Uncovering the New Biology of Giant Cell Arteritis to Guide Therapeutic Strategies
Abstract
1. Introduction
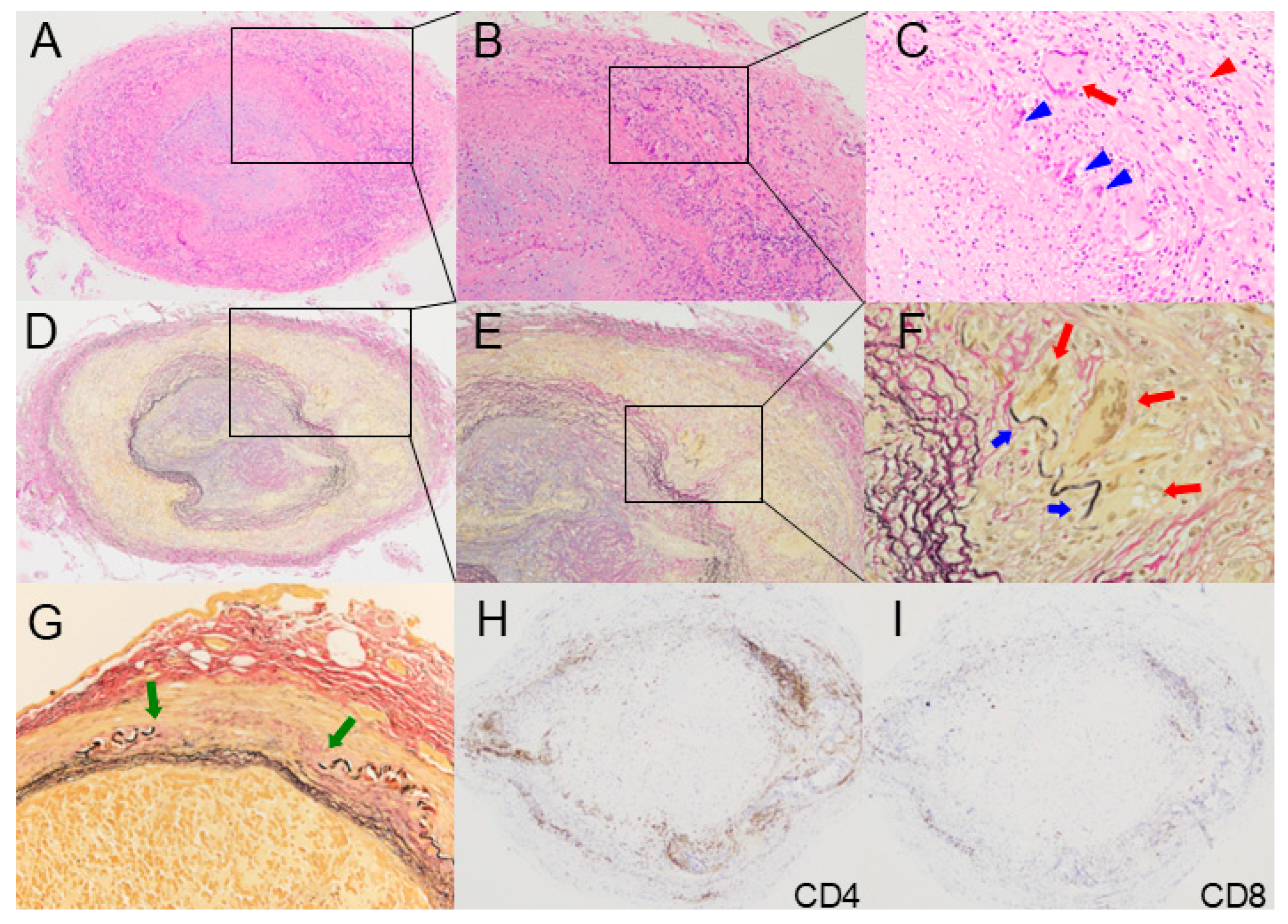
2. Histopathology
3. Pathophysiology
3.1. Genetic Factors
3.2. Environmental Factors
3.3. Immune Checkpoint Dysregulation
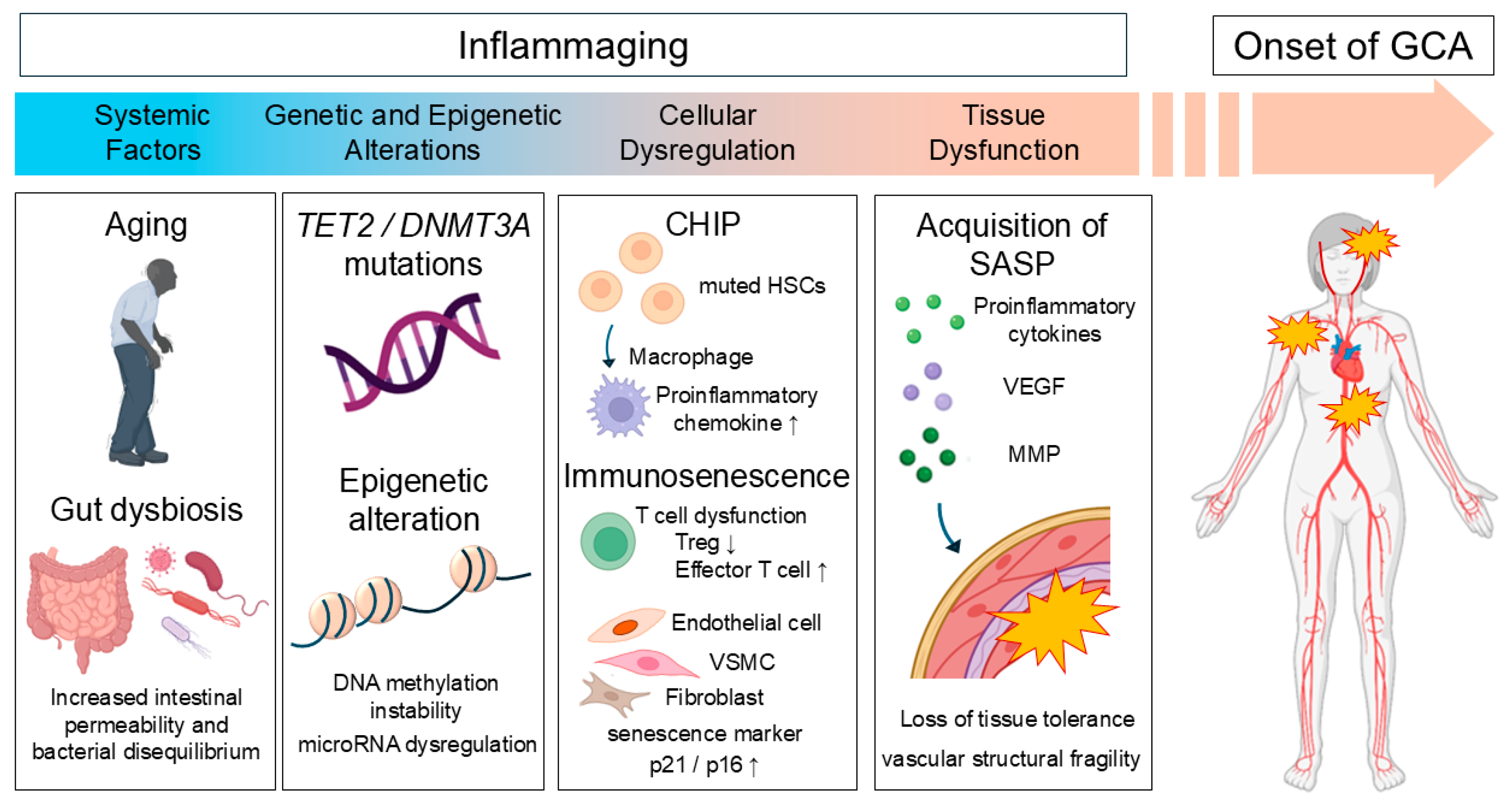
4. Aging and Inflammation
4.1. Epigenetic Alterations
4.2. Clonal Hematopoiesis of Indeterminate Potential (CHIP)
4.3. Immunosenescence
4.4. The Dual Roles of Senescence
5. Omics-Based Pathophysiological Insights
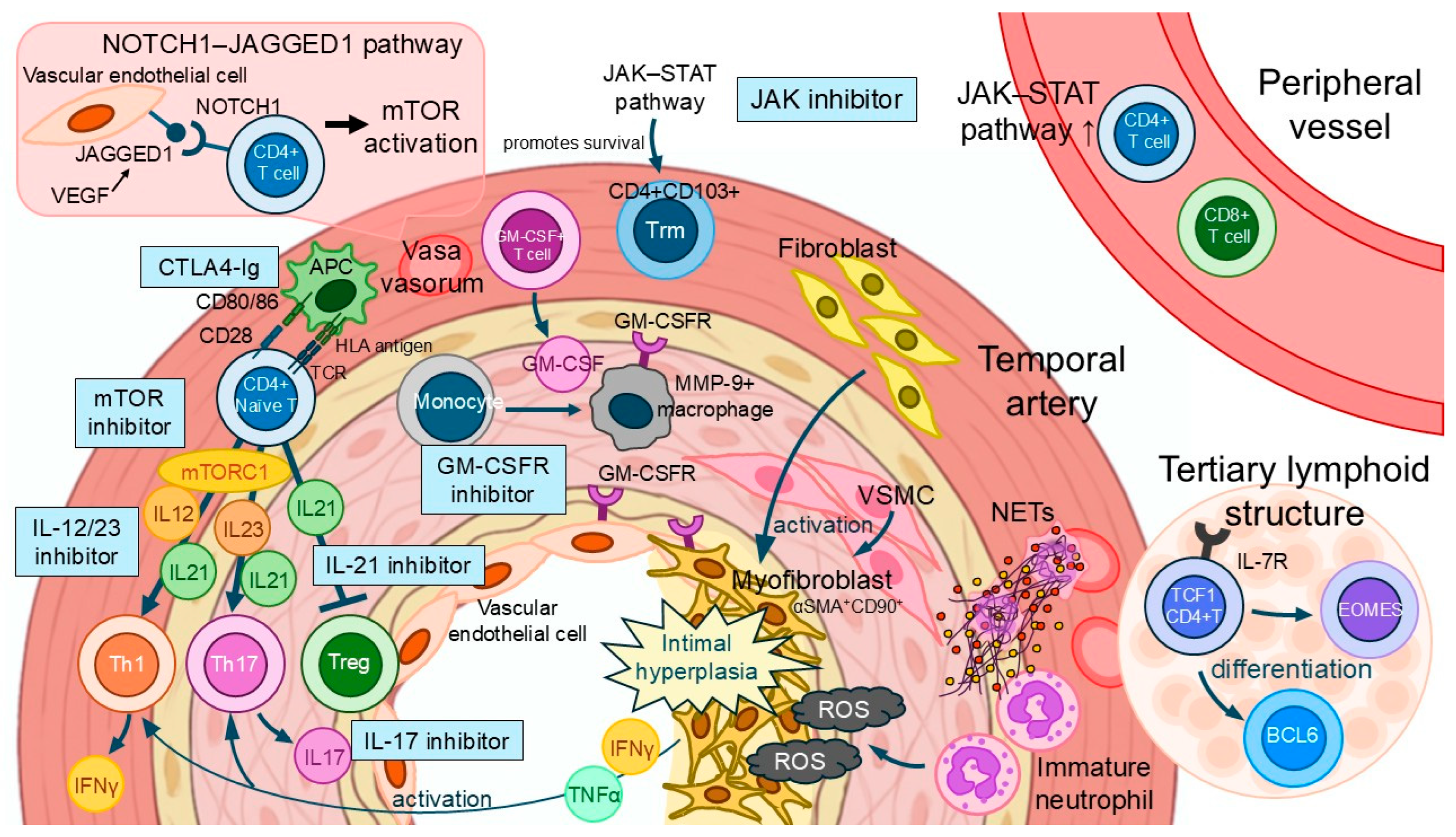
6. Current Therapeutic Strategies and Their Limitations
6.1. Glucocorticoid
6.2. Tocilizumab
6.3. JAK Inhibitor
6.4. GM-CSFR Inhibitor
6.5. CTLA-4 Ig
6.6. IL-17 Inhibitor
6.7. IL-12/IL-23 Inhibitor
6.8. IL-21 Inhibitor
7. Emerging Immune Cells and Potential Therapeutic Targets
7.1. Macrophage
7.2. Tissue-Resident Memory T Cell (Trm)
7.3. Stem-like T Cell
7.4. B Cell
7.5. Vascular Smooth Muscle Cell
7.6. Myofibroblast
7.7. Neutrophil
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weyand, C.M.; Goronzy, J.J. Medium- and large-vessel vasculitis. N. Engl. J. Med. 2003, 349, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lozano, D.; Hernández-Rodríguez, I.; Narvaez, J.; Domínguez-Álvaro, M.; De Miguel, E.; Silva-Díaz, M.; Belzunegui, J.M.; Moriano Morales, C.; Sánchez, J.; Galíndez-Agirregoikoa, E.; et al. Incidence and clinical manifestations of giant cell arteritis in Spain: Results of the ARTESER register. RMD Open 2024, 10, e003824. [Google Scholar] [CrossRef]
- Monti, S.; Milanesi, A.; Klersy, C.; Tomelleri, A.; Dagna, L.; Campochiaro, C.; Farina, N.; Muratore, F.; Galli, E.; Marvisi, C.; et al. Age at diagnosis influences the clinical phenotype, treatment strategies and outcomes in patients with giant cell arteritis: Results from the observational GCAGE study on a large cohort of 1004 patients. Ann. Rheum. Dis. 2023, 82, 1098–1106. [Google Scholar] [CrossRef]
- Hysa, E.; Mantello, E.; Gotelli, E.; Campitiello, R.; Cutolo, C.A.; Matteson, E.L. Environmental factors in polymyalgia rheumatica and giant cell arteritis. J. Environ. Rheumatol. 2025, 2. [Google Scholar] [CrossRef]
- Pugh, D.; Karabayas, M.; Basu, N.; Cid, M.C.; Goel, R.; Goodyear, C.S.; Grayson, P.C.; McAdoo, S.P.; Mason, J.C.; Owen, C.; et al. Large-vessel vasculitis. Nat. Rev. Dis. Primers 2022, 7, 93. [Google Scholar] [CrossRef]
- Karabayas, M.; Ibrahim, H.E.; Roelofs, A.J.; Reynolds, G.; Kidder, D.; De Bari, C. Vascular disease persistence in giant cell arteritis: Are stromal cells neglected? Ann. Rheum. Dis. 2024, 83, 1100–1109. [Google Scholar] [CrossRef]
- Carmona, F.D.; Vaglio, A.; Mackie, S.L.; Hernández-Rodríguez, J.; Monach, P.A.; Castañeda, S.; Solans, R.; Morado, I.C.; Narváez, J.; Ramentol-Sintas, M.; et al. A Genome-wide Association Study Identifies Risk Alleles in Plasminogen and P4HA2 Associated with Giant Cell Arteritis. Am. J. Hum. Genet. 2017, 100, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.D.; Coit, P.; Saruhan-Direskeneli, G.; Hernández-Rodríguez, J.; Cid, M.C.; Solans, R.; Castañeda, S.; Vaglio, A.; Direskeneli, H.; Merkel, P.A.; et al. Analysis of the common genetic component of large-vessel vasculitides through a meta-Immunochip strategy. Sci. Rep. 2017, 7, 43953. [Google Scholar] [CrossRef]
- Borrego-Yaniz, G.; Ortiz-Fernández, L.; Madrid-Paredes, A.; Kerick, M.; Hernández-Rodríguez, J.; Mackie, S.L.; Vaglio, A.; Castañeda, S.; Solans, R.; Mestre-Torres, J.; et al. Risk loci involved in giant cell arteritis susceptibility: A genome-wide association study. Lancet Rheumatol. 2024, 6, e374–e383. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Remuzgo-Martínez, S.; Ocejo-Vinyals, J.G.; Atienza-Mateo, B.; Genre, F.; Muñoz-Jimenez, A.; Ortiz-Sanjuán, F.; Romero-Yuste, S.; Moriano, C.; Galindez-Agirregoikoa, E.; et al. The presence of both HLA-DRB1*04:01 and HLA-B*15:01 increases the susceptibility to cranial and extracranial giant cell arteritis. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S129), 21–26. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, J.; Shan, X.; Jia, B.; Sang, S.; Wang, Y.; Wei, Y.; Hu, Y. Potential Key Genes for Giant Cell Arteritis Revealed Based on Single-Cell Sequencing and Mendelian Randomization Analysis. Int Arch Allergy Immunol 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Watanabe, R.; Zhang, H.; Hashimoto, M.; Morinobu, A.; Matsuda, F. Identification of the VLDLR locus associated with giant cell arteritis and the possible causal role of low-density lipoprotein cholesterol in its pathogenesis. Rheumatology 2024, 63, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Lidar, M.; Lipschitz, N.; Langevitz, P.; Shoenfeld, Y. The infectious etiology of vasculitis. Autoimmunity 2009, 42, 432–438. [Google Scholar] [CrossRef]
- Miyabe, C.; Miyabe, Y.; Miyata, R.; Ishiguro, N. Pathogens in Vasculitis: Is It Really Idiopathic? Jma J. 2021, 4, 216–224. [Google Scholar] [CrossRef]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef]
- Wu, M.; Liao, Z.; Zeng, K.; Jiang, Q. Exploring the causal role of gut microbiota in giant cell arteritis: A Mendelian randomization analysis with mediator insights. Front. Immunol. 2023, 14, 1280249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Vaglio, A.; Liao, Y.J.; Warrington, K.J.; Goronzy, J.J.; Weyand, C.M. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E970–E979. [Google Scholar] [CrossRef]
- Watanabe, R.; Zhang, H.; Berry, G.; Goronzy, J.J.; Weyand, C.M. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1052–H1059. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, W.; Zhao, L.; Zhao, Y.; Wang, F.; Lew, A.M.; Xu, Y. Self-Tolerance of Vascular Tissues Is Broken Down by Vascular Dendritic Cells in Response to Systemic Inflammation to Initiate Regional Autoinflammation. Front. Immunol. 2022, 13, 823853. [Google Scholar] [CrossRef]
- Hysa, E.; Camellino, D.; Dejaco, C.; Bauckneht, M.; Pesce, G.; Morbelli, S.; Bagnasco, M.; Cutolo, M.; Matteson, E.L.; Cimmino, M.A.; et al. Soluble Co-Inhibitory Immune Checkpoint Molecules Are Increased in Patients With Polymyalgia Rheumatica Without Significant Correlations With Clinical Status: A Case-Control Study. ACR Open Rheumatol. 2025, 7, e70045. [Google Scholar] [CrossRef]
- Monjo-Henry, I.; Nieto-Carvalhal, B.; Uyaguari, M.; García-Carazo, S.; Balsa, A.; de Miguel, E.; Miranda-Carús, M.E. Circulating PD-1hi CXCR5- and CXCR5+ CD4 T cells are elevated in patients with newly diagnosed giant cell arteritis, and predict relapse. Rheumatology 2025, 64, 3996–4004. [Google Scholar] [CrossRef]
- Régnier, P.; Le Joncour, A.; Maciejewski-Duval, A.; Darrasse-Jèze, G.; Dolladille, C.; Meijers, W.C.; Bastarache, L.; Fouret, P.; Bruneval, P.; Arbaretaz, F.; et al. CTLA-4 Pathway Is Instrumental in Giant Cell Arteritis. Circ. Res. 2023, 133, 298–312. [Google Scholar] [CrossRef]
- Sato, Y.; Tada, M.; Goronzy, J.J.; Weyand, C.M. Immune checkpoints in autoimmune vasculitis. Best. Pract. Res. Clin. Rheumatol. 2024, 38, 101943. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H., Jr.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Jylhä, M.; Paavilainen, P.; Lehtimäki, T.; Goebeler, S.; Karhunen, P.J.; Hervonen, A.; Hurme, M. Interleukin-1 receptor antagonist, interleukin-6, and C-reactive protein as predictors of mortality in nonagenarians: The vitality 90+ study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H.; Ladelund, S.; Pedersen, A.N.; Schroll, M.; Jørgensen, T.; Pedersen, B.K. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003, 132, 24–31. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Andersen-Ranberg, K.; Hjelmborg, J.; Pedersen, B.K.; Jeune, B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003, 115, 278–283. [Google Scholar] [CrossRef]
- Grants, J.M.; Wegrzyn, J.; Hui, T.; O’Neill, K.; Shadbolt, M.; Knapp, D.; Parker, J.; Deng, Y.; Gopal, A.; Docking, T.R.; et al. Altered microRNA expression links IL6 and TNF-induced inflammaging with myeloid malignancy in humans and mice. Blood 2020, 135, 2235–2251. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Su, T.Y.; Hauenstein, J.; Somuncular, E.; Dumral, Ö.; Leonard, E.; Gustafsson, C.; Tzortzis, E.; Forlani, A.; Johansson, A.S.; Qian, H.; et al. Aging is associated with functional and molecular changes in distinct hematopoietic stem cell subsets. Nat. Commun. 2024, 15, 7966. [Google Scholar] [CrossRef] [PubMed]
- Belizaire, R.; Wong, W.J.; Robinette, M.L.; Ebert, B.L. Clonal haematopoiesis and dysregulation of the immune system. Nat. Rev. Immunol. 2023, 23, 595–610. [Google Scholar] [CrossRef]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef]
- Patricia, H.; Sharon, C.; Robert, H.; David, K.; Richard, C. The role of immune aging in Giant Cell Arteritis. Aging Pathobiology and Therapeutics 2023, 5, 92–100. [Google Scholar] [CrossRef]
- Zhang, H.; Weyand, C.M.; Goronzy, J.J. Hallmarks of the aging T-cell system. Febs J. 2021, 288, 7123–7142. [Google Scholar] [CrossRef]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes. Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Coit, P.; De Lott, L.B.; Nan, B.; Elner, V.M.; Sawalha, A.H. DNA methylation analysis of the temporal artery microenvironment in giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1196–1202. [Google Scholar] [CrossRef]
- Croci, S.; Zerbini, A.; Boiardi, L.; Muratore, F.; Bisagni, A.; Nicoli, D.; Farnetti, E.; Pazzola, G.; Cimino, L.; Moramarco, A.; et al. MicroRNA markers of inflammation and remodelling in temporal arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1527–1533. [Google Scholar] [CrossRef]
- Bolha, L.; Hočevar, A.; Suljič, A.; Jurčić, V. Inflammatory Cell Composition and Immune-Related microRNA Signature of Temporal Artery Biopsies From Patients With Giant Cell Arteritis. Front. Immunol. 2021, 12, 791099. [Google Scholar] [CrossRef] [PubMed]
- Legido, A.; Sarría, A.; Bueno, M.; Garagorri, J.; Fleta, J.; Abós, M.D.; Pérez-González, J. Body fat distribution and hyperinsulinemia in childhood. Am. J. Clin. Nutr. 1988, 48, 686–687. [Google Scholar] [CrossRef]
- Bolha, L.; Hočevar, A.; Jurčić, V. Current state of epigenetics in giant cell arteritis: Focus on microRNA dysregulation. Autoimmun. Rev. 2025, 24, 103739. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.K.; Luo, M.; Rauh, M.J. Clonal hematopoiesis and inflammation: Partners in leukemogenesis and comorbidity. Exp. Hematol. 2020, 83, 85–94. [Google Scholar] [CrossRef]
- Aman, Y. Clonal hematopoiesis driven by microbial metabolite. Nat. Aging 2025, 5, 957. [Google Scholar] [CrossRef]
- Agarwal, P.; Sampson, A.; Hueneman, K.; Choi, K.; Jakobsen, N.A.; Uible, E.; Ishikawa, C.; Yeung, J.; Bolanos, L.; Zhao, X.; et al. Microbial metabolite drives ageing-related clonal haematopoiesis via ALPK1. Nature 2025, 642, 201–211. [Google Scholar] [CrossRef]
- Zeng, S.; Mu, D.; Wang, S. Fighting aging-associated clonal hematopoiesis with microbial metabolite. Trends Mol. Med. 2025, 31, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Park, S.; Martinez, K.; Gray, J.L.; Thowfeik, F.S.; Lundeen, J.A.; Kuhn, A.E.; Charboneau, D.J.; Gerlach, D.L.; Lockart, M.M.; et al. Ruthenium Complexes are pH-Activated Metallo Prodrugs (pHAMPs) with Light-Triggered Selective Toxicity Toward Cancer Cells. Inorg. Chem. 2017, 56, 7519–7532. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Marnell, C.S.; Bick, A.; Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cell Cardiol. 2021, 161, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- von Bonin, M.; Jambor, H.K.; Teipel, R.; Stölzel, F.; Thiede, C.; Damm, F.; Kroschinsky, F.; Schetelig, J.; Chavakis, T.; Bornhäuser, M. Clonal hematopoiesis and its emerging effects on cellular therapies. Leukemia 2021, 35, 2752–2758. [Google Scholar] [CrossRef]
- Pich, O.; Bernard, E.; Zagorulya, M.; Rowan, A.; Pospori, C.; Slama, R.; Huerga Encabo, H.; O’Sullivan, J.; Papazoglou, D.; Anastasiou, P.; et al. Tumor-Infiltrating Clonal Hematopoiesis. N. Engl. J. Med. 2025, 392, 1594–1608. [Google Scholar] [CrossRef]
- Weeks, L.D.; Ebert, B.L. Clonal Hematopoiesis as a Driver of Solid Tumors. N. Engl. J. Med. 2025, 392, 1654–1656. [Google Scholar] [CrossRef]
- Kim, P.G.; Niroula, A.; Shkolnik, V.; McConkey, M.; Lin, A.E.; Słabicki, M.; Kemp, J.P.; Bick, A.; Gibson, C.J.; Griffin, G.; et al. Dnmt3a-mutated clonal hematopoiesis promotes osteoporosis. J. Exp. Med. 2021, 218, e20211872. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Divaris, K.; Pan, B.; Li, X.; Lim, J.H.; Saha, G.; Barovic, M.; Giannakou, D.; Korostoff, J.M.; Bing, Y.; et al. Clonal hematopoiesis driven by mutated DNMT3A promotes inflammatory bone loss. Cell 2024, 187, 3690–3711.e3619. [Google Scholar] [CrossRef]
- Torreggiani, S.; Castellan, F.S.; Aksentijevich, I.; Beck, D.B. Somatic mutations in autoinflammatory and autoimmune disease. Nat. Rev. Rheumatol. 2024, 20, 683–698. [Google Scholar] [CrossRef]
- Gutierrez-Rodrigues, F.; Kusne, Y.; Fernandez, J.; Lasho, T.; Shalhoub, R.; Ma, X.; Alessi, H.; Finke, C.; Koster, M.J.; Mangaonkar, A.; et al. Spectrum of clonal hematopoiesis in VEXAS syndrome. Blood 2023, 142, 244–259. [Google Scholar] [CrossRef]
- Salzbrunn, J.B.; van Zeventer, I.A.; de Graaf, A.O.; Kamphuis, P.; van Bergen, M.; van Sleen, Y.; van der Reijden, B.A.; Schuringa, J.J.; Brouwer, E.; Diepstra, A.; et al. Clonal haematopoiesis and UBA1 mutations in individuals with biopsy-proven giant cell arteritis and population-based controls. Rheumatology 2024, 63, e45–e47. [Google Scholar] [CrossRef]
- Watanabe, R.; Kiji, M.; Hashimoto, M. Vasculitis associated with VEXAS syndrome: A literature review. Front. Med. 2022, 9, 983939. [Google Scholar] [CrossRef] [PubMed]
- Guedon, A.F.; Ouafdi, A.; Belfeki, N.; Dellal, A.; Ghriss, N.; Scheen, M.; Haidar, F.; Espitia, O.; Scoazec, J.Y.; Fain, O.; et al. Higher risk profile among patients with TET2-mutated giant cell arteritis: A cluster analysis. RMD Open 2024, 10, e004694. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rodrigues, F.; Wells, K.V.; Jones, A.I.; Hironaka, D.; Rankin, C.; Gadina, M.; Sikora, K.A.; Alemu, L.; Calado, R.T.; Quinn, K.A.; et al. Clonal haematopoiesis across the age spectrum of vasculitis patients with Takayasu’s arteritis, ANCA-associated vasculitis and giant cell arteritis. Ann. Rheum. Dis. 2024, 83, 508–517. [Google Scholar] [CrossRef]
- Bucala, R.; Tsao, B.P. The Emerging Spectrum of Somatic Mutation in Rheumatic Disease: Clonal Hematopoiesis Connects Aging With Giant Cell Arteritis. Arthritis Rheumatol. 2024, 76, 351–353. [Google Scholar] [CrossRef]
- Robinette, M.L.; Weeks, L.D.; Kramer, R.J.; Agrawal, M.; Gibson, C.J.; Yu, Z.; Sekar, A.; Mehta, A.; Niroula, A.; Brown, J.T.; et al. Association of Somatic TET2 Mutations With Giant Cell Arteritis. Arthritis Rheumatol. 2024, 76, 438–443. [Google Scholar] [CrossRef]
- Greigert, H.; Mounier, M.; Arnould, L.; Creuzot-Garcher, C.; Ramon, A.; Martin, L.; Tarris, G.; Ponnelle, T.; Audia, S.; Bonnotte, B.; et al. Heamatological malignancies in giant cell arteritis: A French population-based study. Rheumatology 2021, 60, 5408–5412. [Google Scholar] [CrossRef] [PubMed]
- Papo, M.; Friedrich, C.; Delaval, L.; Boysson, H.; Viallard, J.F.; Bachmeyer, C.; Sené, T.; Humbert, S.; Duffau, P.; Contis, A.; et al. Myeloproliferative neoplasms and clonal haematopoiesis in patients with giant cell arteritis: A case-control and exploratory study. Rheumatology 2022, 61, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Salman, B.; Ido, N.-M.; Rajesh, D.; Fernando, L.S.; Mehran, B.; Andrea, A.; Tracy, M.; Scott, V.B.; Steven, M.C.; Mark, D.M.; et al. Blood-Based Epigenetic Instability Linked to Human Aging and Disease. bioRxiv 2025. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hashimoto, M. Vasculitogenic T Cells in Large Vessel Vasculitis. Front. Immunol. 2022, 13, 923582. [Google Scholar] [CrossRef]
- Carrasco, E.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Desdín-Micó, G.; Aranda, J.F.; Mittelbrunn, M. The role of T cells in age-related diseases. Nat. Rev. Immunol. 2022, 22, 97–111. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019, 19, 573–583. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Successful and Maladaptive T Cell Aging. Immunity 2017, 46, 364–378. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef]
- Terrier, B.; Geri, G.; Chaara, W.; Allenbach, Y.; Rosenzwajg, M.; Costedoat-Chalumeau, N.; Fouret, P.; Musset, L.; Benveniste, O.; Six, A.; et al. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012, 64, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Parreau, S.; Warrington, K.J.; Koster, M.J.; Berry, G.J.; Goronzy, J.J.; Weyand, C.M. Regulatory T Cells in Autoimmune Vasculitis. Front. Immunol. 2022, 13, 844300. [Google Scholar] [CrossRef] [PubMed]
- Adriawan, I.R.; Atschekzei, F.; Dittrich-Breiholz, O.; Garantziotis, P.; Hirsch, S.; Risser, L.M.; Kosanke, M.; Schmidt, R.E.; Witte, T.; Sogkas, G. Novel aspects of regulatory T cell dysfunction as a therapeutic target in giant cell arteritis. Ann. Rheum. Dis. 2022, 81, 124–131. [Google Scholar] [CrossRef]
- Jiemy, W.F.; van Sleen, Y.; Graver, J.C.; Pringle, S.; Brouwer, E.; van der Geest, K.S.M.; Cornec, D.; Boots, A.M.H.; Sandovici, M. Indication of Activated Senescence Pathways in the Temporal Arteries of Patients With Giant Cell Arteritis. Arthritis Rheumatol. 2023, 75, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Watanabe, R.; Hashimoto, M. Aging-Related Vascular Inflammation: Giant Cell Arteritis and Neurological Disorders. Front. Aging Neurosci. 2022, 14, 843305. [Google Scholar] [CrossRef]
- Veroutis, D.; Argyropoulou, O.D.; Goules, A.V.; Kambas, K.; Palamidas, D.A.; Evangelou, K.; Havaki, S.; Polyzou, A.; Valakos, D.; Xingi, E.; et al. Senescent cells in giant cell arteritis display an inflammatory phenotype participating in tissue injury via IL-6-dependent pathways. Ann. Rheum. Dis. 2024, 83, 342–350. [Google Scholar] [CrossRef]
- Bouzid, H.; Belk, J.A.; Jan, M.; Qi, Y.; Sarnowski, C.; Wirth, S.; Ma, L.; Chrostek, M.R.; Ahmad, H.; Nachun, D.; et al. Clonal hematopoiesis is associated with protection from Alzheimer’s disease. Nat. Med. 2023, 29, 1662–1670. [Google Scholar] [CrossRef]
- Sánchez Vela, P.; Trowbridge, J.J.; Levine, R.L. Clonal hematopoiesis, aging and Alzheimer’s disease. Nat. Med. 2023, 29, 1605–1606. [Google Scholar] [CrossRef]
- López-de-Mesa-Aragón, J.; Silva-Buriticá, C.; Salazar-Londoño, S. Clonal hematopoiesis as the intersection between genetics and resilience in Alzheimer’s disease. Npj Dementia 2025, 1, 11. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Chen, H.; Han, M.; Zhang, M.; Liu, K.; Jin, H.; Liu, X.; Shi, M.; Pu, W.; et al. Identifying specific functional roles for senescence across cell types. Cell 2024, 187, 7314–7334.e7321. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, J.P. Cellular senescence in normal physiology. Science 2024, 384, 1300–1301. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.G.; Callejas-Rubio, J.L.; Raya, E.; Ríos-Fernández, R.; Villanueva-Martín, G.; Cid, M.C.; Hernández-Rodríguez, J.; Ballestar, E.; Timmermann, B.; Ortego-Centeno, N.; et al. Single-cell transcriptomic profiling reveals a pathogenic role of cytotoxic CD4(+) T cells in giant cell arteritis. J. Autoimmun. 2024, 142, 103124. [Google Scholar] [CrossRef] [PubMed]
- Reitsema, R.D.; van der Geest, K.S.M.; Sandovici, M.; Jiemy, W.F.; Graver, J.C.; Abdulahad, W.H.; Boots, A.M.H.; Heeringa, P.; Brouwer, E. Phenotypic, transcriptomic and functional profiling reveal reduced activation thresholds of CD8+ T cells in giant cell arteritis. Rheumatology 2022, 62, 417–427. [Google Scholar] [CrossRef]
- Ferrigno, I.; Bonacini, M.; Rossi, A.; Nicastro, M.; Muratore, F.; Boiardi, L.; Cavazza, A.; Bisagni, A.; Cimino, L.; Ghidini, A.; et al. Genes deregulated in giant cell arteritis by Nanostring nCounter gene expression profiling in temporal artery biopsies. RMD Open 2024, 10, e004600. [Google Scholar] [CrossRef]
- Parreau, S.; Molina, E.; Dumonteil, S.; Goulabchand, R.; Naves, T.; Bois, M.C.; Akil, H.; Terro, F.; Fauchais, A.L.; Liozon, E.; et al. Use of high-plex data provides novel insights into the temporal artery processes of giant cell arteritis. Front. Immunol. 2023, 14, 1237986. [Google Scholar] [CrossRef]
- Su, Y.; Jia, R.L.; Han, L.; Li, Z.G. Role of anti-nucleosome antibody in the diagnosis of systemic lupus erythematosus. Clin. Immunol. 2007, 122, 115–120. [Google Scholar] [CrossRef]
- Matsumoto, K.; Suzuki, K.; Magi, M.; Onishi, S.; Yoshida, H.; Takeshita, M.; Kuramoto, J.; Yazawa, M.; Kato, T.; Shimizu, H.; et al. Trans-omics landscape of systemic vasculitis identified matrix metalloproteinase 12 as a novel signature molecule. Rheumatology 2025, 64, 4766–4775. [Google Scholar] [CrossRef]
- Estupiñán-Moreno, E.; Ortiz-Fernández, L.; Li, T.; Hernández-Rodríguez, J.; Ciudad, L.; Andrés-León, E.; Terron-Camero, L.C.; Prieto-González, S.; Espígol-Frigolé, G.; Cid, M.C.; et al. Methylome and transcriptome profiling of giant cell arteritis monocytes reveals novel pathways involved in disease pathogenesis and molecular response to glucocorticoids. Ann. Rheum. Dis. 2022, 81, 1290–1300. [Google Scholar] [CrossRef]
- Wagner, A.D.; Wittkop, U.; Thalmann, J.; Willmen, T.; Gödecke, V.; Hodam, J.; Ronicke, S.; Zenke, M. Glucocorticoid Effects on Tissue Residing Immune Cells in Giant Cell Arteritis: Importance of GM-CSF. Front. Med. 2021, 8, 709404. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Younge, B.R.; Fritzlen, J.T.; Hunder, G.G.; Goronzy, J.J.; Warrington, K.J.; Weyand, C.M. Clinical and pathological evolution of giant cell arteritis: A prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod. Pathol. 2017, 30, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Henningson, H.; Hammar, B.; Mohammad, A.J. The use of intravenous methylprednisolone in giant cell arteritis: A population-based study. Rheumatology 2025, 64, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Muratore, F.; Marvisi, C.; Castrignanò, P.; Croci, S.; Bonacini, M.; Boiardi, L.; Ricordi, C.; Galli, E.; Besutti, G.; Spaggiari, L.; et al. Effectiveness and safety of a 26-week taper regimen of glucocorticoid in GCA patients: Results from a prospective cohort study. Semin. Arthritis Rheum. 2024, 64, 152351. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; Gewurz, D.; Gewurz-Singer, O. Tocilizumab in giant cell arteritis: An update for the clinician. Curr. Opin. Rheumatol. 2023, 35, 135–140. [Google Scholar] [CrossRef]
- Harigai, M.; Miyamae, T.; Hashimoto, H.; Umetsu, K.; Yamashita, K.; Nakaoka, Y. A multicentre, large-scale, observational study of tocilizumab in patients with giant cell arteritis in Japan. Mod. Rheumatol. 2024, 34, 775–783. [Google Scholar] [CrossRef]
- Unizony, S.; McCulley, T.J.; Spiera, R.; Pei, J.; Sidiropoulos, P.N.; Best, J.H.; Birchwood, C.; Pavlov, A.; Stone, J.H. Clinical outcomes of patients with giant cell arteritis treated with tocilizumab in real-world clinical practice: Decreased incidence of new visual manifestations. Arthritis Res. Ther. 2021, 23, 8. [Google Scholar] [CrossRef]
- Ito, T.; Fukui, S.; Nagase, F.N.; Yamaguchi, T.; Oda, N.; Inokuchi, H.; Suda, M.; Takizawa, N.; Suyama, Y.; Rokutanda, R.; et al. A feasible treatment strategy for tapering subcutaneous tocilizumab in giant cell arteritis: A 24-month multi-center retrospective study. Rheumatol. Int. 2025, 45, 45. [Google Scholar] [CrossRef]
- Moreel, L.; Betrains, A.; Molenberghs, G.; Vanderschueren, S.; Blockmans, D. Epidemiology and predictors of relapse in giant cell arteritis: A systematic review and meta-analysis. Jt. Bone Spine 2023, 90, 105494. [Google Scholar] [CrossRef]
- Ricordi, C.; Marvisi, C.; Macchioni, P.; Boiardi, L.; Cavazza, A.; Croci, S.; Bonacini, M.; Malchiodi, G.; Durmo, R.; Versari, A.; et al. Does tocilizumab eliminate inflammation in GCA? A cohort study on repeated temporal artery biopsies. RMD Open 2024, 10, e005132. [Google Scholar] [CrossRef]
- Loricera, J.; Tofade, T.; Prieto-Peña, D.; Romero-Yuste, S.; de Miguel, E.; Riveros-Frutos, A.; Ferraz-Amaro, I.; Labrador, E.; Maiz, O.; Becerra, E.; et al. Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature. Arthritis Res. Ther. 2024, 26, 116. [Google Scholar] [CrossRef]
- Blockmans, D.; Penn, S.K.; Setty, A.R.; Schmidt, W.A.; Rubbert-Roth, A.; Hauge, E.M.; Keen, H.I.; Ishii, T.; Khalidi, N.; Dejaco, C.; et al. A Phase 3 Trial of Upadacitinib for Giant-Cell Arteritis. N. Engl. J. Med. 2025, 392, 2013–2024. [Google Scholar] [CrossRef]
- Nash, P.; Kerschbaumer, A.; Konzett, V.; Aletaha, D.; Dörner, T.; Fleischmann, R.; McInnes, I.; Primdahl, J.; Sattar, N.; Tanaka, Y.; et al. Expert consensus statement on the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: 2024 update. Ann. Rheum. Dis. 2025, 84, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Corbera-Bellalta, M.; Alba-Rovira, R.; Muralidharan, S.; Espígol-Frigolé, G.; Ríos-Garcés, R.; Marco-Hernández, J.; Denuc, A.; Kamberovic, F.; Pérez-Galán, P.; Joseph, A.; et al. Blocking GM-CSF receptor α with mavrilimumab reduces infiltrating cells, pro-inflammatory markers and neoangiogenesis in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2022, 81, 524–536. [Google Scholar] [CrossRef]
- Cid, M.C.; Unizony, S.H.; Blockmans, D.; Brouwer, E.; Dagna, L.; Dasgupta, B.; Hellmich, B.; Molloy, E.; Salvarani, C.; Trapnell, B.C.; et al. Efficacy and safety of mavrilimumab in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2022, 81, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Langford, C.A.; Cuthbertson, D.; Ytterberg, S.R.; Khalidi, N.; Monach, P.A.; Carette, S.; Seo, P.; Moreland, L.W.; Weisman, M.; Koening, C.L.; et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Giant Cell Arteritis. Arthritis Rheumatol. 2017, 69, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Thiel, J. Giant cell arteritis-New treatment targets at the horizon. Semin. Arthritis Rheum. 2025, 72s, 152686. [Google Scholar] [CrossRef]
- Hur, B.; Koster, M.J.; Jang, J.S.; Weyand, C.M.; Warrington, K.J.; Sung, J. Global Transcriptomic Profiling Identifies Differential Gene Expression Signatures Between Inflammatory and Noninflammatory Aortic Aneurysms. Arthritis Rheumatol. 2022, 74, 1376–1386. [Google Scholar] [CrossRef]
- Zander, R.; Kasmani, M.Y.; Chen, Y.; Topchyan, P.; Shen, J.; Zheng, S.; Burns, R.; Ingram, J.; Cui, C.; Joshi, N.; et al. Tfh-cell-derived interleukin 21 sustains effector CD8(+) T cell responses during chronic viral infection. Immunity 2022, 55, 475–493.e475. [Google Scholar] [CrossRef]
- Liu, Y.W.; Fu, S.H.; Chien, M.W.; Hsu, C.Y.; Lin, M.H.; Dong, J.L.; Lu, R.J.; Lee, Y.J.; Chen, P.Y.; Wang, C.H.; et al. Blimp-1 molds the epigenetic architecture of IL-21-mediated autoimmune diseases through an autoregulatory circuit. JCI Insight 2022, 7, e151614. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; Segarra, M.; Vilardell, C.; Sánchez, M.; García-Martínez, A.; Esteban, M.J.; Queralt, C.; Grau, J.M.; Urbano-Márquez, A.; Palacín, A.; et al. Tissue production of pro-inflammatory cytokines (IL-1beta, TNFalpha and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology 2004, 43, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Maeda, T.; Zhang, H.; Berry, G.J.; Zeisbrich, M.; Brockett, R.; Greenstein, A.E.; Tian, L.; Goronzy, J.J.; Weyand, C.M. MMP (Matrix Metalloprotease)-9-Producing Monocytes Enable T Cells to Invade the Vessel Wall and Cause Vasculitis. Circ. Res. 2018, 123, 700–715. [Google Scholar] [CrossRef]
- Watanabe, R.; Hashimoto, M. Pathogenic role of monocytes/macrophages in large vessel vasculitis. Front. Immunol. 2022, 13, 859502. [Google Scholar] [CrossRef]
- Weinberger, T.; Esfandyari, D.; Messerer, D.; Percin, G.; Schleifer, C.; Thaler, R.; Liu, L.; Stremmel, C.; Schneider, V.; Vagnozzi, R.J.; et al. Ontogeny of arterial macrophages defines their functions in homeostasis and inflammation. Nat. Commun. 2020, 11, 4549. [Google Scholar] [CrossRef]
- Stock, A.T.; Parsons, S.; Sharma, V.J.; James, F.; Starkey, G.; D’Costa, R.; Gordon, C.L.; Wicks, I.P. Intimal macrophages develop from circulating monocytes during vasculitis. Clin. Transl. Immunol. 2022, 11, e1412. [Google Scholar] [CrossRef]
- Jiemy, W.F.; van Sleen, Y.; van der Geest, K.S.; Ten Berge, H.A.; Abdulahad, W.H.; Sandovici, M.; Boots, A.M.; Heeringa, P.; Brouwer, E. Distinct macrophage phenotypes skewed by local granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) are associated with tissue destruction and intimal hyperplasia in giant cell arteritis. Clin. Transl. Immunol. 2020, 9, e1164. [Google Scholar] [CrossRef]
- Watanabe, N.; Hara, Y.; Nishito, Y.; Kounoe, M.; Sekiyama, K.; Takamasu, E.; Kise, T.; Chinen, N.; Shimada, K.; Sugihara, M.; et al. Tissue degrading and remodelling molecules in giant cell arteritis. Rheumatology 2025, 64, 3095–3103. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Tian, L.; Goronzy, J.J.; Weyand, C.M. Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis. Circulation 2018, 137, 1934–1948. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Nadler, S.G.; Goronzy, J.J.; Weyand, C.M. CD28 Signaling Controls Metabolic Fitness of Pathogenic T Cells in Medium and Large Vessel Vasculitis. J. Am. Coll. Cardiol. 2019, 73, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hashimoto, M. Perspectives of JAK Inhibitors for Large Vessel Vasculitis. Front. Immunol. 2022, 13, 881705. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Y.; Ma, X.; Zhang, Y. Causal genes identification of giant cell arteritis in CD4+ Memory t cells: An integration of multi-omics and expression quantitative trait locus analysis. Inflamm. Res. 2025, 74, 3. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Jain, A.; Ohtsuki, S.; Okuyama, H.; Sturmlechner, I.; Takashima, Y.; Le, K.C.; Bois, M.C.; Berry, G.J.; Warrington, K.J.; et al. Stem-like CD4(+) T cells in perivascular tertiary lymphoid structures sustain autoimmune vasculitis. Sci. Transl. Med. 2023, 15, eadh0380. [Google Scholar] [CrossRef] [PubMed]
- Graver, J.C.; Jiemy, W.F.; Altulea, D.H.A.; van Sleen, Y.; Xu, S.; van der Geest, K.S.M.; Verstappen, G.; Heeringa, P.; Abdulahad, W.H.; Brouwer, E.; et al. Cytokine producing B-cells and their capability to polarize macrophages in giant cell arteritis. J. Autoimmun. 2023, 140, 103111. [Google Scholar] [CrossRef] [PubMed]
- Pesce, E.; Bombaci, M.; Croci, S.; Bonacini, M.; Marvisi, C.; Ricordi, C.; Monti, S.; Muratore, F.; Abrignani, S.; Caporali, R.; et al. Identification of two autoantigens recognised by circulating autoantibodies as potential biomarkers for diagnosing giant cell arteritis. Clin. Exp. Rheumatol. 2024, 42, 1317–1320. [Google Scholar] [CrossRef]
- Lozano, E.; Segarra, M.; García-Martínez, A.; Hernández-Rodríguez, J.; Cid, M.C. Imatinib mesylate inhibits in vitro and ex vivo biological responses related to vascular occlusion in giant cell arteritis. Ann. Rheum. Dis. 2008, 67, 1581–1588. [Google Scholar] [CrossRef]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef]
- Greigert, H.; Genet, C.; Ramon, A.; Bonnotte, B.; Samson, M. New Insights into the Pathogenesis of Giant Cell Arteritis: Mechanisms Involved in Maintaining Vascular Inflammation. J. Clin. Med. 2022, 11, 2905. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Immune mechanisms in medium and large-vessel vasculitis. Nat. Rev. Rheumatol. 2013, 9, 731–740. [Google Scholar] [CrossRef]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [CrossRef]
- Xu, S.; Jiemy, W.F.; Brouwer, E.; Burgess, J.K.; Heeringa, P.; van der Geest, K.S.M.; Alba-Rovira, R.; Corbera-Bellalta, M.; Boots, A.H.; Cid, M.C.; et al. Current evidence on the role of fibroblasts in large-vessel vasculitides: From pathogenesis to therapeutics. Autoimmun. Rev. 2024, 23, 103574. [Google Scholar] [CrossRef]
- Parreau, S.; Vedrenne, N.; Regent, A.; Richard, L.; Sindou, P.; Mouthon, L.; Fauchais, A.L.; Jauberteau, M.O.; Ly, K.H. An immunohistochemical analysis of fibroblasts in giant cell arteritis. Ann. Diagn. Pathol. 2021, 52, 151728. [Google Scholar] [CrossRef] [PubMed]
- Greigert, H.; Ramon, A.; Genet, C.; Cladière, C.; Gerard, C.; Cuidad, M.; Corbera-Bellalta, M.; Alba-Rovira, R.; Arnould, L.; Creuzot-Garcher, C.; et al. Neointimal myofibroblasts contribute to maintaining Th1/Tc1 and Th17/Tc17 inflammation in giant cell arteritis. J. Autoimmun. 2024, 142, 103151. [Google Scholar] [CrossRef]
- Wang, L.; Luqmani, R.; Udalova, I.A. The role of neutrophils in rheumatic disease-associated vascular inflammation. Nat. Rev. Rheumatol. 2022, 18, 158–170. [Google Scholar] [CrossRef]
- Wang, L.; Ai, Z.; Khoyratty, T.; Zec, K.; Eames, H.L.; van Grinsven, E.; Hudak, A.; Morris, S.; Ahern, D.; Monaco, C.; et al. ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI Insight 2020, 5, e139163. [Google Scholar] [CrossRef]
- Palamidas, D.A.; Argyropoulou, O.D.; Georgantzoglou, N.; Karatza, E.; Xingi, E.; Kapsogeorgou, E.K.; Anagnostopoulos, C.D.; Lazaris, A.C.; Ritis, K.; Goules, A.V.; et al. Neutrophil extracellular traps in giant cell arteritis biopsies: Presentation, localization and co-expression with inflammatory cytokines. Rheumatology 2022, 61, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, D.; Duvvuri, B.; Kuley, R.; Cuthbertson, D.; Grayson, P.C.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; McAlear, C.A.; Moreland, L.W.; et al. Neutrophil activation in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis and large-vessel vasculitis. Arthritis Res. Ther. 2022, 24, 160. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Argento, F.R.; Fini, E.; Bello, F.; Di Scala, G.; Taddei, N.; Emmi, G.; Prisco, D.; Becatti, M.; Fiorillo, C. ROS-driven structural and functional fibrinogen modifications are reverted by interleukin-6 inhibition in Giant Cell Arteritis. Thromb. Res. 2023, 230, 1–10. [Google Scholar] [CrossRef]

| Year | Specimen | N | C | Modality/Platform | Key Findings/Potential Therapeutic Target | PMID |
|---|---|---|---|---|---|---|
| 2022 | GCA PBMC | 3 | 3 | scRNA-seq /10x Chromium | Genes associated with antiviral defense and immune activation (KLRD1, IFITM1) were upregulated, whereas those involved in cytotoxicity and proliferation control (GNLY, ZFP36L2) were downregulated. | 35460236 |
| 2022 | GCA Arterial tissue | 3 | None | scRNA-seq, TCR repertoire /10x Chromium | TCF1+IL7R+ stem-like CD4+ T cells expand clonally in TLSs, suggesting IL-7R+ CD4+ T cells as pathogenic and therapeutic targets. | 37672564 |
| 2023 | GCA TAB | 9 | 7 | Spatial transcriptomics /NanoString GeoMx DSP | CD74 and macrophage-associated pathways—including MMP2, MMP9, and CXCR4—were identified as key upregulated targets in GCA, particularly within the intima and media layers. | 37744332 |
| 2024 | GCA PBMC | 8 | 8 | scRNA-seq, TCR repertoire /10x Genomics Chromium | Clonally expanded cytotoxic CD4+ T cells in active GCA highly expressed GZMB and CCL5, suggesting Maraviroc as a potential therapy. | 37952293 |
| 2024 | GCA TAB | 49 | 7 | Gene expression profiling /NanoString nCounter | In TMI lesions, genes encoding TNF superfamily members, immune checkpoints, chemokines and their receptors, toll-like receptors, complement components, Fc receptors for IgG, signaling lymphocytic activation molecules, as well as JAK3, STAT1, and STAT4 were upregulated. | 39317454 |
| 2024 | GCA PBMC * | (a) 40 (b) 14 | (a) 31 (b) 6 | (a) Bulk RNA-seq (b) scRNA-seq /Illumina, 10x Chromium | Targeting DDIT4, a causal gene for GCA, may suppress persistent inflammation in CD4+ memory T cells. | 39762453 |
| 2024 | GCA TAB | 10 | 6 | Gene expression profiling /Agilent microarray | MMP12, ACP5, and TREM2 are potential therapeutic targets in GCA, associated with macrophage-driven tissue destruction, while LRRC15 marks myofibroblasts contributing to intimal hyperplasia in granulomatous inflammation. | 39837478 |
| 2025 | SV (c) Serum, whole blood, tissue | 332 (c) GCA2, GPA1 | 30 | Proteome analysis, RNA sequencing, Spatial transcriptomics /Olink Proteomics, Illumina. 10x Genomics | MMP12 is selectively elevated in vasculitis and specifically expressed in CD206-positive macrophages and multinucleated giant cells within lesions. It reflects disease activity even under IL-6 blockade and helps predict relapse. | 40139687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiomi, M.; Watanabe, R.; Ishihara, R.; Tanaka, S.; Kageyama, G.; Hashimoto, M. Uncovering the New Biology of Giant Cell Arteritis to Guide Therapeutic Strategies. J. Clin. Med. 2025, 14, 6350. https://doi.org/10.3390/jcm14186350
Shiomi M, Watanabe R, Ishihara R, Tanaka S, Kageyama G, Hashimoto M. Uncovering the New Biology of Giant Cell Arteritis to Guide Therapeutic Strategies. Journal of Clinical Medicine. 2025; 14(18):6350. https://doi.org/10.3390/jcm14186350
Chicago/Turabian StyleShiomi, Mayu, Ryu Watanabe, Ryuhei Ishihara, Sayaka Tanaka, Goichi Kageyama, and Motomu Hashimoto. 2025. "Uncovering the New Biology of Giant Cell Arteritis to Guide Therapeutic Strategies" Journal of Clinical Medicine 14, no. 18: 6350. https://doi.org/10.3390/jcm14186350
APA StyleShiomi, M., Watanabe, R., Ishihara, R., Tanaka, S., Kageyama, G., & Hashimoto, M. (2025). Uncovering the New Biology of Giant Cell Arteritis to Guide Therapeutic Strategies. Journal of Clinical Medicine, 14(18), 6350. https://doi.org/10.3390/jcm14186350






