CAPTURE and PUMA as Case-Finding Tools in Patients at Risk of COPD—A Multicenter Mexican Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pulmonary Function Testing
2.3. COPD Definition
2.4. Questionnaires
2.5. Patient and Public Involvement Statement
2.6. Statistical Analysis
2.6.1. Propensity Score (PS) Matching
2.6.2. Sample Estimation
3. Results
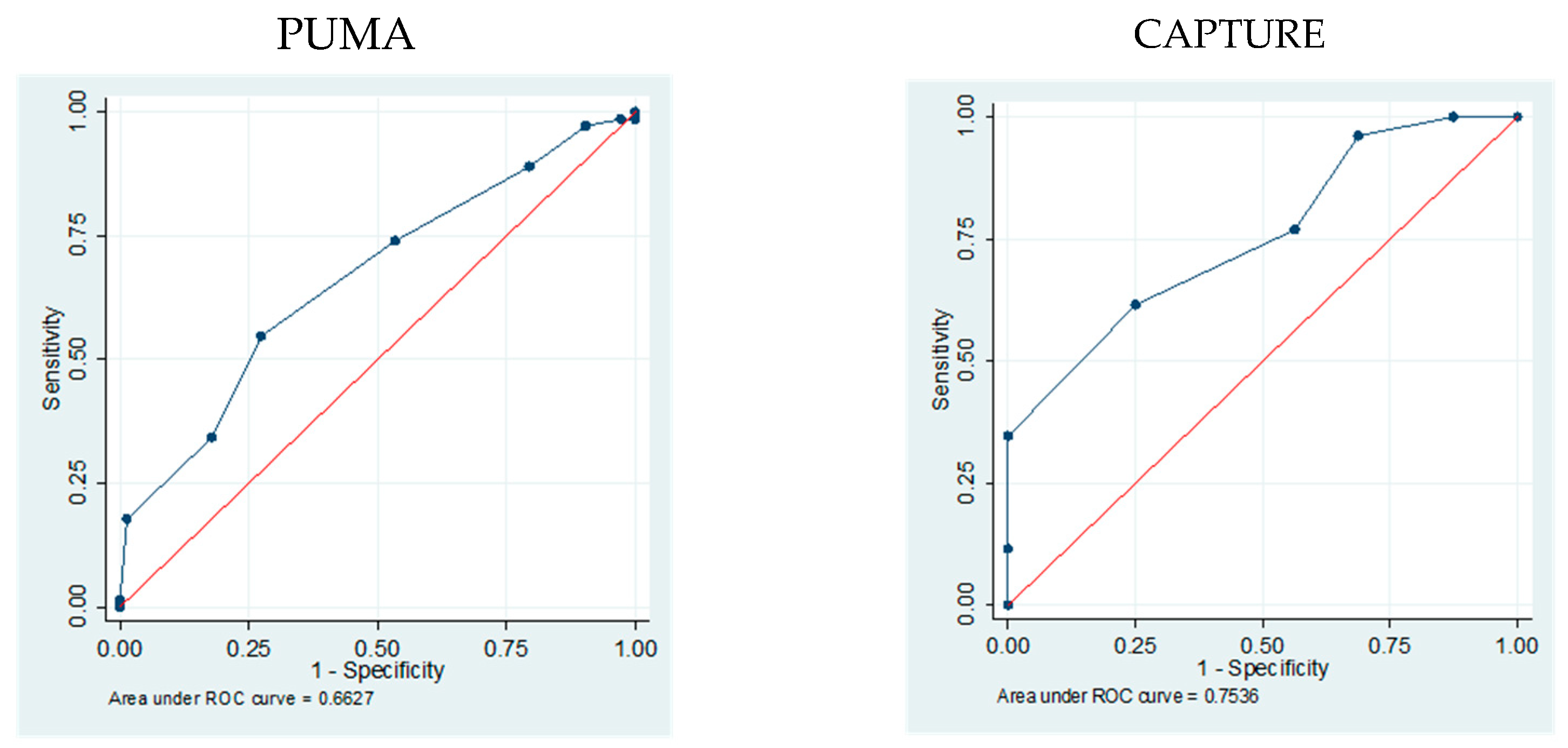
3.1. Accuracy of PUMA and CAPTURE to Identify Patients with High Risk of COPD
3.2. Linear Regression Analysis (LRA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATS/ERS | American Thoracic Society/European Respiratory Society |
| AUC | area under the curve |
| CAPTURE | COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk |
| CDQ | COPD Diagnostic Questionnaire |
| CI | confidence intervals |
| COPD | Chronic obstructive pulmonary disease |
| COPS-PS | COPD Population Screener Questionnaire |
| FEV1 | Forced Expiratory Volume in 1 s |
| FEV1/FVC | ratio by dividing FEV1 by FV |
| FVC | Forced Vital Capacity |
| IQR | interquartile range |
| L-MICs | low- and middle-income countries |
| ORs | odds ratios |
| PC | primary care |
| PCP | primary care physicians |
| post-BD | post-bronchodilator |
| PSM | Propensity score matching |
| ROC | receiver operator characteristics |
References
- Bahadori, K.; FitzGerald, J.M. Risk factors of hospitalization and readmission of patients with COPD exacerbation—Systematic review. Int. J. COPD 2007, 2, 241–251. [Google Scholar]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210, Erratum in Lancet 2017, 390, e38. https://doi.org/10.1016/S0140-6736(17)32646-6. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report. Bethesda: GOLD. Available online: https://goldcopd.org/2024-gold-report (accessed on 7 November 2024).
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global Health Epidemiology Reference Group (GHERG). Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.M.; Perez-Padilla, R.; Jardim, J.R.; Muiño, A.; Lopez, M.V.; Valdivia, G.; Montes de Oca, M.; Talamo, C.; Hallal, P.C.; Victora, C.G.; et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): A prevalence study. Lancet 2005, 366, 1875–1881. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Gebke, K.B. Activity restriction in mild COPD: A challenging clinical problem. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 577–588. [Google Scholar] [CrossRef]
- Ho, T.; Cusack, R.P.; Chaudhary, N.; Satia, I.; Om, P.K. Under and over-diagnosis of COPD: A global perspective. Breathe 2019, 15, 24–35. [Google Scholar] [CrossRef]
- Lamprecht, B.; Soriano, J.B.; Studnicka, M.; Kaiser, B.; Vanfleteren, L.E.; Gnatiuc, L.; Burney, P.; Miravitlles, M.; García-Rio, F.; Akbari, K.; et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest 2015, 148, 971–985. [Google Scholar] [CrossRef]
- Casas-Herrera, A.; de Oca, M.M.; López-Varela, M.V.; Aguirre, C.; Schiavi, E.; Jardim, J.R.; PUMA team. COPD Underdiagnosis and Misdiagnosis in a High-Risk Primary Care Population in Four Latin American Countries. A Key to Enhance Disease Diagnosis: The PUMA Study. PLoS ONE 2016, 11, e0152266. [Google Scholar] [CrossRef]
- Martinez, F.J.; Raczek, A.E.; Seifer, F.D.; Conoscenti, C.S.; Curtice, T.G.; D’Eletto, T.; Cote, C.; Hawkins, C.; Phillips, A.L. Development and Initial Validation of a Self-Scored COPD Population Screener Questionnaire (COPD-PS). COPD: J. Chronic Obstr. Pulm. Dis. 2008, 5, 85–95. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases, Rehabilitation and Disability. Chronic Respiratory Diseases Programme. Available online: https://www.who.int/teams/noncommunicable-diseases/ncds-management/chronic-respiratory-diseases-programme (accessed on 7 November 2024).
- Lin, C.H.; Cheng, S.L.; Chen, C.Z.; Chen, C.H.; Lin, S.H.; Wang, H.C. Current Progress of COPD Early Detection: Key Points and Novel Strategies. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1511–1524. [Google Scholar] [CrossRef]
- Diab, N.; Gershon, A.S.; Sin, D.D.; Tan, W.C.; Bourbeau, J.; Boulet, L.P.; Aaron, S.D. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ichinose, M.; Inoue, H.; Shirato, K.; Hattori, T.; Takishima, T. Underdiagnosis and undertreatment of COPD in primary care settings. Respirology 2003, 8, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, M.; Maciejewski, J.; Wozniak, M.; Kuca, P.; Zielinski, J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008, 63, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, T.; Pollard, S.L.; Quaderi, S.A.; Rykiel, N.A.; Wosu, A.C.; Alupo, P.; Barber, J.A.; Cárdenas, M.K.; Chandyo, R.K.; Flores-Flores, O.; et al. Discriminative Accuracy of Chronic Obstructive Pulmonary Disease Screening Instruments in 3 Low- and Middle-Income Country Settings. JAMA 2022, 327, 151–160. [Google Scholar] [CrossRef]
- López-Varela, M.V.; Montes de Oca, M.; Rey, A.; Casas, A.; Stirbulov, R.; Di Boscio, V.; PUMA Team. Development of a simple screening tool for opportunistic COPD case finding in primary care in Latin America: The PUMA study. Respirology 2016, 21, 1227–1234. [Google Scholar] [CrossRef]
- Au-Doung, P.L.W.; Wong, C.K.M.; Chan, D.C.C.; Chung, J.W.H.; Wong, S.Y.S.; Leung, M.K.W. PUMA screening tool to detect COPD in high-risk patients in Chinese primary care–A validation study. PLoS ONE 2022, 17, e0274106. [Google Scholar] [CrossRef]
- Martinez, F.J.; Mannino, D.; Leidy, N.K.; Malley, K.G.; Bacci, E.D.; Barr, R.G.; Bowler, R.P.; Han, M.K.; Houfek, J.F.; Make, B.; et al. A New Approach for Identifying Patients with Undiagnosed Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 195, 748–756. [Google Scholar] [CrossRef]
- Benítez-Pérez, R.E.; Vázquez-García, J.C.; Sánchez-Gallén, E.; Salas-Hernández, J.; Pérez-Padilla, R.; Reyes-Herrera, A.; Ruiz-Ascencio, D.; Camargo-Ángeles, R.; Irineo-González, L. Impacto de un programa educativo de espirometría en el primer nivel de atención en México. NCT Neumol. Cirugía Tórax 2021, 80, 29–38. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quezada, W.A.; Whippo, B.A.; Jellen, P.A.; Leidy, N.K.; Mannino, D.M.; Kim, K.J.; Han, M.K.; Houfek, J.F.; Make, B.; Malley, K.G.; et al. How Well Does CAPTURE Translate?: An Exploratory Analysis of a COPD Case-Finding Method for Spanish-Speaking Patients. Chest 2017, 152, 761–770. [Google Scholar] [CrossRef]
- Martinez, F.J.; Han, M.K.; Lopez, C.; Murray, S.; Mannino, D.; Anderson, S.; Brown, R.; Dolor, R.; Elder, N.; Joo, M.; et al. Discriminative Accuracy of the CAPTURE Tool for Identifying Chronic Obstructive Pulmonary Disease in US Primary Care Settings. JAMA 2023, 329, 490–501. [Google Scholar] [CrossRef]
- Leidy, N.K.; Martinez, F.J.; Malley, K.G.; Mannino, D.M.; Han, M.K.; Bacci, E.D.; Brown, R.W.; Houfek, J.F.; Labaki, W.W.; Make, B.J.; et al. Can CAPTURE be used to identify undiagnosed patients with mild-to-moderate COPD likely to benefit from treatment? Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Sebayang, R.R.; Pandia, P.; Pradana, A.; Tarigan, A.P.; Wahyuni, A.S. Comparative analysis between PUMA and CAPTURE questionnaires for chronic obstructive pulmonary disease (COPD) screening in smokers. Narra J. 2024, 4, e654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-H.; McClish, D.K.; Obuchowski, N.A. Statistical Methods in Diagnostic Medicine, 2nd ed.; Wiley: Hoboken, NJ, USA, 2011; p. 205. [Google Scholar]
- Hawass, N.E. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br. J. Radiol. 1997, 70, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Florman, K.E.H.; Siddharthan, T.; Pollard, S.L.; Alupo, P.; Barber, J.A.; Chandyo, R.K.; Flores-Flores, O.; Kirenga, B.; Mendes, R.G.; Miranda, J.J.; et al. Additional GECo Study Investigators. Unmet Diagnostic and Therapeutic Opportunities for Chronic Obstructive Pulmonary Disease in Low- and Middle-Income Countries. Am. J. Respir. Crit. Care Med. 2023, 208, 442–450. [Google Scholar] [CrossRef]
- Tyagi, J.; Moola, S.; Bhaumik, S. Diagnostic accuracy of screening tools for chronic obstructive pulmonary disease in primary health care: Rapid evidence synthesis. J. Fam. Med. Prim. Care. 2021, 10, 2184–2194. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Senger, C.A.; Webber, E.M.; Mularski, R.A.; Whitlock, E.P. Screening for Chronic Obstructive Pulmonary Disease: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 315, 1378–1393. [Google Scholar] [CrossRef]
- Reyes-Garcia, A.; Torre-Bouscoulet, L.; Pérez-Padilla, R. Controversies and Limitations in the Diagnosis of Chronic Obstructive Pulmonary Disease. Rev. Investig. Clin. 2019, 71, 28–35. [Google Scholar] [CrossRef]

| Score | Sensitivity (%) | Specificity (%) | Correct COPD Classification (%) | LR+ | LR− |
|---|---|---|---|---|---|
| PUMA (n = 197) | |||||
| ≥5 | 89.0 | 29.8 | 51.8 | 1.52 | 0.50 |
| ≥6 | 74.0 | 51.6 | 59.9 | 2.12 | 0.60 |
| ≥7 | 54.8 | 74.2 | 67.0 | 2.49 | 0.76 |
| CAPTURE (n = 48) | |||||
| ≥2 | 96.1 | 22.7 | 62.5 | 1.24 | 0.16 |
| ≥3 | 76.9 | 40.9 | 60.4 | 1.30 | 0.56 |
| ≥4 | 61.5 | 72.7 | 66.6 | 2.25 | 0.58 |
| Before PS Matching (n = 197) | After PS Matching (n = 146) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Without COPD (n = 124) | with COPD (n = 73) | p | Without COPD (n = 73) | with COPD (n = 73) | p |
| Age, years. Median (IQR) | 64 (57, 70) | 72 (63, 79) | <0.001 | 67 (61, 74) | 72 (63, 79) | 0.030 |
| Age, years; frequency (%) | 0.177 | 0.913 | ||||
| 40–49 | 11 (8.9) | 3 (4.1) | 4 (5.5) | 3 (4.1) | ||
| 50–59 | 31 (25.0) | 13 (17.8) | 12 (16.4) | 13 (17.8) | ||
| >60 | 82 (66.1) | 78.1 (78.1) | 57 (78.1) | 57 (78.1) | ||
| Gender, frequency (%) | 0.475 | 0.864 | ||||
| Female | 54 (43.6) | 28 (38.4) | 27 (37.0) | 28 (38.4) | ||
| Male | 70 (56.4) | 45 (61.6) | 46 (63.0) | 45 (61.6) | ||
| Comorbidities, frequency (%) | 104 (83.8) | 61 (83.6) | 0.955 | 55 (75.3) | 61 (83.6) | 0.219 |
| Arterial hypertension, frequency (%) | 55 (44.4) | 35 (47.9) | 0.625 | 29 (39.7) | 35 (47.9) | 0.317 |
| Diabetes Mellitus 2, frequency (%) | 38 (30.6) | 16 (21.9) | 0.185 | 11 (15.1) | 16 (21.9) | 0.286 |
| Cardiovascular, frequency (%) | 18 (14.5) | 16 (21.9) | 0.184 | 13 (17.8) | 16 (21.9) | 0.534 |
| Asthma, frequency (%) | 2 (1.6) | 7 (9.6) | 0.014 | 2 (2.7) | 7 (9.6) | 0.085 |
| Smoking, frequency (%) | 111 (89.5) | 69 (94.5) | 0.227 | 65 (89.0) | 69 (94.5) | 0.228 |
| History of smoking, years; Median (IQR) | 21 (15, 40) | 33 (20, 43) | 0.001 | 20 (15, 40) | 33 (20, 43) | 0.008 |
| Biomass, frequency (%) | 21 (16.9) | 7 (9.6) | 0.154 | 13 (17.8) | 7 (9.6) | 0.149 |
| FEV1, liters; Median (IQR) | 2.15 (1.70, 2.71) | 1.34 (1.01, 1.78) | <0.001 | 2.19 (1.75, 2.66) | 1.34 (1.01, 1.78) | <0.001 |
| FEV1, %; Median (IQR) | 84.0 (73.0, 95.8) | 61.0 (47.0, 70.0) | <0.001 | 84.0 (75.0, 100) | 61.0 (47.0, 70.0) | <0.001 |
| FEV1 Z Score, Median (IQR) | −0.91 (−1.61, 0.0) | −2.33 (−2.92, −1.64) | <0.001 | −0.77 (−1.50, 0.10) | −2.33 (−2.92, −1.64) | <0.001 |
| FVC, liters; Median (IQR) | 2.79 (2.05, 3.40) | 2.40 (1.70, 3.23) | 0.021 | 2.91 (2.11, 3.38) | 2.40 (1.70, 3.23) | 0.026 |
| FVC, %; Median (IQR) | 82.0 (72.0, 92.4) | 78.0 (59.0, 89.0) | 0.023 | 84.0 (76.0, 94.0) | 78.0 (59.0, 89.0) | 0.013 |
| FVC Z Score, Median (IQR) | −1.25 (−1.85, −0.47) | −1.46 (−2.13, −0.71) | 0.188 | −1.21 (−1.71, −0.31) | −1.46 (−2.13, −0.71) | 0.076 |
| Relation FEV1/FVC, Median (IQR) | 0.78 (0.74, 0.82) | 0.60 (0.52, 0.65) | <0.001 | 0.77 (0.74, 0.81) | 0.60 (0.52, 0.65) | <0.001 |
| PUMA Score | 5 (4, 7) | 7 (5, 8) | <0.001 | 6 (5, 7) | 7 (5, 8) | <0.001 |
| CAPTURE Score | 3 (2, 4) | 4 (3, 5) | 0.009 | 3 (0, 3.5) | 4 (3, 5) | 0.005 |
| Sensitivity (%) | Specificity (%) | Correct Classification (%) | LR+ | LR− | |
|---|---|---|---|---|---|
| PUMA score | |||||
| ≥5 | 89.0 | 20.5 | 54.7 | 1.12 | 0.53 |
| ≥6 | 73.9 | 46.6 | 60.3 | 1.38 | 0.55 |
| ≥7 | 54.8 | 72.6 | 63.7 | 2.00 | 0.62 |
| CAPTURE score | |||||
| ≥2 | 96.1 | 31.2 | 71.4 | 1.39 | 0.12 |
| ≥3 | 76.9 | 43.7 | 64.2 | 1.36 | 0.52 |
| ≥4 | 61.5 | 75.0 | 66.6 | 2.46 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortes-Telles, A.; Juarez-de-Dios, I.; Figueroa-Hurtado, E.; Ortiz-Farias, D.L.; Alvarez-Pinto, J.; Olaya-López, E. CAPTURE and PUMA as Case-Finding Tools in Patients at Risk of COPD—A Multicenter Mexican Experience. J. Clin. Med. 2025, 14, 6433. https://doi.org/10.3390/jcm14186433
Cortes-Telles A, Juarez-de-Dios I, Figueroa-Hurtado E, Ortiz-Farias DL, Alvarez-Pinto J, Olaya-López E. CAPTURE and PUMA as Case-Finding Tools in Patients at Risk of COPD—A Multicenter Mexican Experience. Journal of Clinical Medicine. 2025; 14(18):6433. https://doi.org/10.3390/jcm14186433
Chicago/Turabian StyleCortes-Telles, Arturo, Ismael Juarez-de-Dios, Esperanza Figueroa-Hurtado, Diana Lizbeth Ortiz-Farias, Jonathan Alvarez-Pinto, and Enrique Olaya-López. 2025. "CAPTURE and PUMA as Case-Finding Tools in Patients at Risk of COPD—A Multicenter Mexican Experience" Journal of Clinical Medicine 14, no. 18: 6433. https://doi.org/10.3390/jcm14186433
APA StyleCortes-Telles, A., Juarez-de-Dios, I., Figueroa-Hurtado, E., Ortiz-Farias, D. L., Alvarez-Pinto, J., & Olaya-López, E. (2025). CAPTURE and PUMA as Case-Finding Tools in Patients at Risk of COPD—A Multicenter Mexican Experience. Journal of Clinical Medicine, 14(18), 6433. https://doi.org/10.3390/jcm14186433






