neuroinflammation 2025, 22, 57. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Peng, X.; Yang, L.; Miao, J.; Yue, Y.; Wang, Y.; Wang, X.; Zhu, C.; Song, J. Targeting Neuroinflammation in Preterm White Matter Injury: Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes. Cell. Mol. Neurobiol. 2025, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Strunk, T.; Doherty, D.; Mbiostat, A.J.; Simmer, K.; Richmond, P.; Kohan, R.; Charles, A.; Burgner, D. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 2012, 129, e134–e141. [Google Scholar] [CrossRef] [PubMed]
- Lachin, J.M. Biostatistical Methods: The Assessment of Relative Risks, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 1–636. [Google Scholar] [CrossRef]



| Variable | N = 125 | p-Value | ||
|---|---|---|---|---|
| FIRS (n = 75) | Non-FIRS (n = 50) | |||
| Gestational age (weeks) (SD) | 31.17 (3.86) | 35.32 (1.91) | 0.001 | |
| Birth weight (grams) (SD) | 1660 (1147.50–2292.50) * | 2600 (2200–2930) * | 0.001 | |
| Length at birth (cm) (SD) | 40.24 (5.68) | 46.37 (3.54) | 0.001 | |
| Head circumference (cm) (SD) | 28.19 (3.91) | 32.19 (2.31) | 0.001 | |
| APGAR 1 min | 6.50 (4.00–8.00) * | 9.00 (8.00–9.00) * | 0.001 | |
| APGAR 5 min | 8.00 (7.00–9.00) * | 9.00 (8.50–9.00) * | 0.001 | |
| Gender (n = 125) | Male | 41 (32.80%) | 32 (25.6%) | 0.30 |
| Female | 34 (27.20%) | 18 (14.40%) | ||
| Vaginal delivery | 23 (82.14%) | 5 (17.86%) | 0.007 | |
| Intrauterine growth restriction (n = 16) | 13 (81.25%) | 3 (18.75%) | 0.06 | |
| Meconium-stained amniotic fluid (n = 14) | 13 (92.85%) | 1 (7.15%) | 0.008 | |
| Umbilical Astrup—pH | 7.28 (0.10) | 7.32 (0.09) | 0.04 | |
| Umbilical Astrup—Base excess | −5.19 (4.03) | −2.36 (3.52) | 0.001 | |
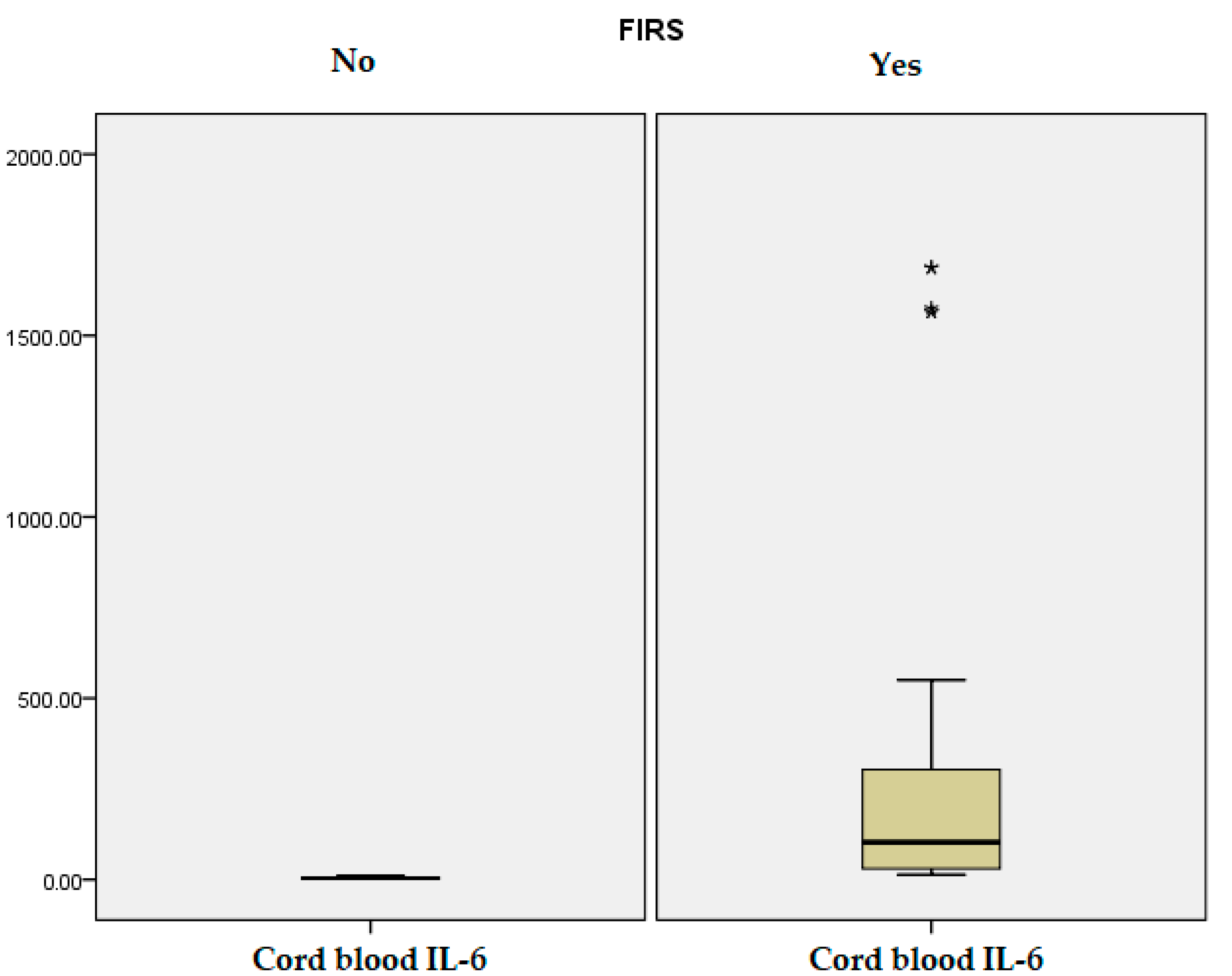
| Umbilical cord IL-6 (pg/mL) | 259.65 (43.40) | 4.13 (2.30) | 0.001 | |
| IL-6 at 24 h (pg/mL) | 47.84 (6.39) | 21.94 (3.41) | 0.01 | |
| CRP at 24 h (mg/L) | 6.00 (15.88) | 2.20 (2.65) | 0.09 | |
| PCT at 24 h (ng/mL) | 20.44 (27.46) | 9.32 (11.05) | 0.008 | |
| Leucocyte (/µL) | 14,366.93 (10061.90) | 14,534 (4710.15) | 0.91 | |
| Hematocrit level (%) | 44.99 (7.74) | 48.52 (6.03) | 0.008 | |
| RDW (%) | 16.86 (2.46) | 17.51 (1.55) | 0.10 | |
| Platelet count (/µL) | 256,000 (86516.36) | 279,340 (70,520.11) | 0.11 | |
| ANC (/µL) | 6.10 (4.17–9.75) * | 8.10 (5.40–12.05) * | 0.07 | |
| Lymphocyte (%) | 30.05 (20.80–41.20) * | 28.30 (21.50–36.75) * | 0.70 | |
| Lymphocyte absolute number (/µL) | 3.96 (1.96) | 4.22 (1.45) | 0.43 | |
| NLR | 2.59 (2.67) | 2.38 (1.56) | 0.61 | |
| ALT (U/L) | 9.24 (8.70) | 11.69 (9.10) | 0.13 | |
| AST (U/L) | 57.73 (31.86) | 49.78 (33.62) | 0.18 | |
| Blood glucose (mg/dL) | 74.91 (44.29) | 55.80 (15.94) | 0.004 | |
| Positive blood culture (n = 4) | 3 (75%) | 1 (25%) | 0.95 | |
| Variable | N = 125 | p-Value | |
|---|---|---|---|
| FIRS (n = 75) | Non-FIRS (n = 50) | ||
| Hours of noninvasive ventilation | 107.64 (182.60) | 12.68 (35.59) | 0.001 |
| Hours of invasive ventilation | 99.80 (245.80) | 4.80 (30.66) | 0.008 |
| Oxygen therapy hours | 223.71 (370.51) | 31.30 (87.37) | 0.001 |
| Parenteral nutrition days | 16.76 (14.81) | 3.08 (4.80) | 0.001 |
| Tube feeding days | 20.40 (21.91) | 2.50 (6.91) | 0.001 |
| Antibiotic therapy days | 16.23 (15.16) | 2.92 (3.88) | 0.001 |
| NICU days | 20.25 (18.18) | 3.16 (4.16) | 0.001 |
| Total hospitalization days | 30.00 (12.00–50.00) * | 5.00 (3.00–13.00) * | 0.001 |
| Phototherapy hours | 27.63 (23.76) | 10.40 (14.48) | 0.001 |
| Caffeine treatment days | 185.15 (19.98) | 2.74 (6.53) | 0.001 |
| Fresh Frozen Plasma transfusion | 0.56 (1.03) | 0.041 (0.19) | 0.001 |
| Red blood cell transfusion | 1.21 (2.00) | 0.001 (0.001) | 0.001 |
| Surfactant administration (n = 28) | 27 (96.42%) | 1 (3.58%) | 0.001 |
| Need for inotrope (n = 31) | 30 (96.77%) | 1 (3.23%) | 0.001 |
| Human Immunoglobulin (n = 28) | 27 (96.42%) | 1 (3.58%) | 0.001 |
| Sedation (n = 32) | 30 (93.75%) | 2 (6.25%) | 0.001 |
| Corticotherapy (n = 21) | 19 (90.47%) | 2 (9.53%) | 0.002 |
| Variable | N = 125 | p-Value | ||
|---|---|---|---|---|
| FIRS (n = 75) | Non-FIRS (n = 50) | |||
| Mother’s age | 31.24 (6.94) | 32.34 (5.28) | 0.34 | |
| PROM hours | 75.51 (195.70) | 2.76 (8.20) | 0.01 | |
| Leucocyte count (/µL) | 13,440.13 (5932.24) | 11,074 (3520.13) | 0.01 | |
| Neutrophil % | 78.10 (72.80–84.10) * | 73.85 (69.67–79.17) * | 0.006 | |
| ANC level (/µL) | 10.91 (7.19) | 8.48 (3.81) | 0.03 | |
| Lymphocyte % | 14.61 (5.83) | 17.86 (6.90) | 0.005 | |
| Lymphocyte count (/µL) | 1.43 (0.68) | 1.98 (1.08) | 0.04 | |
| Platelet count (/µL) | 249,822.66 (78,263.76) | 228,360 (76,693.33) | 0.13 | |
| CRP (mg/L) | 25.47 (38.81) | 11.20 (21.27) | 0.01 | |
| Fibrinogen (mg/dL) | 471 (423–629) * | 476 (446.25–588.25) * | 0.59 | |
| ALT (U/L) | 15 (11–21) * | 12 (8–17.25) * | 0.18 | |
| AST (U/L) | 20 (17–31) * | 18 (15–25) * | 0.09 | |
| Antibiotic therapy during pregnancy (n = 89) | 48 (53.93%) | 41 (46.07%) | 0.02 | |
| Antenatal corticosteroids (n = 41) | 31 (75.60%) | 10 (24.40%) | 0.01 | |
| Corioamnionitis (n = 42) | 37 (88.1%) | 5 (11.9%) | 0.001 | |
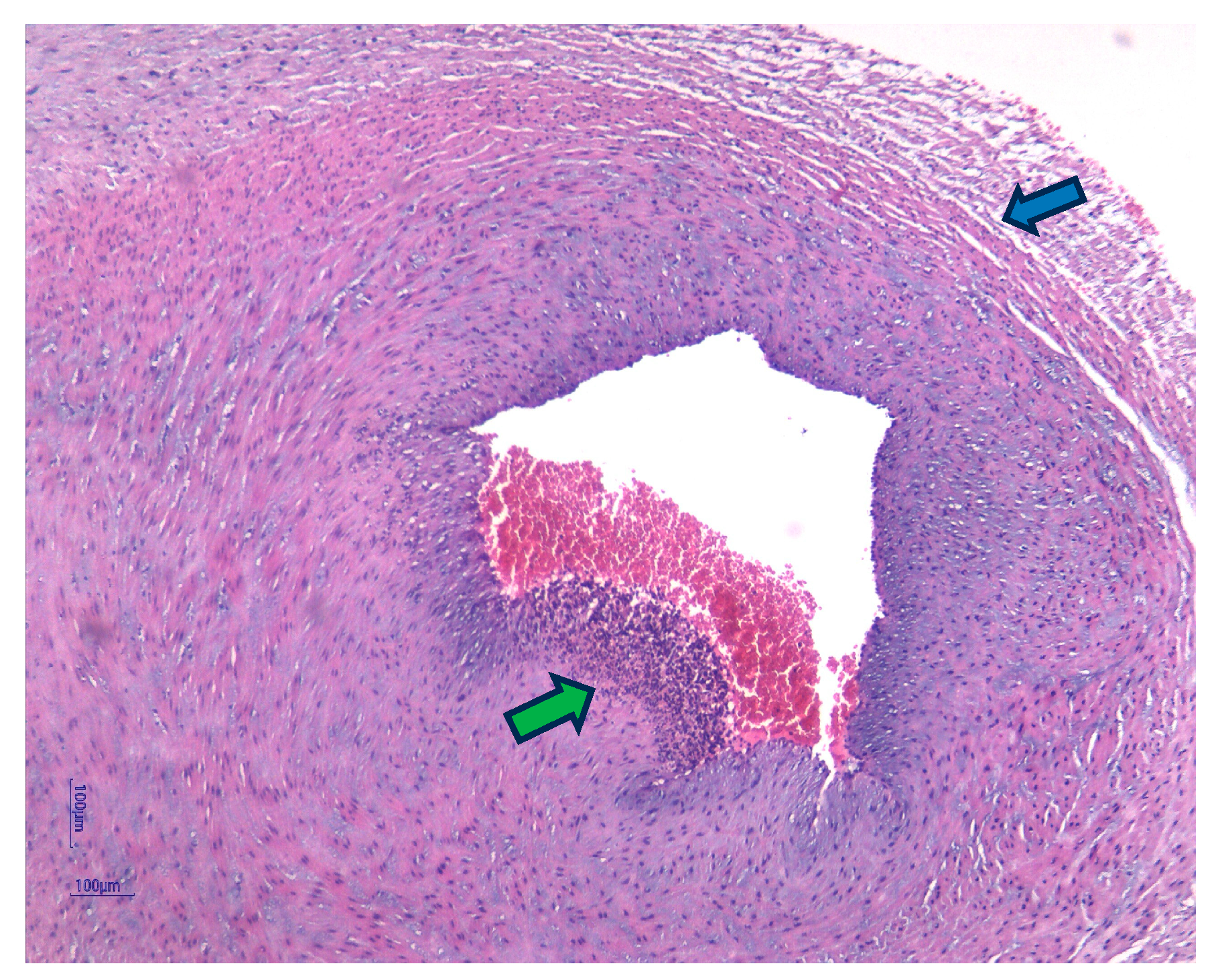
| Funisitis (n = 37) | 33 (89.2%) | 4 (10.8%) | 0.001 | |
| Smoking (n = 48) | 34 (70.84%) | 14 (29.16%) | 0.051 | |
| Socio-economic status (n = 125) | low | 30 (24%) | 10 (8%) | 0.059 |
| middle | 23 (18.4%) | 22 (17.59%) | ||
| high | 22 (17.59%) | 18 (14.42%) | ||
| Residential area (n = 125) | Urban | 33 (26.40%) | 32 (25.6%) | 0.08 |
| Rural | 42 (33.6%) | 18 (14.40%) | ||
| Marital status: married (n = 85) | 48 (56.47%) | 37 (43.53%) | 0.24 | |
| Variable | FIRS (n = 75) | Non-FIRS (n = 50) | p-Value |
|---|---|---|---|
| Group B Streptococcus (n = 36) | 11 (30.55%) | 25 (69.45%) | 0.45 |
| Ureaplasma spp. (n = 16) | 9 (56.25%) | 7 (43.75%) | 0.92 |
| Chlamydia trachomatis (n = 10) | 9 (90%) | 1 (10%) | 0.02 |
| Klebsiella pneumoniae (n = 8) | 6 (75%) | 2 (25%) | 0.29 |
| Escherichia coli (n = 31) | 19 (61.29%) | 12 (38.71%) | 0.54 |
| Variable | FIRS (n = 75) | Non-FIRS (n = 50) | p-Value |
|---|---|---|---|
| EOS (n = 65) | 62 (95.38%) | 3 (4.62%) | 0.001 |
| LOS (n = 13) | 13 (100%) | 0 (0%) | 0.002 |
| Respiratory distress (n = 66) | 55 (83.33%) | 11 (16.67%) | 0.001 |
| BPD (n = 19) | 18 (94.73%) | 1 (5.27%) | 0.001 |
| NEC (n = 6) | 6 (100%) | 0 (0%) | 0.04 |
| PDA (n = 39) | 33 (84.62%) | 6 (15.38%) | 0.001 |
| ROP (n = 15) | 15 (100%) | 0 (0%) | 0.001 |
| IVH/PVL (n = 39) | 33 (84.62%) | 6 (15.38%) | 0.001 |
| Apnea (n = 53) | 45 (84.90%) | 8 (15.10%) | 0.001 |
| Feeding intolerance (n = 67) | 52 (77.61%) | 15 (22.39%) | 0.001 |
| Seizures (n = 12) | 12 (100%) | 0 (0%) | 0.003 |
| Bleeding (n = 15) | 15 (100%) | 0 (0%) | 0.001 |
| Death (n = 4) | 4 (100%) | 0 (0%) | 0.09 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| EOS | 74.71 (20.13–277.29) | 0.001 |
| Respiratory distress | 9.75 (4.19–22.63) | 0.001 |
| BDP | 15.47 (1.99–120.14) | 0.009 |
| PDA | 5.76 (2.19–15.15) | 0.001 |
| IVH | 5.76 (2.19–15.15) | 0.001 |
| Variable | AUC | 95% CI | p-Value | Cut-Off | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
| EOS | 0.93 | 0.89–0.98 | 0.001 | 11.92 | 95.4% | 21.7% |
| LOS | 0.85 | 0.75–0.94 | 0.001 | 9.92 | 100% | 56.3% |
| BPD | 0.78 | 0.67–0.89 | 0.001 | 13.38 | 94.7% | 52.8% |
| NEC | 0.77 | 0.60–0.53 | 0.02 | 13.53 | 100% | 56.3% |
| PDA | 0.70 | 0.60–0.79 | 0.001 | 13.76 | 84.6% | 45.3% |
| ROP | 0.80 | 0.69–0.90 | 0.001 | 14.22 | 100% | 50.9% |
| IVH | 0.74 | 0.65–0.83 | 0.001 | 14.62 | 84.6% | 43.0% |
| Death | 0.85 | 0.64–1.00 | 0.01 | 17.32 | 75% | 50.4% |
| Variable | GA = 22–27 Weeks of Gestation (n = 13) | GA = 28–31 Weeks of Gestation (n = 26) | GA = 32–36 Weeks of Gestation (n = 86) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FIRS (n = 13) | Non-FIRS (n = 0) | p-Value | FIRS (n = 24) | Non-FIRS (n = 2) | p-Value | FIRS (n = 38) | Non-FIRS (n = 48) | p-Value | |
| Birth weight grams | 910.38 (257.92) | - | - | 1353.33 (374.01) | 1575.00 (459.61) | 0.44 | 2299.2 (553.27) | 2711.46 (701.38) | 0.006 |
| Length at birth cm | 33.23 (3.46) | - | - | 37.18 (3.38) | 39.50 (3.53) | 0.39 | 44.56 (3.32) | 46.65 (3.27) | 0.002 |
| Head circumference cm | 23.19 (2.43) | - | - | 26.31 (2.29) | 27.00 (1.41) | 0.55 | 31.09 (2.39) | 32.40 (2.08) | 0.018 |
| APGAR 1 min | 3.00 (1.91) | - | - | 5.17 (2.31) | 6.50 (0.70) | 0.55 | 7.47 (1.03) | 8.31 (1.18) | 0.001 |
| APGAR 5 min | 5.54 (1.85) | - | - | 6.79 (2.08) | 7.50 (0.70) | 0.88 | 8.42 (0.91) | 8.96 (0.74) | 0.003 |
| Gender Male Female | 8 (61.53%) | - | - | 15 (57.69%) | 1 (3.84%) | 0.72 | 18 (20.93%) | 31 (36.04%) | 0.10 |
| 5 (38.47%) | - | 9 (34.63%) | 1 (3.84%) | 20 (23.25%) | 17 (19.78%) | ||||
| Umbilical cord IL-6 (pg/mL) | 401.61 (548.16) | - | - | 332.92 (527.60) | 2.23 (1.13) | 0.006 | 159.14 (186.35) | 4.27 (2.28) | 0.001 |
| IL-6 at 24 h (pg/mL) | 39.77 (44.58) | - | - | 47.81 (65.87) | 32.59 (3.79) | 0.30 | 51.78 (78.07) | 21.82 (31.23) | 0.001 |
| CRP at 24 h (mg/L) | 4.41 (7.18) | - | - | 7.69 (25.41) | 3.32 (0.96) | 0.22 | 4.99 (8.73) | 2.16 (2.72) | 0.03 |
| PCT at 24 h (ng/mL) | 21.49 (33.23) | - | - | 22.02 (31.53) | 8.08 (10.88) | 0.61 | 19.08 (23.30) | 9.28 (11.27) | 0.11 |
| EOS | 13 (100%) | - | - | 21 (95.45%) | 1 (4.54%) | 0.15 | 28 (93.33%) | 2 (6.67%) | 0.001 |
| LOS | 10 (100%) | - | - | 3 (100%) | 0 (0%) | 0.59 | 0 (0%) | 0 (0%) | - |
| Respiratory distress | 13 (100%) | - | - | 24 (92.30%) | 2 (7.70%) | - | 18 (66.66%) | 9 (33.34%) | 0.005 |
| BPD | 10 (100%) | - | - | 8 (88.88%) | 1 (11.12%) | 0.63 | 0 (0%) | 0 (0%) | - |
| NEC | 3 (100%) | - | - | 2 (100%) | 0 (0%) | 0.67 | 1 (100%) | 0 (0%) | 0.25 |
| PDA | 10 (100%) | - | - | 13 (92.85%) | 1 (7.15%) | 0.91 | 10 (66.66%) | 5 (33.34%) | 0.054 |
| ROP | 7 (100%) | - | - | 6 (100%) | 0 (0%) | 0.42 | 2 (100%) | 0 (0%) | 0.10 |
| IVH/PVL | 10 (100%) | - | - | 16 (94.11%) | 1 (5.89%) | 0.63 | 7 (58.33%) | 5 (41.67%) | 0.28 |
| Apnea | 12 (100%) | - | - | 23 (95.83%) | 1 (4.17%) | 0.01 | 10 (58.82%) | 7 (41.18%) | 0.17 |
| Feeding intolerance | 12 (100%) | - | - | 21 (91.30%) | 2 (8.70%) | 0.59 | 19 (59.37%) | 13 (40.63%) | 0.002 |
| Seizures | 5 (100%) | - | - | 4 (100%) | 0 (0%) | 0.53 | 3 (100%) | 0 (0%) | 0.04 |
| Bleeding | 4 (100%) | - | - | 8 (100%) | 0 (0%) | 0.32 | 3 (100%) | 0 (0%) | 0.04 |
| Death | 3 (100%) | - | - | 1 (100%) | 0 (0%) | 0.76 | 0 (0%) | 0 (0%) | - |
| Funisitis | 12 (100%) | - | - | 10 (90.90%) | 1 (9.10%) | 0.81 | 11 (78.57%) | 3 (21.43%) | 0.005 |
| Chorioamnionitis | 12 (100%) | - | - | 13 (92.85%) | 1 (7.15%) | 0.91 | 12 (75%) | 4 (25%) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilescu, D.I.; Dan, A.M.; Dragomir, I.; Vasilescu, S.L.; Dumitru, A.V.; Dima, V.; Cîrstoiu, M.M. Predicting Neonatal Morbidity and Correlations with Maternal and Neonatal Biomarkers in Connection with Fetal Inflammatory Response Syndrome in Premature Births. J. Clin. Med. 2025, 14, 6440. https://doi.org/10.3390/jcm14186440
Vasilescu DI, Dan AM, Dragomir I, Vasilescu SL, Dumitru AV, Dima V, Cîrstoiu MM. Predicting Neonatal Morbidity and Correlations with Maternal and Neonatal Biomarkers in Connection with Fetal Inflammatory Response Syndrome in Premature Births. Journal of Clinical Medicine. 2025; 14(18):6440. https://doi.org/10.3390/jcm14186440
Chicago/Turabian StyleVasilescu, Diana Iulia, Adriana Mihaela Dan, Ion Dragomir, Sorin Liviu Vasilescu, Adrian Vasile Dumitru, Vlad Dima, and Monica Mihaela Cîrstoiu. 2025. "Predicting Neonatal Morbidity and Correlations with Maternal and Neonatal Biomarkers in Connection with Fetal Inflammatory Response Syndrome in Premature Births" Journal of Clinical Medicine 14, no. 18: 6440. https://doi.org/10.3390/jcm14186440
APA StyleVasilescu, D. I., Dan, A. M., Dragomir, I., Vasilescu, S. L., Dumitru, A. V., Dima, V., & Cîrstoiu, M. M. (2025). Predicting Neonatal Morbidity and Correlations with Maternal and Neonatal Biomarkers in Connection with Fetal Inflammatory Response Syndrome in Premature Births. Journal of Clinical Medicine, 14(18), 6440. https://doi.org/10.3390/jcm14186440





