Colchicine and Atherosclerotic Coronary Artery Disease: An Updated Review
Abstract
1. Introduction
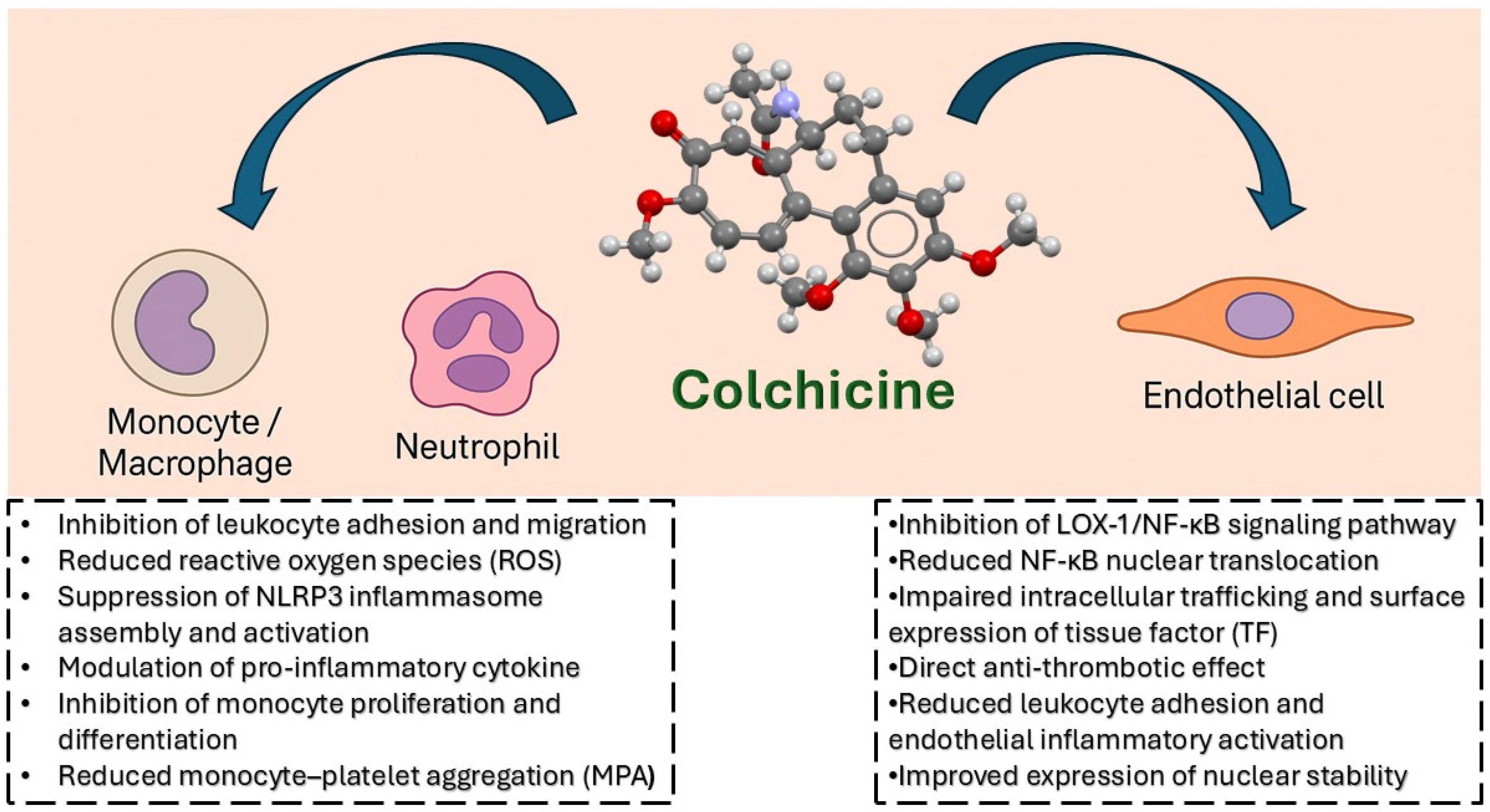
2. Mechanism of Action of Colchicine in Atherosclerosis
2.1. Effects on Neutrophils and Monocytes
2.2. Inhibition of the NLRP3 Inflammasome
2.3. Modulation of Cytokines and Endothelial Function
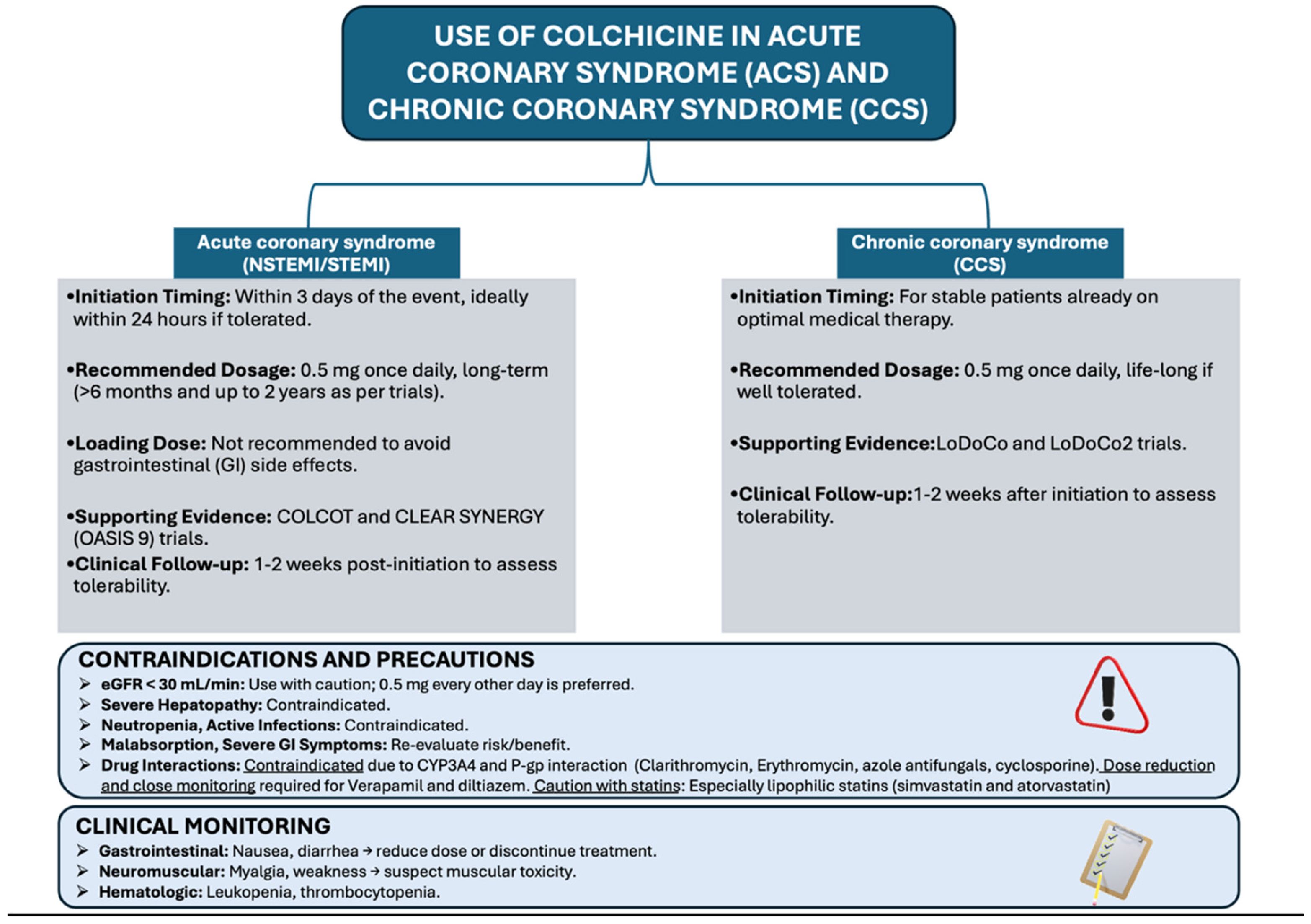
3. Clinical Evidence in Acute Coronary Syndromes
4. Clinical Evidence in Chronic Coronary Syndromes
5. Safety Profile and Drug Interactions
5.1. Gastrointestinal Side Effects and Dose Consideration
5.2. Risks in Patients with Hepatic or Renal Impairment
5.3. Interaction with Concomitant Medications
6. Limitations and Controversies
7. Practical Considerations for Clinical Use
Monitoring and Follow-Up
8. Future Perspectives and Unmet Needs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Braunwald, E.; Catapano, A.L. The LDL cumulative exposure hypothesis: Evidence and practical applications. Nat. Rev. Cardiol. 2024, 21, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaman, S.; Wasfy, J.H.; Kapil, V.; Ziaeian, B.; A Parsonage, W.; Sriswasdi, S.; A Chico, T.J.; Capodanno, D.; Colleran, R.; Sutton, N.R.; et al. The Lancet Commission on rethinking coronary artery disease: Moving from ischaemia to atheroma. Lancet 2025, 405, 1264–1312. [Google Scholar] [CrossRef]
- Alenghat, F.J. The prevalence of atherosclerosis in those with inflammatory connective tissue disease by race, age, and traditional risk factors. Sci. Rep. 2016, 6, 20303. [Google Scholar] [CrossRef]
- Shah, A.S.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: Systematic review and meta-analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; E Nissen, S. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Fuster, V.; Ridker, P.M. Low-Dose Colchicine for Secondary Prevention of Coronary Artery Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 82, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Hartung, E.F. History of the use of colchicum and related medicaments in gout; with suggestions for further research. Ann. Rheum. Dis. 1954, 13, 190–200. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 1187, https://doi.org/10.1002/acr.24401. Erratum in: Arthritis Care Res. 2021, 73, 458. https://doi.org/10.1002/acr.24566. Erratum in: Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Lancieri, M.; Bustaffa, M.; Palmeri, S.; Prigione, I.; Penco, F.; Papa, R.; Volpi, S.; Caorsi, R.; Gattorno, M. An Update on Familial Mediterranean Fever. Int. J. Mol. Sci. 2023, 24, 9584. [Google Scholar] [CrossRef] [PubMed]
- Gracheva, I.A.; Shchegravina, E.S.; Schmalz, H.-G.; Beletskaya, I.P.; Fedorov, A.Y. Colchicine Alkaloids and Synthetic Analogues: Current Progress and Perspectives. J. Med. Chem. 2020, 63, 10618–10651. [Google Scholar] [CrossRef]
- Taylor, E.W. The Mechanism of Colchicine Inhibition of Mitosis: I. Kinetics of Inhibition and the Binding of H3-Colchicine. J. Cell Biol. 1965, 25, 145–160. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Asako, H.; Kubes, P.; Baethge, B.A.; Wolf, R.E.; Granger, D.N. Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation 1992, 16, 45–56. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Molad, Y.; Reibman, J.; Balakhane, E.; I Levin, R.; Weissmann, G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Investig. 1995, 96, 994–1002. [Google Scholar] [CrossRef]
- Paschke, S.; Weidner, A.F.; Paust, T.; Marti, O.; Beil, M.; Ben-Chetrit, E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J. Leukoc. Biol. 2013, 94, 1091–1096. [Google Scholar] [CrossRef]
- Roberge, C.J.; Gaudry, M.; de Médicis, R.; Lussier, A.; E Poubelle, P.; Naccache, P.H. Crystal-induced neutrophil activation. IV. Specific inhibition of tyrosine phosphorylation by colchicine. J. Clin. Investig. 1993, 92, 1722–1729. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.; Toledo-Flores, D.; Fernando, S.; Di Bartolo, B.A.; S, J.N.; Psaltis, P.J. Pro-Inflammatory Effects of Colchicine on Macrophages Stimulated with Atherogenic Stimuli in Vitro. Heart Lung Circ. 2016, 25, S89. [Google Scholar] [CrossRef]
- Shah, B.; Allen, N.; Harchandani, B.; Pillinger, M.; Katz, S.; Sedlis, S.P.; Echagarruga, C.; Samuels, S.K.; Morina, P.; Singh, P.; et al. Effect of Colchicine on Platelet-Platelet and Platelet-Leukocyte Interactions: A Pilot Study in Healthy Subjects. Inflammation 2016, 39, 501, https://doi.org/10.1007/s10753-015-0266-2. Erratum in: Inflammation 2016, 39, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Kanneganti, T.D. Inflammasomes and the fine line between defense and disease. Curr. Opin. Immunol. 2020, 62, 39–44. [Google Scholar] [CrossRef]
- Grebe, A.; Latz, E. Cholesterol crystals and inflammation. Curr. Rheumatol. Rep. 2013, 15, 313. [Google Scholar] [CrossRef]
- Pope, R.M.; Tschopp, J. The role of interleukin-1 and the inflammasome in gout: Implications for therapy. Arthritis Rheum. 2007, 56, 3183–3188. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Park, Y.H.; Wood, G.; Kastner, D.L.; Chae, J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016, 17, 914–921. [Google Scholar] [CrossRef]
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013, 14, 454–460. [Google Scholar] [CrossRef]
- Robertson, S.; Martínez, G.J.; Payet, C.A.; Barraclough, J.Y.; Celermajer, D.S.; Bursill, C.; Patel, S. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin. Sci. 2016, 130, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.T.; Do, H.M.; Nguyen, T.T. Evaluation of Colchicine’s interaction with the ATP-binding region of mice NLRP3-NACHT domain using molecular docking and dynamics simulation. J. Phys. Conf. Ser. 2022, 2269, 012012. [Google Scholar] [CrossRef]
- Marques-Da-Silva, C.; Chaves, M.; Castro, N.; Coutinho-Silva, R.; Guimaraes, M. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: Implications for its therapeutic action. Br. J. Pharmacol. 2011, 163, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Bulnes, J.F.; Orellana, M.P.; Munoz Venturelli, P.; Martinez Rodriguez, G. The Role of Colchicine in Ather-Osclerosis: From Bench to Bedside. Pharmaceutics 2022, 14, 1395. [Google Scholar] [CrossRef]
- Cimmino, G.; Loffredo, F.S.; De Rosa, G.; Cirillo, P. Colchicine in Athero-Thrombosis: Molecular Mechanisms and Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 2483. [Google Scholar] [CrossRef]
- Pamuk, B.O.; Sari, I.; Selcuk, S.; Gokce, G.; Kozaci, D.L. Evaluation of circulating endothelial biomarkers in familialMediterranean fever. Rheumatol. Int. 2013, 33, 1967–1972. [Google Scholar] [CrossRef]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc. Pharmacol. 2016, 84, 1–7. [Google Scholar] [CrossRef]
- Kanuri, S.H.; Mehta, J.L. Role of Ox-LDL and LOX-1 in Atherogenesis. Curr. Med. Chem. 2019, 26, 1693–1700. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Gul, A. Behcet’s disease: An update on the pathogenesis. Clin. Exp. Rheumatol. 2001, 19, S6–12. [Google Scholar]
- Oeth, P.; Parry, G.C.; Mackman, N. Regulation of the tissue factor gene in human monocytic cells. Role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arter. Thromb. Vasc. Biol. 1997, 17, 365–374. [Google Scholar] [CrossRef]
- Kamal, A.; Goldstein, L.S. Connecting vesicle transport to the cytoskeleton. Curr. Opin. Cell Biol. 2000, 12, 503–508. [Google Scholar] [CrossRef]
- Cimmino, G.; Conte, S.; Morello, A.; Pellegrino, G.; Marra, L.; Calì, G.; Golino, P.; Cirillo, P. Colchicine inhibits the prothrombotic effects of oxLDL in human endothelial cells. Vasc. Pharmacol. 2020, 137, 106822. [Google Scholar] [CrossRef]
- Jackman, R.W.; Rhoads, M.G.; Cornwell, E.; Kandarian, S.C. Microtubule-mediated NFkappaB activation in the TNF-alpha signaling pathway. Exp. Cell Res. 2009, 315, 3242–3249. [Google Scholar] [CrossRef]
- Zhou, H.; Khan, D.; Hussain, S.M.; Gerdes, N.; Hagenbeck, C.; Rana, M.; Cornelius, J.F.; Muhammad, S. Colchicine prevents oxidative stress-induced endothelial cell senescence via blocking NF-κB and MAPKs: Implications in vascular diseases. J. Inflamm. 2023, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine After Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation. Eur. Heart J. 2023, 44, 2805–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Nakamura, M.; Wang, N.P.; Wilcox, J.N.; Shearer, S.; Ronson, R.S.; Guyton, R.A.; Vinten-Johansen, J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc. Res. 2000, 45, 651–666. [Google Scholar] [CrossRef]
- Robertson, S.; Martínez, G.J.; Payet, C.A.; Barraclough, J.Y.; Celermajer, D.S.; Bursill, C.; Patel, S. Colchicine Therapy in Patients with Coronary Disease: Mechanistic Insights and Clinical Implications. Circ. Cardiovasc. Interv. 2020, 13, e008888. [Google Scholar]
- Roubille, F.; Bouabdallaoui, N.; Kouz, S.; Waters, D.D.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Grégoire, J.C.; Gamra, H.; Kiwan, G.S.; et al. Low-Dose Colchicine in Patients with Type 2 Diabetes and Recent Myocardial Infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Diabetes Care 2024, 47, 467–470. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Tardif, J.-C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jolly, S.S.; d’Entremont, M.A.; Lee, S.F.; Mian, R.; Tyrwhitt, J.; Kedev, S.; Montalescot, G.; Cornel, J.H.; Stanković, G.; Moreno, R.; et al. Colchicine in acute myocardial infarction. N. Engl. J. Med. 2025, 392, 633–642. [Google Scholar] [CrossRef]
- Samuel, M.; Berry, C.; Dubé, M.-P.; Koenig, W.; López-Sendón, J.; Maggioni, A.P.; Pinto, F.J.; Roubille, F.; Tardif, J.-C. Long-term trials of colchicine for secondary prevention of vascular events: A meta-analysis. Eur. Heart J. 2025, 46, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, A.T.L.; Lin, A.; Kwiecinski, J.; Nolthenius, J.T.; McElhinney, P.; Grodecki, K.; Kietselaer, B.; Opstal, T.S.; Cornel, J.H.; Knol, R.J.; et al. Effect of low-dose colchicine on pericoronary inflammation and coronary plaque composition in chronic coronary disease: A subanalysis of the LoDoCo2 trial. Heart 2025, heartjnl-2024-325527. [Google Scholar] [CrossRef]
- Silvis, M.J.; Fiolet, A.T.; Opstal, T.S.; Dekker, M.; Suquilanda, D.; Zivkovic, M.; Duyvendak, M.; The, S.H.; Timmers, L.; Bax, W.A.; et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: A LoDoCo2 biomarker substudy. Atherosclerosis 2021, 334, 93–100. [Google Scholar] [CrossRef]
- Vidal-Gómez, X.; Vergori, L.; Dubois, S.; Gagnadoux, F.; Henni, S.; Veerapen, R.; Meilhac, O.; Muñoz-Picos, M.; Peiró, C.; Martinez, M.C.; et al. NLRP3, conveyed via extracellular vesicles from metabolic syndrome patients, is involved in atherosclerosis development. Cell Commun. Signal. 2025, 23, 284. [Google Scholar] [CrossRef]
- Opstal, T.S.; van Broekhoven, A.; Fiolet, A.T.; Mosterd, A.; Eikelboom, J.W.; Nidorf, S.M.; Thompson, P.L.; Budgeon, C.A.; Bartels, L.; de Nooijer, R.; et al. Long-Term Efficacy of Colchicine in Patients with Chronic Coronary Disease: Insights from LoDoCo2. Circulation 2022, 145, 626–628. [Google Scholar] [CrossRef]
- Burger, P.M.; Dorresteijn, J.A.N.; Fiolet, A.T.L.; Koudstaal, S.; Eikelboom, J.W.; Nidorf, S.M.; Thompson, P.L.; Cornel, J.H.; A Budgeon, C.; Westendorp, I.C.D.; et al. LoDoCo2 Trial Investigators; UCC-SMART Study Group; REACH Registry Investigators. Individual Lifetime Benefit from Low-Dose Colchicine in Patients with Chronic Coronary Artery Disease. Eur. J. Prev. Cardiol. 2023, 30, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Nidorf, M. Colchicine and the heart. Eur. Heart J. 2021, 42, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, V.; Ansell, G.; Galler, D. Colchicine overdose: The devil is in the detail. N. Zealand Med. J. 2007, 120, U2402. [Google Scholar]
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and new. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef]
- Putterman, C.; Ben-Chetrit, E.; Caraco, Y.; Levy, M. Colchicine intoxication: Clinical pharmacology, risk factors, features, and management. Semin. Arthritis Rheum. 1991, 21, 143–155. [Google Scholar] [CrossRef]
- Caraco, Y.; Putterman, C.; Rahamimov, R.; Ben-Chetrit, E. Acute colchicine intoxication—Possible role of erythromycin administration. J. Rheumatol. 1992, 19, 494–496. [Google Scholar]
- Donovan, J.W. Colchicine. In Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose; Shannon, M.W., Borron, S.W., Burns, M.J., Eds.; Saunders/Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Tan, M.S.; Gomez-Lumbreras, A.; Villa-Zapata, L.; Malone, D.C. Colchicine and macrolides: A cohort study of the risk of adverse outcomes associated with concomitant exposure. Rheumatol. Int. 2022, 42, 2253–2259. [Google Scholar] [CrossRef]
- Schwier, N.C.; Cornelio, C.K.; Boylan, P.M. A systematic review of the drug-drug interaction between statins and colchicine: Patient characteristics, etiologies, and clinical management strategies. Pharmacotherapy 2022, 42, 320–333. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, M.-M.; Zhao, H.; Qiu, X.-Y.; Zhu, D. Rhabdomyolysis associated with concomitant use of colchicine and statins in the real world: Identifying the likelihood of drug-drug interactions through the FDA adverse event reporting system. Front. Pharmacol. 2024, 15, 1445324. [Google Scholar] [CrossRef]
- Tong, D.C.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; Stub, D. Colchicine in Patients with Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation 2020, 142, 1890–1900. [Google Scholar] [CrossRef]
- D’aMario, D.; Cappetta, D.; Cappannoli, L.; Princi, G.; Migliaro, S.; Diana, G.; Chouchane, K.; Borovac, J.A.; Restivo, A.; Arcudi, A.; et al. Colchicine in ischemic heart disease: The good, the bad and the ugly. Clin. Res. Cardiol. 2021, 110, 1531–1542. [Google Scholar] [CrossRef]
- Imazio, M.; Andreis, A.; De Ferrari, G.M. Colchicine for cardiovascular diseases: From mechanisms to clinical applications. Eur. Heart J. 2021, 42, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Opstal, T.S.; Nidorf, S.M.; Fiolet, A.T.; Eikelboom, J.W.; Mosterd, A.; Bax, W.A.; Budgeon, C.A.; Ronner, E.; Prins, F.J.; Tijssen, J.G.; et al. Drivers of mortality in patients with chronic coronary disease in the low-dose colchicine 2 trial. Int. J. Cardiol. 2023, 372, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Ben-Chetrit, E.; Ridker, P.M. Low-dose colchicine for atherosclerosis: Long-term safety. Eur. Heart J. 2024, 45, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Livneh, A.; Vivante, A.; Afek, A.; Shamiss, A.; Derazne, E.; Tzur, D.; Ben-Zvi, I.; Tirosh, A.; Barchana, M.; et al. Mortality risk factors associated with familial Mediterranean fever among a cohort of 1.25 million adolescents. Ann. Rheum. Dis. 2014, 73, 704–709. [Google Scholar] [CrossRef]
- Libby, P. Targeting inflammation in atherosclerosis: The evolving role of interleukin-1β inhibition. Circ. Res. 2021, 129, 232–248. [Google Scholar]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Colchicine and the Heart—An Old Drug with New Tricks. N. Engl. J. Med. 2020, 383, 1881–1884. [Google Scholar]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537, https://doi.org/10.1093/eurheartj/ehae177. Erratum in: Eur. Heart J. 2025, 46, 1565. [Google Scholar] [CrossRef] [PubMed]
- Deftereos Deftereos, S.G.; Beerkens, F.J.; Shah, B.; Giannopoulos, G.; Vrachatis, D.A.; Giotaki, S.G.; Siasos, G.; Nicolas, J.; Arnott, C.; Patel, S.; et al. Colchicine in cardiovascular disease: In-depth review. Circulation 2022, 145, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Opstal Tjerk, S.J.; Hoogeveen Renate, M.; Fiolet Aernoud, T.L.; Silvis Max, J.M.; The Salem, H.K.; Bax Willem, A.; de Kleijn Dominique, P.V.; Mosterd, A.; Stroes Erik, S.G.; Cornel Jan, H. Colchicine Attenuates Inflammation Beyond the Inflammasome in Chronic Coronary Artery Disease. Circulation 2020, 142, 1996–1998. [Google Scholar] [CrossRef]
- Dubé, M.-P.; Legault, M.-A.; Lemaçon, A.; Perreault, L.-P.L.; Fouodjio, R.; Waters, D.D.; Kouz, S.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; et al. Pharmacogenomics of the Efficacy and Safety of Colchicine in COLCOT. Circ. Genom. Precis. Med. 2021, 14, e003183. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.L.; Rohde, P.D.; Dahl, J.N.; Rasmussen, L.D.; Nissen, L.; Schmidt, S.E.; McGilligan, V.; Gudbjartsson, D.F.; Stefansson, K.; Holm, H.; et al. Predicting the presence of coronary plaques featuring high-risk characteristics using polygenic risk scores and targeted proteomics in patients with suspected coronary artery disease. Genome Med. 2024, 16, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Keefe, J.H., Jr.; McCallister, B.D.; Bateman, T.M.; Kuhnlein, D.L.; Ligon, R.W.; Hartzler, G.O. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J. Am. Coll. Cardiol. 1992, 19, 1597–1600. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Raisakis, K.; Kossyvakis, C.; Kaoukis, A.; Panagopoulou, V.; Driva, M.; Hahalis, G.; Pyrgakis, V.; Alexopoulos, D.; et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J. Am. Coll. Cardiol. 2013, 61, 1679–1685. [Google Scholar] [CrossRef]
- Shah, B.; Pillinger, M.; Zhong, H.; Cronstein, B.; Xia, Y.; Lorin, J.D.; Smilowitz, N.R.; Feit, F.; Ratnapala, N.; Keller, N.M.; et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020, 13, e008717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaidya, K.; Arnott, C.; Martínez, G.J.; Ng, B.; McCormack, S.; Sullivan, D.R.; Celermajer, D.S.; Patel, S. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: A CT coronary angiography study. JACC Cardiovasc. Imaging 2018, 11, 305–316. [Google Scholar] [CrossRef]
- Tong, D.C.; Bloom, J.E.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; et al. Colchicine in patients with acute coronary syndrome: Two-year follow-up of the Australian COPS randomized clinical trial. Circulation 2021, 144, 1584–1586. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Imazio, M.; Rizzello, V.; Gatto, L.; Spinelli, A.; Aquilani, S.; Riccio, C.; Caldarola, P.; Nardi, F.; De Luca, L.; et al. Position paper ANMCO: La colchicina come agente terapeutico nelle sindromi coronariche [ANMCO Position paper: Colchicine as a therapeutic agent in coronary syndromes]. G. Ital. Cardiol. 2003, 24, 665–674, In Italian. [Google Scholar] [CrossRef]
- Tucker, B.; Tucker, W.J.; Chung, J.S.; A Figtree, G.; Keech, A.; Patel, S. The efficacy and safety of low dose colchicine in atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2025, zwaf302. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, J.; Jin, K.; Chen, X. Colchicine and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front. Cardiovasc. Med. 2022, 9, 947959. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; A Gemmato-Valecillos, M.; Martín, D.M.; De Sanctis, J.B.; Martí-Amarista, C.E.; Hidalgo, R.; Alegría-Barrero, E.; Lizardo, R.J.R.; Correa-Pérez, A. Colchicine for the primary prevention of cardiovascular events. Cochrane Database Syst. Rev. 2025, 2025, CD015003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dimitriadis, K.; Pyrpyris, N.; Iliakis, P.; Beneki, E.; Adamopoulou, E.; Papanikolaou, A.; Konstantinidis, D.; Fragkoulis, C.; Kollias, A.; Aznaouridis, K.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Patients Following Acute Coronary Syndromes: From Lipid Lowering and Plaque Stabilization to Improved Outcomes. J. Clin. Med. 2024, 13, 5040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurozumi, A.; Shishido, K.; Yamashita, T.; Sato, D.; Uchida, S.; Koyama, E.; Tamaki, Y.; Hayashi, T.; Miyashita, H.; Yokoyama, H.; et al. Sodium-Glucose Cotransporter-2 Inhibitors Stabilize Coronary Plaques in Acute Coronary Syndrome with Diabetes Mellitus. Am. J. Cardiol. 2024, 214, 47–54. [Google Scholar] [CrossRef] [PubMed]
| Trial | Setting | No. of Patients | Follow-Up | Primary Endpoint | Result |
|---|---|---|---|---|---|
| COLCOT [47] | Post-MI (<30 days) | 4745 | 22 months | CV death, MI, stroke, angina → revascularization | −23% (HR 0.77; p = 0.02) |
| LoDoCo [55] | Chronic CAD | 532 | 3 years | ACS, fatal or nonfatal out-of-hospital cardiac arrest, non-cardioembolic ischemic stroke | −67% (HR 0.33, p < 0.001) |
| LoDoCo2 [56] | Chronic CAD | 5522 | 28.6 months | CV death, MI, ischemic stroke | −31% (HR 0.69; p < 0.001) |
| CLEAR SYNERGY [53] | Acute MI with PCI | 7000+ | 3 years | CV death, MI, stroke | No significant benefit |
| Meta-analysis [54] | RCTs (21,800 patients) | 10,871 colchicine 10,929 placebo | 12–34 months | Composite MACE | −25% MACE vs. placebo |
| Drug Class/Interaction Type | Examples | Mechanism | Clinical Risk | Preventive Strategies |
|---|---|---|---|---|
| CYP3A4 Inhibitors | Clarithromycin, Erythromycin, Ketoconazole, Fluconazole, Diltiazem, Grapefruit juice, Nefazodone | Inhibit hepatic metabolism of colchicine | Increased colchicine levels Enhanced risk of myopathy, GI toxicity, multiorgan failure | Avoid combination in patients with renal/hepatic impairment; consider dose reduction or temporary discontinuation of colchicine in others |
| P-gp Inhibitors | Ciclosporin, Azithromycin, Carvedilol, Erythromycin, Lopinavir, Propafenone, Tacrolimus | Inhibit colchicine efflux Increased intracellular levels | Enhanced risk of colchicine accumulation and toxicity | Contraindicated in renal/hepatic impairment; monitor closely and reduce colchicine dose if co-use is necessary |
| Dual CYP3A4 and P-gp Inhibitors | Amiodarone, Verapamil, Clarithromycin, Cyclosporine, Dronedarone, Itraconazole, Ketoconazole, Ranolazine | Block both metabolism and excretion | High potential for severe colchicine toxicity, incl. rhabdomyolysis and bone marrow suppression | Avoid colchicine or use very low doses with close monitoring; avoid repeat dosing for ≥2 weeks in case of flares |
| Statins (Lipophilic) | Atorvastatin, Simvastatin, Lovastatin | Compete for CYP3A4 and P-gp; additive myotoxicity | Enhanced risk of myopathy, rhabdomyolysis | Prefer hydrophilic statins (e.g., rosuvastatin, pravastatin); monitor CK and muscle symptoms |
| Immunosuppressants | Tacrolimus, Cyclosporine | P-gp inhibition; impaired colchicine clearance | Enhanced risk of systemic colchicine toxicity | Avoid combination if possible; otherwise, use minimal colchicine dose and monitor closely |
| Antifungals (Azoles) | Itraconazole, Ketoconazole | Strong CYP3A4 inhibitors | Enhanced risk of colchicine overexposure | Avoid combination or significantly reduce colchicine dose |
| Calcium Channel Blockers | Verapamil, Diltiazem | CYP3A4 and P-gp inhibition | Enhanced colchicine toxicity | Use alternative agents or reduce colchicine dose; monitor closely |
| Antivirals | Ritonavir, Lopinavir | Strong CYP3A4/P-gp inhibition | Increased colchicine levels, especially in COVID-19 treatment | Use alternative anti-inflammatory agents; avoid colchicine unless no alternative exists |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giubilato, S.; Ciliberti, G.; Scicchitano, P.; Di Monaco, A.; Fortuni, F.; Zilio, F.; Ciampi, C.M.; Cangemi, S.; Spinelli, A.; Gatto, L.; et al. Colchicine and Atherosclerotic Coronary Artery Disease: An Updated Review. J. Clin. Med. 2025, 14, 6396. https://doi.org/10.3390/jcm14186396
Giubilato S, Ciliberti G, Scicchitano P, Di Monaco A, Fortuni F, Zilio F, Ciampi CM, Cangemi S, Spinelli A, Gatto L, et al. Colchicine and Atherosclerotic Coronary Artery Disease: An Updated Review. Journal of Clinical Medicine. 2025; 14(18):6396. https://doi.org/10.3390/jcm14186396
Chicago/Turabian StyleGiubilato, Simona, Giuseppe Ciliberti, Pietro Scicchitano, Antonio Di Monaco, Federico Fortuni, Filippo Zilio, Claudio Mario Ciampi, Stefano Cangemi, Antonella Spinelli, Laura Gatto, and et al. 2025. "Colchicine and Atherosclerotic Coronary Artery Disease: An Updated Review" Journal of Clinical Medicine 14, no. 18: 6396. https://doi.org/10.3390/jcm14186396
APA StyleGiubilato, S., Ciliberti, G., Scicchitano, P., Di Monaco, A., Fortuni, F., Zilio, F., Ciampi, C. M., Cangemi, S., Spinelli, A., Gatto, L., Franchin, L., Cornara, S., Magnesa, M., Sorini Dini, C., Vitale, E., Gasparetto, N., Geraci, G., Rossini, R., Della Bona, R., ... Imazio, M. (2025). Colchicine and Atherosclerotic Coronary Artery Disease: An Updated Review. Journal of Clinical Medicine, 14(18), 6396. https://doi.org/10.3390/jcm14186396











